10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2020; 16(9):1526-1535. doi:10.7150/ijbs.42966 This issue Cite
Research Paper
PPARγ inhibition boosts efficacy of PD-L1 Checkpoint Blockade Immunotherapy against Murine Melanoma in a sexually dimorphic manner
1. Department of Biochemistry and Molecular Medicine, School of Medicine and Health Sciences, The George Washington University, Washington, DC 20037, USA.
2. Department of Molecular Medicine, University of Texas Health San Antonio, San Antonio, TX 78229, USA.
3. Department of Medicine, University of Texas Health San Antonio, San Antonio, TX 78229, USA.
Abstract
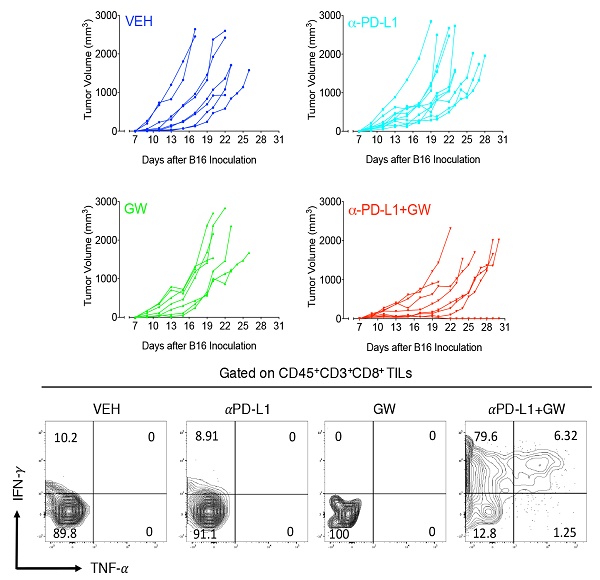
Immune checkpoint blockade-based immunotherapy has become standard of care for multiple cancer types. However, the overall response rates among various cancer types still remain unsatisfactory. There is a pressing clinical need to identify combination therapies to improve efficacy of anticancer immunotherapy. We previously showed that pharmacologic inhibition of PPARγ by GW9662 boosts αPD-L1 and αPD-1 antibody efficacy in treating murine mammary tumors. In addition, we defined sexually dimorphic αPD-L1 efficacy in B16 melanoma. Here, we show a sexually dimorphic response to the combination of GW9662 and αPD-L1 immunotherapy in B16 melanoma. Combination effects were observed in female, but not male hosts. Neither female oöphorectomy impairs, nor does male castration rescue the combination effects, suggesting a sex hormone-independent response to this combination therapy. In diet-induced obese females, melanoma growth remained responsive to the combination treatment, albeit less robustly than lean females. These findings are informative for future design and application of immunotherapy-related combination therapy for treating human melanoma patients by taking gender and obesity status into consideration.
Keywords: Melanoma, immunotherapy, PD-L1, PPARγ, obesity, sexual dimorphism

 Global reach, higher impact
Global reach, higher impact