10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2020; 16(4):633-643. doi:10.7150/ijbs.38414 This issue Cite
Research Paper
Tumor Cell-associated Exosomes Robustly Elicit Anti-tumor Immune Responses through Modulating Dendritic Cell Vaccines in Lung Tumor
1. Department of anatomy, School of Medicine, Jinan University, Guangzhou 510632, China
2. Shenzhen Key Laboratory of Stem cell research and clinical transformation, Guangdong Engineering Technology Research Center of Stem cell and Cell therapy, Translational Medicine Collaborative Innovation Center, The Second Clinical Medical College (Shenzhen People's Hospital), Jinan University, Shenzhen 518020, China
Abstract
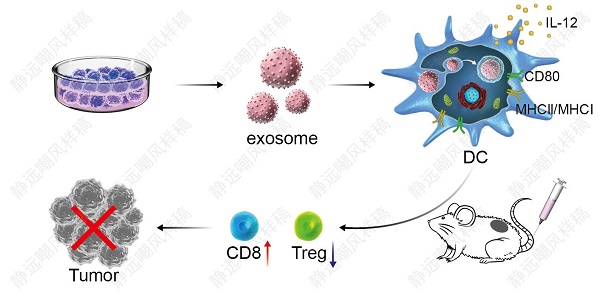
DC vaccine-based immunotherapy is emerging as a novel therapeutic strategy for cancer treatment, however, antitumor effect of DC vaccines based on tumor cell lysates (TCLs) remains unsatisfactory due to poor immunogenicity of tumor antigens. Although tumor-associated exosomes (TAEs) have been reported as a promising antigen for DC vaccines, it remains unclear how TAE-based DC vaccine induced antitumor immunity in lung cancer.
Methods: In the present study, we extracted TAEs from the supernatant of tumor cell culture medium, and compared the effect of TAEs with TCLs on DCs. To further evaluate the therapeutic effect of DCTAE, we used immunofluorescence and flow cytometry to evaluate the apoptosis of tumor tissue, tumor-infiltrating CD8+ T cells and Tregs in TDLNs and spleen. Then the levels of cytokines of IL-12, IFN-γ, L-10 and TGF-β were quantified by ELISA assays.
Results: Our data showed that TAEs were more potent than TCLs to promote DC maturation and enhance MHC cross presentation, which directly contributed to more robust tumor-specific cytotoxic T lymphocyte (CTL) response. More importantly, TAEs reduced the expression of PD-L1 of DCs, thereby led to down-regulated population of Tregs in vitro. Moreover, DCTAE remarkably suppressed the tumor growth and prolonged survival rate in vivo, due to participance of CD8+ T cells and decreased Tregs in TDLNs and spleen.
Conclusion: TAEs could serve to improve vaccine-elicited immunotherapy by triggering stronger DC-mediated immune responses and decreasing Tregs in the tumor microenvironment.
Keywords: tumor cell-associated exosome, immune response, DC vaccine, immunosuppression, lung tumor

 Global reach, higher impact
Global reach, higher impact