10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2019; 15(5):1020-1029. doi:10.7150/ijbs.27825 This issue Cite
Research Paper
Inflammatory and Senescent Phenotype of Pancreatic Stellate Cells Induced by Sqstm1 Downregulation Facilitates Pancreatic Cancer Progression
1. Department of Hepatopancreatobiliary Surgery, the Fifth Affiliated Hospital of Wenzhou Medical University, Lishui Hospital of Zhejiang University, School of Medicine, Lishui, Zhejiang 323000, P.R. China
2. Department of Gastrointestinal Surgery, The First Affiliated Hospital of Zhejiang Chinese Medicine University, Hangzhou, Zhejiang, China
3. Department of Hepatopancreatobiliary Surgery, Jiaxing Second Hospital, Jiaxing, Zhejiang, China
4. Department of Surgery, the Second Affiliated Hospital of Zhejiang University, Hangzhou, Zhejiang, China
5. Cancer Institute of Integrated traditional Chinese and Western Medicine, Key laboratory of cancer prevention and therapy combining traditional Chinese and Western Medicine, Zhejiang Academy of Traditional Chinese Medicine, Hangzhou, Zhejiang, 310012, China
6. Department of Medical Oncology, Tongde hospital of Zhejiang Province, Hangzhou, Zhejiang, 310012, China
*These authors contributed equally to this work.
Abstract
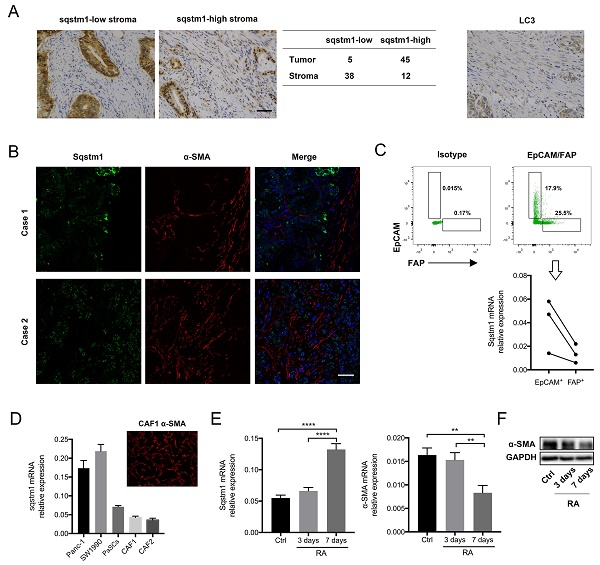
Pancreatic ductal adenocarcinoma (PDAC) has unique microenvironment with extensive infiltration of fibroblasts, which are mainly derived from the resident pancreatic stellate cells (PaSCs). As activated PaSCs constitute a major contributor to pancreatic cancer progression, the mechanisms underlying their activation have been being intensively studied. Previous studies showed that Sequestosome-1 (sqstm1) can modulate the functional status of fibroblasts in cancer. Here, we further delineated the role of sqstm1 in PaSCs. The analysis of PDAC patient samples revealed reduction of sqstm1 expression in activated PaSCs in both mRNA and protein level. Downregulated sqstm1 via shRNA in PaSCs led to an inflammatory and senescent phenotype with increased IL8, CXCL1, and CXCL2 expression. Further analysis demonstrated that increased intracellular reactive oxygen species level contributed to the senescence in sqstm1-downregulated PaSCs. This was mediated via impaired NRF2 activity since reduced sqstm1 resulted in accumulation of KEAP1. Meanwhile, we found that sqstm1 degradation caused by enhanced autophagy was not associated with transformation of senescent phenotype. At last, the data revealed that sqstm1-downregulated PaSCs promoted pancreatic tumor cell growth, invasion, and macrophage phenotype transformation. Collectively, the current study indicated that sqstm1 controlled transformation of senescent phenotype of PaSCs, which in turn is pro-tumorigenic.
Keywords: sqstm1, pancreatic stellate cells, pancreatic adenocarcinoma, reactive oxygen species, senescence

 Global reach, higher impact
Global reach, higher impact