10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2021; 17(14):3898-3910. doi:10.7150/ijbs.65488 This issue Cite
Research Paper
ADAM9 functions as a transcriptional regulator to drive angiogenesis in esophageal squamous cell carcinoma
1. Graduate Institute of Clinical Medical Science, China Medical University, Taichung 404, Taiwan.
2. Division of Thoracic Surgery, China Medical University Hospital, Taichung 404, Taiwan.
3. Center for Molecular Medicine, China Medical University Hospital, Taichung 404, Taiwan.
4. Graduate Institute of Biomedical Sciences, China Medical University, Taichung 404, Taiwan.
5. Department of Anatomic Pathology, Nantou Hospital of the Ministry of Health and Welfare, Nantou 540, Taiwan.
6. Division of Thoracic Surgery, Department of Surgery, Taipei Veterans General Hospital, Taipei 112, Taiwan.
7. Institute of Emergency and Care Medicine, School of Medicine, National Yang-Ming University, Taipei 112, Taiwan.
8. Graduate Institute of Molecular and Comparative Pathobiology, School of Veterinary Medicine, National Taiwan University, Taipei 106, Taiwan.
9. School of Pharmacy, China Medical University, Taichung 404, Taiwan.
10. Division of Hematology and Oncology, China Medical University Hospital, Taichung 404, Taiwan.
11. Graduate Institute of Physiology, College of Medicine, National Taiwan University, Taipei 100, Taiwan.
12. Chinese Medicine Research Center, China Medical University, Taichung 404, Taiwan.
Abstract
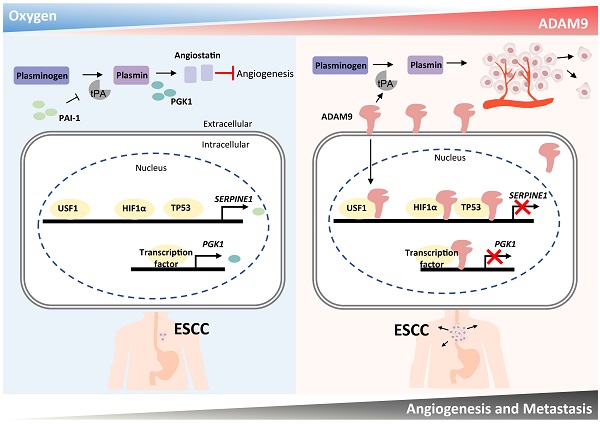
Hypoxia and angiogenesis play key roles in the pathogenesis of esophageal squamous cell carcinoma (ESCC), but regulators linking these two pathways to drive tumor progression remain elusive. Here we provide evidence of ADAM9's novel function in ESCC progression. Increasing expression of ADAM9 was correlated with poor clinical outcomes in ESCC patients. Suppression of ADAM9 function diminished ESCC cell migration and in vivo metastasis in ESCC xenograft mouse models. Using cellular fractionation and imaging, we found a fraction of ADAM9 was present in the nucleus and was uniquely associated with gene loci known to be linked to the angiogenesis pathway demonstrated by genome-wide ChIP-seq. Mechanistically, nuclear ADAM9, triggered by hypoxia-induced translocation, functions as a transcriptional repressor by binding to promoters of genes involved in the negative regulation of angiogenesis, and thereby promotes tumor angiogenesis in plasminogen/plasmin pathway. Moreover, ADAM9 suppresses plasminogen activator inhibitor-1 gene transcription by interacting with its transcription factors at the promoter. Our findings uncover a novel regulatory mechanism of ADAM9 as a transcriptional regulator in angiogenesis and highlight ADAM9 as a promising therapeutic target for ESCC treatment.
Keywords: ESCC, ADAM9, PAI-1, angiogenesis, transcriptional regulator

 Global reach, higher impact
Global reach, higher impact