10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2019; 15(9):1962-1976. doi:10.7150/ijbs.35440 This issue Cite
Research Paper
Microtubule-associated protein 4 phosphorylation regulates epidermal keratinocyte migration and proliferation
1. Institute of Burn Research, Southwest Hospital, Third Military Medical University (Army Medical University), Chongqing, China.
2. State Key Laboratory of Trauma, Burns and Combined Injury, Southwest Hospital, Third Military Medical University (Army Medical University), Chongqing, China.
3. Endocrinology Department, Southwest Hospital, Third Military Medical University (Army Medical University), Chongqing, China.
* These authors contributed equally to this work.
Abstract
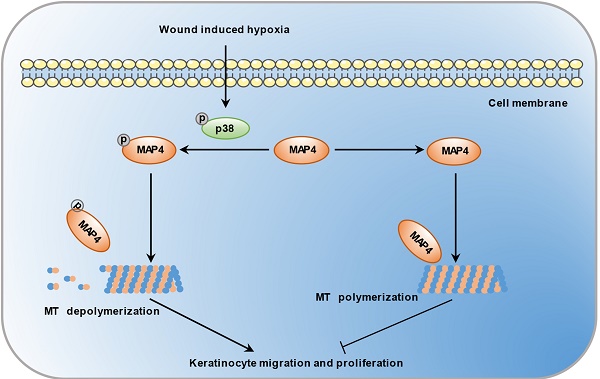
Both cell migration and proliferation are indispensable parts of reepithelialization during skin wound healing, which is a complex process for which the underlying molecular mechanisms are largely unknown. Here, we identify a novel role for microtubule-associated protein 4 (MAP4), a cytosolic microtubule-binding protein that regulates microtubule dynamics through phosphorylation modification, as a critical regulator of epidermal wound repair. We showed that MAP4 phosphorylation was induced in skin wounds. In an aberrant phosphorylated MAP4 mouse model, hyperphosphorylation of MAP4 (S737 and S760) accelerated keratinocyte migration and proliferation and skin wound healing. Data from both primary cultured keratinocytes and HaCaT cells in vitro revealed the same results. The promigration and proproliferation effects of MAP4 phosphorylation depended on microtubule rearrangement and could be abolished by MAP4 dephosphorylation. We also identified p38/MAPK as an upstream regulator of MAP4 phosphorylation in keratinocytes. Our findings provide new insights into the molecular mechanisms underlying wound-associated keratinocyte migration and proliferation and identify potential targets for the remediation of defective wound healing.
Keywords: keratinocyte, MAP4, phosphorylation, p38/MAPK, migration, proliferation

 Global reach, higher impact
Global reach, higher impact