10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2021; 17(12):3091-3103. doi:10.7150/ijbs.55689 This issue Cite
Research Paper
URI1 suppresses irradiation-induced reactive oxygen species (ROS) by activating autophagy in hepatocellular carcinoma cells
1. Department of Cell Biology, Institute of Bioengineering, School of Medicine, Soochow University, Suzhou 215123, China
2. Department of Endocrinology, Children's Hospital affiliated to Soochow University, Suzhou, 215000, China
Abstract
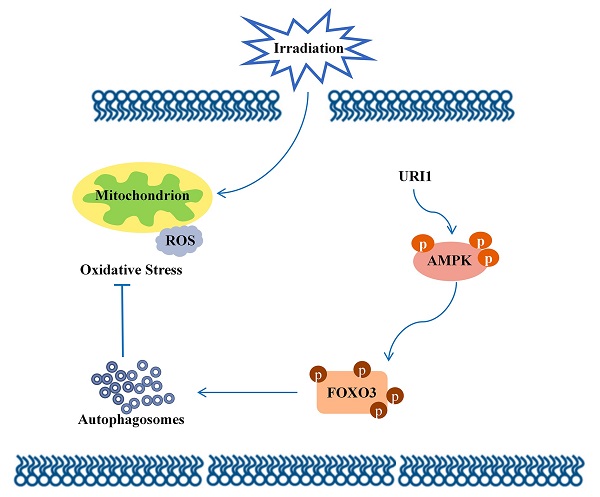
Radiotherapy has been extensively applied in cancer treatment. However, this treatment is ineffective in Hepatocellular carcinoma (HCC) due to lack of radiosensitivity. Unconventional prefoldin RPB5 interactor 1 (URI1) exhibits characteristics similar to those oncoproteins, which promotes survival of cancer cells. As a consequence of the irradiation, the levels of endogenous reactive oxygen species (ROS) rise. In the current study, we analyzed the role of URI1 in the control of ROS levels in HepG2 cells. Upon URI1 overexpression, HepG2 cells significantly suppressed irradiation-induced ROS, which may help cells escape from oxidative toxicity. And our data demonstrated that overexpression of URI1 not only resulted in an increase of autophagic flux, but also resulted in an further increased capacity of autophagy to eliminate ROS. It indicated that URI1 suppressed irradiation-induced ROS through activating autophagy. Moreover, URI1 activated autophagy by promoting the activities of AMP-activated protein kinase (AMPK). Results showed that overexpression of URI1 increased the phosphorylation of AMPKα at the Thr172 residue and the activated-AMPK promoted the phosphorylation of forkhead box O3 (FOXO3) at the Ser253 residue, which significantly induced autophagy. Taken together, our findings provide a mechanism that URI1 suppresses irradiation-induced ROS by activating autophagy through AMPK/FOXO3 signaling pathway. These new molecular insights will provide an important contribution to our better understanding about irradiation insensitivity of HCC.
Keywords: URI1, autophagy, ROS, HCC, irradiation insensitivity

 Global reach, higher impact
Global reach, higher impact