10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2019; 15(5):1010-1019. doi:10.7150/ijbs.29680 This issue Cite
Research Paper
Metformin Inhibits the NLRP3 Inflammasome via AMPK/mTOR-dependent Effects in Diabetic Cardiomyopathy
1. Department of Endocrinology, The Second Affiliated Hospital of Harbin Medical University, Harbin, China;
2. Translational Medicine Research and Cooperation Center of Northern China, Heilongjiang Academy of Medical Sciences, Harbin, China;
3. Department of Geriatrics, The Second Affiliated Hospital of Harbin Medical University, Harbin, China;
4. Department of Cardiology, The Second Affiliated Hospital of Harbin Medical University, Harbin, China;
5. Department of Pharmacology (State-Province Key Laboratories of Biomedicine-Pharmaceutics of China, Key Laboratory of Cardiovascular Medicine Research, Ministry of Education), College of Pharmacy, Harbin Medical University, Harbin, China.
Abstract
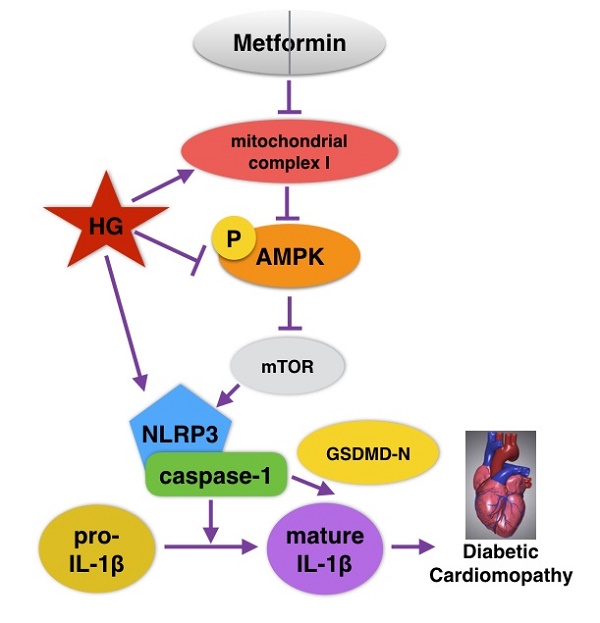
Metformin is a widely used antidiabetic drug for type 2 diabetes that can play a cardioprotective role through multiple pathways. It is a recognized agonist of AMP-activated protein kinase (AMPK) that blocks mitochondrial complex I. The NLRP3 inflammasome has been demonstrated to be activated in diabetic cardiomyopathy (DCM). However, the role of metformin in regulating the NLRP3 signaling pathway in DCM remains unclear. It has been reported that AMPK can inhibit NLRP3 by activating autophagy. The aim of this study was to investigate whether metformin can inhibit the NLRP3 inflammasome by activating the AMPK/mTOR pathway in DCM. In this study, streptozotocin-induced C57BL/6 mice and high glucose-treated primary cardiomyocytes from neonatal mice were treated with metformin or an AMPK inhibitor compound C. Echocardiography, hematoxylin-eosin and Masson staining showed that the function and morphology of the diabetic hearts were improved after metformin treatment, whereas these parameters deteriorated after intervention with an AMPK inhibitor. Immunohistochemical staining, immunofluorescence staining and western blot assays indicated that the expression levels of mTOR, NLRP3, caspase-1, IL-1β and GSDMD-N were decreased in the diabetic model treated with metformin and were reversed after the administration of an AMPK inhibitor in vivo and in vitro. Mechanistically, our results demonstrated that metformin can activate AMPK, thus improving autophagy via inhibiting the mTOR pathway and alleviating pyroptosis in DCM. Thus, we provide novel information for the treatment of DCM.
Keywords: metformin, AMPK, autophagy, NLRP3 inflammasome, diabetic cardiomyopathy

 Global reach, higher impact
Global reach, higher impact