10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2022; 18(2):873-888. doi:10.7150/ijbs.68093 This issue Cite
Review
Secreted Phospholipases A2 - not just Enzymes: Revisited
1. Department of Molecular and Biomedical Sciences, Jožef Stefan Institute, Jamova 39, 1000 Ljubljana, Slovenia.
2. Faculty of Medicine, University of Ljubljana, Vrazov trg 2, 1000 Ljubljana, Slovenia.
Received 2021-10-15; Accepted 2021-12-2; Published 2022-1-1
Abstract
Secreted phospholipases A2 (sPLA2s) participate in a very broad spectrum of biological processes through their enzymatic activity and as ligands for membrane and soluble receptors. The physiological roles of sPLA2s as enzymes have been very well described, while their functions as ligands are still poorly known. Since the last overview of sPLA2-binding proteins (sPLA2-BPs) 10 years ago, several important discoveries have occurred in this area. New and more sensitive analytical tools have enabled the discovery of additional sPLA2-BPs, which are presented and critically discussed here. The structural diversity of sPLA2-BPs reveals sPLA2s as very promiscuous proteins, and we offer some structural explanations for this nature that makes these proteins evolutionarily highly advantageous. Three areas of physiological engagement of sPLA2-BPs have appeared most clearly: cellular transport and signalling, and regulation of the enzymatic activity of sPLA2s. Due to the multifunctionality of sPLA2s, they appear to be exceptional pharmacological targets. We reveal the potential to exploit interactions of sPLA2s with other proteins in medical terms, for the development of original diagnostic and therapeutic procedures. We conclude this survey by suggesting the priority questions that need to be answered.
Keywords: Secreted phospholipase A2, binding protein, promiscuity, cell transport, signalling, phospholipase activity regulation
Introduction
Secreted phospholipases A2 (sPLA2s) (EC 3.1.1.4) are a structurally related group of low-molecular-mass enzymes (14-18 kDa) that catalyse the hydrolysis of glycerophospholipids (phospholipids hereafter) at their sn-2 position, to produce lysophospholipids and free fatty acids. sPLA2s contain 6 to 8 disulphide bonds, a highly conserved His/Asp catalytic dyad, and a Ca2+-binding loop [1]. Several sPLA2 isoforms have been described in mammals. Depending on their structural characteristics, the mammalian sPLA2s are divided into 11 groups (G): IB, IIA, IIC, IID, IIE, IIF, III, V, X, XIIA and XIIB [2]. Snake venom sPLA2s are orthologous to mammalian GIIA, GIIB or GIIE sPLA2s, or they belong to the unique GIA sPLA2s [3]. sPLA2s from bee and lizard venoms are homologous to mammalian GIII sPLA2s. Finally, the further GIX sPLA2s are found in venom of marine snails, and GXIA and GXIB sPLA2s are plant proteins.
The importance of sPLA2s as enzymes
The enzymatic activity of sPLA2s define their participation in a very broad spectrum of biological processes. Generally, sPLA2s are secreted from cells and require micromolar to millimolar Ca2+ to be catalytically competent. They predominantly target phospholipids in the extracellular space, although they also act intracellularly.
sPLA2s show distinct substrate specificities in terms of the phospholipid polar headgroups and the fatty acids esterified at the sn-2 position of the glycerol backbone. For instance, GIII, GV and GX sPLA2s efficiently hydrolyse phosphatidylcholine, while GIIA sPLA2 has higher activity against negatively charged phospholipid substrates, and in particular, phosphatidylserine, phosphatidylglycerol and phosphatidylethanolamine. For their sn-2 fatty acid tail specificities, GIB, GIIA and GIIE sPLA2s are promiscuous. GV sPLA2 preferentially targets sn-2 fatty acyls with low number of double bonds, such as oleyl, while GIID, GIIF, GIII and GX sPLA2s target polyunsaturated fatty acyls, such as arachidonyl.
sPLA2 isoforms have unique tissue and cellular distributions, and therefore it is evident that individual sPLA2s have distinct enzyme-activity-related biological functions. These include the generation of a variety of lipid mediators, along with membrane remodelling, modification of extracellular non-cellular phospholipid components of pulmonary surfactant, microparticles and lipoproteins, and degradation of microbial membranes and dietary phospholipids. The pathophysiological aspects of sPLA2s as enzymes have been comprehensively reviewed recently (e.g., [2,4]), and therefore we will not focus on these here. Instead, our attention here is focussed on sPLA2s acting as ligands, where fewer of the details have been described.
The existence of sPLA2s without enzymatic activity but with pharmacological activity was an early indication that sPLA2s can participate in physiological settings not just as enzymes, but also as ligands for membrane and soluble receptors. Indeed, in 1982, the first specific sPLA2-binding protein (sPLA2-BP) was discovered [5]. Since then, the number of newly characterized sPLA2-BPs has expanded considerably (Table 1), and due to the development of more sensitive analytical methodologies, this process continues. Several reviews on sPLA2-BPs have been published over the years [6-9], although the most recent is 10 years old [9]. Therefore, now is an appropriate time to survey the advances in this important area of research, and to critically discuss new insights and suggest research directions.
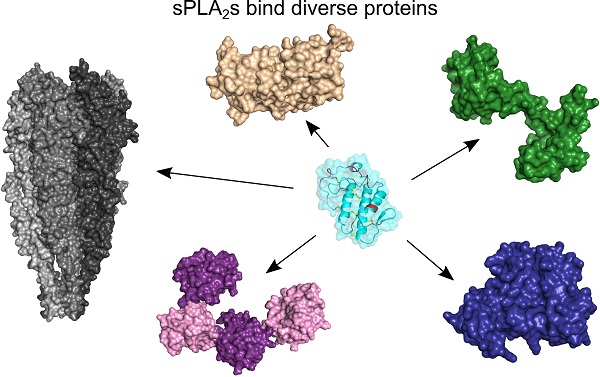
sPLA2s bind very structurally diverse proteins
There are two types of sPLA2-BPs: integral membrane proteins and soluble proteins [9]. The former belong to one of the following protein families: (1) muscle-type sPLA2 receptors (M-type sPLA2Rs); (2) heparan sulphate proteoglycans (HSPGs); (3) integrins; (4) vascular endothelial growth factor receptors (VEGFRs); and (5) ion channels. Two new integral membrane sPLA2-BPs have been described recently, a G-protein-coupled receptor (GPCR), and cytochrome c oxidase (CCOX) [35, 37]. CCOX is the only known intracellular membrane sPLA2-BP, as all of the other integral membrane sPLA2-BPs are located in the plasma membrane. On the other hand, soluble sPLA2-BPs are more frequently found inside cells, in the endoplasmic reticulum (ER), cytosol or nucleus, as well as in the extracellular space. The ER-resident sPLA2-BPs, disulphide isomerase (PDI), taipoxin-associated 49-kDa Ca2+-binding protein (TCBP-49) and crocalbin, and the cytosolic calmodulin (CaM) and 14-3-3 proteins, have been known for some time. Two new soluble sPLA2-BPs were only described more recently: vimentin [26] and nucleolin [27]. Vimentin is an intermediate filament protein, as a constituent of the cytoskeleton, while nucleolin is a nucleolar protein; however, both of these proteins are also found on the cell surface, which is where they are most likely to interact with sPLA2s. Extracellular sPLA2-BPs include pentraxins, α-, β- and γ-type sPLA2 inhibitors (PLIs), blood coagulation factors, pulmonary surfactant protein A (SP-A), metalloproteinase inhibitor DM64, and a serine protease inhibitor (C1 inhibitor protein). The main characteristics of the sPLA2-BPs are shown in Table 1.
Structural aspects of sPLA2 protein-binding-promiscuity
sPLA2-BPs are structurally so diverse that attempts to define their common sPLA2-binding attributes have remained unsuccessful. To solve this problem, it would be most helpful to determine the three-dimensional structures of complexes between sPLA2s and the sPLA2-BPs, and thus to analyse their interaction areas. However, contemporary methodologies are still not optimal for this kind of approach.
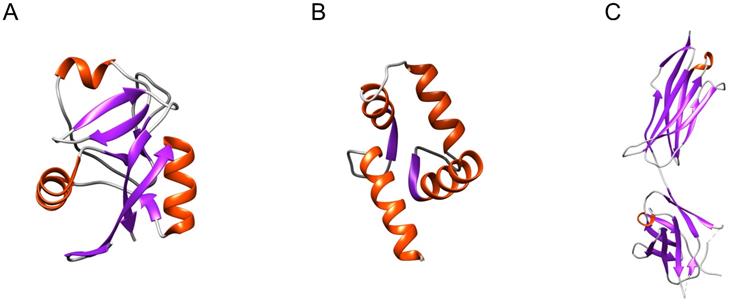
The focus has thus mainly been on well-defined structural elements that are present in more than one type of sPLA2-BP, such as the C-type carbohydrate recognition domain (CRD) and CRD-like folds (in M-type sPLA2Rs, SP-A, α-PLIs), the EF-hand Ca2+-binding motif (in CaM, crocalbin, TCBP-49), and the immunoglobulin-like (Ig-like) domain (in VEGFRs, DM64) (Figure 1).
Only the CRD fold (Figure 1A) has been confirmed as an sPLA2-binding structure [10]. Using radio-iodinated snake venom GIA sPLA2 (125I-OS1), it was established that one of the eight CRDs in M-type sPLA2R (i.e., CRD5) includes the sPLA2 binding site. This also revealed that not all of the CRDs can bind sPLA2s. M-type sPLA2R bound sPLA2s only under neutral and basic conditions, which implies that positively charged amino acids have important roles in this interaction. At pH 6 or lower, M-type sPLA2R also underwent conformational changes that prevented the binding of sPLA2s [41]. For the other two CRD-fold-containing sPLA2-BPs, as lung SP-A [13] and soluble α-PLIs from sera [12], the sPLA2-binding characteristics are not very clear yet. As M-type sPLA2R and α-PLIs do not require Ca2+ to bind sPLA2s [12, 42], while SP-A does require Ca2+, this indicates that CRDs bind sPLA2s in different ways. To date, Ca2+-dependent binding of sPLA2s has been reported only in a case of a single CRD-containing sPLA2-BP, SP-A, while Ca2+-independent binding of sPLA2s has been shown for sPLA2-BPs with multiple CRDs, where more than one CRD constitutes the sPLA2-binding site. For M-type sPLA2R, three consecutive CRD repeats (repeats 4, 5, 6) were shown to influence sPLA2 binding [10], and consistently, in α-PLIs, the trimeric CRD proteins, the central part, where three CRDs are associated together, has been suggested to provide the sPLA2-binding site [43].
Some characteristics of sPLA2-BPs.
| sPLA2-BP | Location | Defined structural element | Interacting part of sPLA2 | Binding affinity | Effect of interaction |
|---|---|---|---|---|---|
| M-type sPLA2Rs [10] | Plasma membrane, extracellular | CRD-like fold | Ca2+-binding loop | 38 pM (OS1) [11] | Inhibition, clearance, translocation of sPLA2 |
| α-PLIs [12] | Blood | n.d. | n.d. | Inhibition of sPLA2 | |
| SP-A [13] | Extracellular | n.d. | n.d. | Inhibition of sPLA2 | |
| β-PLIs [14] | Blood | Leucine-rich repeat | N-terminal region, β-wing | n.d. | Inhibition of sPLA2 |
| γ-PLIs [15] | Blood | Three-finger motif | N-terminal region, β-wing | n.d. | Inhibition of sPLA2 |
| CaM [16] | Cytosol | EF-hand Ca2+-binding motif | C-terminal region, α-helices C, E | 3.3 nM (Atx) [17] | Stabilization of sPLA2, augmentation of sPLA2 enzymatic activity |
| TCBP-49 [18] | Endoplasmic reticulum | n.d. | n.d. | Translocation of sPLA2 (proposed) | |
| Crocalbin [19] | Endoplasmic reticulum | n.d. | n.d. | Translocation of sPLA2 (proposed) | |
| PDI [20] | Endoplasmic reticulum | Thioredoxin-like fold | IBS | 1.27 µM (Atx) [21] | Translocation of sPLA2 (proposed) |
| VEGFR-1/Flt-1 [22] | Plasma membrane | Ig-like domain | C-terminal region | 74 nM (Lys49 GIIA) [23] | Competitive inhibition of VEGFR |
| VEGFR-2/KDR [22] | Plasma membrane | 10 nM (Lys49 GIIA) [23] | |||
| DM64 [24] | Blood | n.d. | n.d. | Neutralization of sPLA2 | |
| HSPGs [25] | Plasma membrane | Negatively charged carbohydrate moiety | Clusters of basic amino acids at C- and N-terminal regions | n.d. | Clearance and translocation of sPLA2 |
| Vimentin [26] | Cytosol, plasma membrane | Rod domain | IBS | n.d. | Internalization and translocation of sPLA2 |
| Nucleolin [27] | Nucleolus, cytoplasm, plasma membrane | RNA recognition motif | n.d. | n.d. | Internalization and translocation of sPLA2 |
| NP1, NP2, NPR [28] | Extracellular | Pentraxin domain | n.d. | n.d. | Translocation of sPLA2 (proposed) |
| 14-3-3γ/ε [29] | Cytosol | 14-3-3 domain | C-terminal region | 1 µM (Atx) [29] | Positioning of sPLA2 on plasma membrane |
| L-type voltage-dependent Ca2+ channel [30] | Plasma membrane | α domain | n.d. | n.d. | Activation of L-type voltage-dependent Ca2+ channel (proposed) |
| nAChR [31] | Plasma membrane | - | n.d. | 120 nM (crotoxin) [31] | Negative allosteric modulation of nAChR |
| GLIC [32] | Plasma membrane | ECD | IBS | 125 nM (CBc) [32] | Negative allosteric modulation of GLIC |
| CFTR/ ΔF508CFTR [33] | Plasma membrane | NBD1 | IBS, Ca2+-binding loop, C-terminal region | 4 nM (CBc) [33] | Potentiation and correction of ΔF508CFTR |
| EGFR [34] | Plasma membrane | L domain | n.d. | n.d. | Activation of EGFR |
| PAR-1 [35] | Plasma membrane | - | n.d. | n.d. | Activation of PAR-1 |
| Integrins [36] | Plasma membrane | - | C-terminal region, α-helices D, E | 200 nM (hGIIA) [36] | Induction of integrin-mediated signalling |
| CCOX-II [37] | Inner mitochondrial membrane | - | C-terminal region | 15 nM (Atx) [38] | Inhibition of CCOX (proposed) |
| C1 inhibitor protein [39] | Extracellular | - | n.d. | n.d. | Impairment of C1 inhibitor protein activity (proposed) |
| FX, FXa, FIIa (thrombin) [40] | Blood | EGF-like domain of light chain, interface regions I-V, exosite of heavy chain | Basic amino acids in C-terminal region, IBS, loop preceding β-wing | 0.6 nM (CBc) [40] | Noncompetitive inhibition of FX, FXa, FIIa (thrombin) |
sPLA2-BP, sPLA2-binding protein; M-type, muscle-type; CRD, carbohydrate recognition domain; OS1, sPLA2 from Oxyuranus s. scutellatus venom; PLIs, sPLA2 inhibitors; n.d., not defined; SP-A, pulmonary surfactant protein A; CaM, calmodulin; Atx, sPLA2 from Vipera a. ammodytes venom; TCBP-49, taipoxin-associated 49-kDa Ca2+-binding protein; PDI, protein disulphide isomerase; VEGFR, vascular endothelial growth factor receptor; DM64, metalloproteinase inhibitor from Didelphis marsupialis blood; HSPGs, heparan sulphate proteoglycans; IBS, interfacial binding surface; NP, neuronal pentraxin; nAChR, nicotinic acetylcholine receptor; GLIC, proton-gated ion channel from Gloeobacter violaceus; ECD, extracellular domain; CB, basic sPLA2 subunit of crotoxin; CFTR, cystic fibrosis transmembrane conductance regulator; NBD; nucleotide-binding domain; EGF(R), epidermal growth factor (receptor); PAR, protease-activated receptor; hGIIA; human group IIA sPLA2; CCOX, cytochrome c oxidase; FX/FXa/FIIa, blood coagulation factors.
Distinct structural elements present in more than one type of sPLA2-BP. (A) CRD (PDB ID: 6JLI) and the CRD-like fold are structural elements found in M-type sPLA2Rs, SP-A and α-PLIs. (B) The EF-hand Ca2+-binding motif (PDB ID: 1CLL) is found in CaM, crocalbin and TCBP-49, which all bind sPLA2s. (C) The Ig-like domain (PDB ID: 2X1X) is found in VEGFRs and DM64. To date, only the CRD has been experimentally demonstrated to be a sPLA2-binding structure. Red, α-helices; violet, β-sheets; grey, loops. The Figure was prepared using UCSF Chimera v1.15.
There are plenty of other CRD-containing proteins, such as the mannose receptor, Endo180, DEC205 and FcRY. At present, only the human mannose receptor has been demonstrated to interact with bee venom GIII sPLA2 [44], while FcRY did not bind any of the sPLA2s tested [45].
To recognize sPLA2-binding attributes of sPLA2-BPs it is important to also consider the characteristics of the sites on sPLA2s that interact with sPLA2-BPs, as these are complementary to the sPLA2-binding sites on sPLA2-BPs. It has to be borne in mind, however, that a single sPLA2 molecule might harbour multiple protein-binding sites [46], and therefore it is reasonable to consider separately binding sites directed towards particular types of sPLA2-BPs, or those sPLA2s that induce the same interaction-dependent physiological effects.
The interaction of an sPLA2 with a CRD fold is primarily governed by the structure of the interfacial binding surface (IBS) of the sPLA2 [47]. The IBS is defined as the molecular surface with which sPLA2 contacts a phospholipid membrane - its substrate. The IBS is formed by a collar of hydrophobic amino acids around the entrance to the catalytic site of the enzyme, and by mainly positively charged amino acids in more remote positions. The amino acids within or close to the Ca2+-binding loop in sPLA2s were also shown to be involved in the interactions between sPLA2s and CRDs [42]; however, not profoundly, consistent with the evidence that the Ca2+-binding loop is highly conserved among sPLA2s that show considerably different affinities for M-type sPLA2R.
sPLA2-BPs that contain Ig-like domains, such as VEGFRs and DM64, have been shown to associate with catalytically inactive myotoxic sPLA2s. VEGFRs are receptor tyrosine kinases, and they interact with sPLA2s through their extracellular region, which is composed of seven Ig-like domains. Although the interaction of myotoxic sPLA2s and VEGFRs has not been functionally linked with myotoxic effects yet, the C-terminal region of these sPLA2s (known as the 'myotoxic site') was shown to also be responsible for binding to VEGFRs [22, 23]. The myotoxic site of the snake venom myotoxic sPLA2s has been attributed to amino acids 115-129, which form a single α-helical turn at the C-terminus [48-50]. This C-terminal region is firmly attached to the body of the protein by two disulphide bonds, Cys27-Cys126 and Cys50-Cys133, thus constraining its position and orientation. The myotoxicity of sPLA2s has been associated with a cluster of positively charged amino acids in this region (i.e., conserved Lys115 and Arg118, and possibly also Lys122 and Lys127), and with hydrophobic amino acids at positions 121 and 125 [51, 52].
The sPLA2-BP DM64 was originally isolated from marsupial (Didelphis marsupialis) blood, and it was shown to inhibit the myotoxic activity of several sPLA2s [24]. DM64 contains five Ig-like domains. Its myotoxicity-inhibitory activity is most likely due to its binding to the myotoxic site of sPLA2s, to thus obstruct their association with the 'myotoxicity receptor', which would be VEGFR. Contrary to what is seen for CRD in M-type sPLA2R, binding of sPLA2s to DM64 does not inhibit their enzymatic activity. This is in agreement with the involvement of the C-terminal of these sPLA2s in the interaction with this Ig-like domain-containing sPLA2-BP, rather than the IBS.
Identification of the PDZ-binding domain at the C-terminus of some myotoxic sPLA2s [53] suggested that there is another group of sPLA2-BPs, as PDZ-domain-containing proteins. In muscle cells, these proteins include, e.g., LDB3 and α-1-syntrophin [54, 55]; however, their potential sPLA2 binding remains to be tested.
The third type of well-defined structural element present in more than one sPLA2-BP is the EF-hand Ca2+-binding motif. These sPLA2-BPs include CaM, crocalbin and TCBP-49, although the structural aspects of sPLA2 binding have been studied only for CaM. A three-dimensional model of a complex between CaM and the snake venom GIIA sPLA2 ammodytoxin (Atx) [16] revealed that CaM 'clamps' Atx between its N-terminal and C-terminal domains (Figure 2A). Atx contacts CaM most extensively through a distinct patch of hydrophobic and charged amino acids at its C-terminus, which interact with the central part of CaM, and through the α-helices C and E, which contact the C-terminal globular domain of CaM. In the complex, Atx is oriented in such a way that the entrance to its enzymatic pocket remains open wide. In the complex, a new larger membrane-contacting area (IBS) is formed; this comprises parts of both Atx and CaM, and explains the increased enzymatic activity of complexed Atx.
Secreted PLA2s are ligands of proteins that are structurally very diverse. The three-dimensional models, generated by molecular docking, are showing complexes between the sPLA2 Atx and CaM (green, PDB ID: 1CLL) (A), Atx and FXa (blue, PDB ID: 2BOH) (B), CBb and ΔF508NBD1 of CFTR (PDB ID: 1XMJ) (C), Atx and PDI (purple/pink, PDB ID: 4EL1) (D), and between the sPLA2 vurtoxin and nAChR (grey, PDB ID: 2BG9) (E). Centre: The sPLA2 Atx, showing its main structural elements. Red, interfacial binding surface; yellow, disulphide bonds; violet, C-terminal region. Note that the sPLA2s interact with these different sPLA2-BPs in very different ways; i.e., they have multiple protein binding sites, as is characteristic of promiscuous proteins. The Figure was prepared by adaptation of Figures from Kovačič et al. (2010) (A), Faure and Saul (2011) (B), Faure et al. (2016) (C), Oberčkal et al. (2015) (D) and Vulfius et al. (2014) (E), using PyMOL.
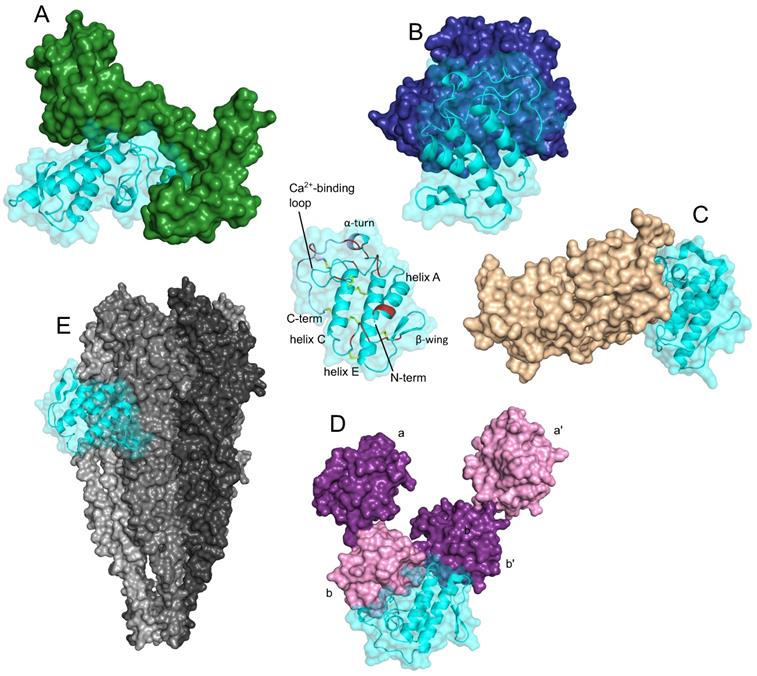
Considerable structural insight has also been gained for the interactions between the anticoagulant GIIA sPLA2s Atx, CB (basic sPLA2 subunit of crotoxin, from venom of the South American rattlesnake [Crotalus durissus terrificus]) and daboxin P (from venom of the Indian viper [Daboia r. russelii]), and human activated blood coagulation factor X (FXa) [40, 56, 57, 58]. The interaction area between the anticoagulant sPLA2s and FXa is extensive, and encompasses the heavy and light chains of FXa. As evident from the model of the complex between Atx and FXa shown in Figure 2B, Atx interacts with the FXa heavy chain through its Ca2+-binding loop, α-helix C, β-wing, and C-terminal region, and with the FXa light chain through its β-wing and α-helices A and B. Electrostatic interactions importantly participate in the efficient binding of sPLA2s to FXa, including in particular some positively charged amino acids of Atx (e.g., Arg72, Lys74, His76, Arg77 in the β-wing; Arg118, Lys128, Lys127, Lys132 in the C-terminal region). However, not all of the basic sPLA2s are strong anticoagulants, which are consistent with the importance also of certain hydrophobic and aromatic sPLA2 amino acids for optimal interactions with FXa (e.g., Atx: Phe124 in the C-terminal region; CBa2: Trp70 in the 65-72 loop).
Molecular docking allowed to propose a model of the complex between ΔF508-NBD1 (nucleotide-binding domain 1), the sPLA2-binding domain of ΔF508CFTR (Phe508 deletion mutant of cystic fibrosis transmembrane conductance regulator), and CBb (Figure 2C) [33]. From the model, which applies also to the wild type NBD1 (i.e. CFTR), it can be seen that the binding interface of CB with ΔF508NBD1/NBD1 is predominately composed of the hydrophobic residues located in its α-helices A and B, Ca2+-binding loop and the C-terminal region. Hydrophobic interactions are clearly very important for holding both proteins together, nevertheless, also some polar amino acid residues are involved in the interaction. Forming ionic contacts with ΔF508NBD1/NBD1 of ΔF508CFTR/CFTR, the N-terminal His1 and the Asp112 in the C-terminal region of CB are two examples.
A three-dimensional model of the complex between Atx and PDI has also been reported [20]. PDI consists of multiple domains (i.e., a, a', b, b'), and according to the model shown in Figure 2D, the Atx-binding site on PDI is situated between domains b and b'. Atx interacts with PDI across an extensive area that also includes the Atx IBS. The basic amino acids are important for Atx binding to PDI, especially Arg77 and Arg118, but also Lys69, Arg72, Lys74 and Lys86. However, Atx also contacts PDI with some hydrophobic amino acids, Leu3, Leu19 and Phe24, and through two polar amino acids, Asn17 and Asn119.
Molecular docking has also provided some structural insights into the interactions between vurtoxin, another snake venom GIIA sPLA2, and the nicotinic acetylcholine receptor (nAChR) [59]. According to their model, vurtoxin binds at the interface of the α and γ subunits of nAChR, and is oriented with its active site towards the lipid bilayer (Figure 2E). Vurtoxin does not occupy the nAChR binding sites for its classical agonists and competitive antagonists; however, it is located very close to these.
Several structural features allow the sPLA2s to bind to structurally very diverse targets (Table 1; Figure 2), which is characteristic of promiscuous proteins [60, 61]. Despite the generally compact structure of sPLA2s, the flexibility of the exposed side chains of the amino acids at the IBS promotes their optimal binding to phospholipid aggregates, as their substrates, for efficient hydrolysis [62]. As indicated above, the IBS is critically involved also in the interactions of sPLA2s with the CRD-containing sPLA2-BPs and FXa, as also for PDI [20] and vimentin [26]. The association of the sPLA2 IBS with a variety of different protein surfaces is most likely driven by the same principles as the association of the sPLA2 IBS with phospholipid membranes. The flexibility of the sPLA2 β-wing might be another feature that favours their high adaptability for different protein partners. For example, the β-wing of sPLA2s should be involved in the interactions of sPLA2s with β-PLIs and γ-PLIs [63]. sPLA2s are also characterized by patches of basic and hydrophobic amino acids that might also greatly broaden the spectrum of their protein interaction partners. We have already shown that such patches are part of the IBS, but they are also located in other parts of sPLA2s. Indeed, those at the C-terminus [62] are implicated in the binding of sPLA2s to CaM and VEGFR.
However, only the atomic structure of an sPLA2-sPLA2-BP complex will fully disclose the structural requirements for particular association. The present-day rapid advances in techniques for the determination of the three-dimensional structures of proteins might enable this in the near future; e.g., cryo-electron microscopy.
Pathophysiological significance of sPLA2 binding to sPLA2-BPs
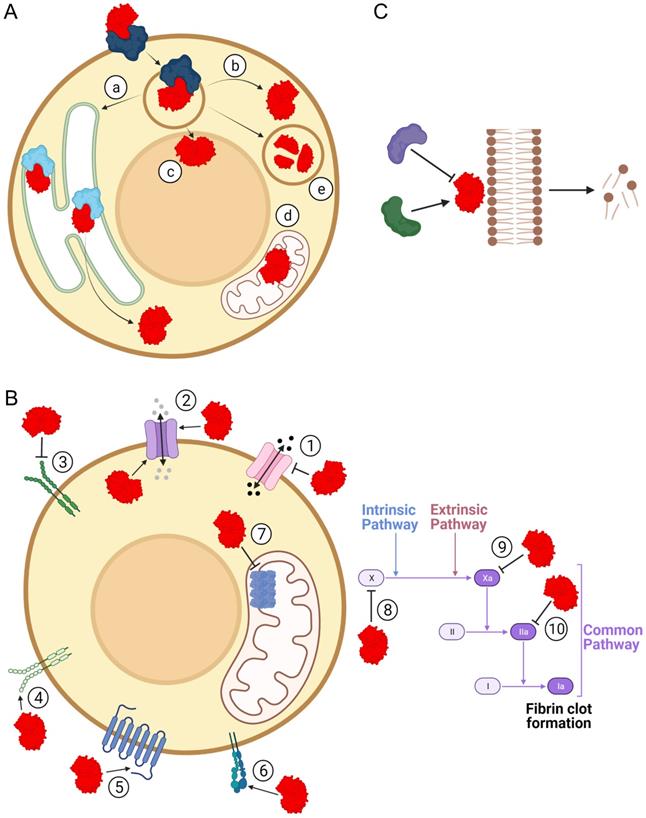
The structural diversity of sPLA2-BPs is consistent with the wide range of pathophysiological activities that have been associated with sPLA2s. By binding to sPLA2-BPs, sPLA2s have been suggested to be involved in inflammation [64, 65], hormone release [66, 67], neurotoxicity [20, 37, 68, 69] and myotoxicity [22]. It has already been confirmed that sPLA2s are ligands for specific sPLA2-BPs in cytokine production [70], cell proliferation [71-73], cell migration [74], lipid mediator production [75], antibacterial activity [73] and blood coagulation [40]. These (patho)physiological effects of sPLA2s might arise through: (1) specific cellular translocation and associated actions of sPLA2s after their binding to sPLA2-BPs; (2) triggering of specific signalling following their binding to sPLA2-BPs; and/or (3) regulation of sPLA2 enzymatic activity by sPLA2-BPs (Figure 3). Particular mechanistic possibilities for sPLA2 actions as ligands for sPLA2-BPs are exemplified and discussed below.
Binding of sPLA2s to some sPLA2-BPs does not have any obvious physiological function. Some functional implications of sPLA2s acting as ligands certainly remain to be discovered, while others might be latent. When environmental conditions change radically, these interactions might become functional. Promiscuous proteins, which include sPLA2s, can acquire new functions much more readily than non-promiscuous proteins, which can provide swifter adaptation of the organism to new situations [76, 77].
Pathophysiological implications of binding of sPLA2s to sPLA2-BPs. Physiological and/or pathological effects of sPLA2s (red) are also the consequence of their binding to sPLA2-BPs. (A) By binding to sPLA2-BPs (blue/cyan), sPLA2s can be translocated to specific intracellular compartments, such as the endoplasmic reticulum (a), cytosol (b), nucleus (c), mitochondria (d) or lysosomes (e). In each of these compartments, they can act as enzymes or ligands for receptors, or they can undergo proteolytic degradation in the lysosome. (B) As ligands for receptors, sPLA2s have been specifically implicated in molecular signalling through decreased (1) or increased (2) permeabilities of certain ion channels, inhibition (3) or activation (4) of activities of receptor tyrosine kinases, modulation of activities of GPCR (5), interference in integrin-mediated functions (6), attenuation of ATP production (7) and inhibition of blood coagulation, at different stages (8, 9, 10). (C) Binding of sPLA2 to a sPLA2-BP might inhibit or potentiate phospholipolytic activity. The Figure was created with BioRender.com.
Cellular transport of sPLA2s after binding to sPLA2-BPs
Although sPLA2s appear to be secreted into the extracellular space after being synthesized inside the cell, convincing evidence of their intracellular localization and activities has already been provided [69, 71, 78, 79]. sPLA2s can re-enter cells after their synthesis and secretion, to pass into the cytosol and to different organelles, such as the nucleus and mitochondria [80]. Indeed, several sPLA2-BPs have been suggested to assist sPLA2s in their retrograde cellular transport and intracellular translocation (Figure 3A).
The sPLA2-BP in the lumen of the ER, PDI, has been proposed to be involved in such retro-transport of the Atx snake venom GIIA sPLA2, which is a neurotoxin from nose-horned viper venom that acts presynaptically [20]. Molecular docking and heterologous competition assays have suggested that PDI acts in a similar way as on Atx also on some mammalian sPLA2s, such as GIB, GIIA and GV sPLA2s.
Two other candidates for the same function are the ER luminal proteins TCBP-49 and crocalbin, which were discovered through their binding to the hetero-oligomeric snake venom sPLA2s taipoxin and crotoxin [9]. As for PDI, these two EF-hand Ca2+-binding proteins have the characteristic ER-retention motif at their C-termini, which has been suggested to be essential for retention and concentration of sPLA2s in the ER before they are translocated into the cytosol. Some other EF-hand Ca2+-binding proteins might also interact with sPLA2s. For instance, the Miro proteins are mitochondrial adaptor proteins that promote the transportation of mitochondria by connecting them to motor proteins [81]. Binding of sPLA2s to the Miro proteins might concentrate the sPLA2s on the outer mitochondrial membrane, which would explain their colocalization with mitochondria after entering cells [78, 79].
Cellular retro-transport of sPLA2s might also be associated with two recently discovered sPLA2-BPs, vimentin and nucleolin. Vimentin belongs to the family of intermediate filaments, although it is also present on the cell surface and in extracellular fluids. Indeed, in recent years, vimentin has been shown to have a much wider role in cell physiology, rather than just being an inert scaffold protein [82]. Vimentin was identified as the receptor for GIIA sPLA2 on the surface of apoptotic human T lymphocytes [65]. Interactions between these two proteins was also shown in rheumatoid fibroblast-like synoviocytes, which associated rapid internalization of sPLA2 with arachidonic acid metabolism in synovial inflammation [83]. Recently, vimentin was reported to bind an acidic sPLA2 (NnPLA2-I) from venom of the Indian cobra (Naja naja) [26]. Binding of NnPLA2-I to vimentin resulted in its internalization into partially differentiated myoblasts. The involvement of vimentin in cellular uptake mechanisms has already been shown for C3, a Clostridium botulinum toxin [84, 85], and several viruses, such as human immunodeficiency virus type 1, vaccinia virus and the severe acute respiratory syndrome coronavirus [86]. In all of these cases, vimentin acts as a component of the cellular attachment mechanism, either as a receptor or a co-receptor. In a similar way, vimentin might mediate internalization of sPLA2s. This is further supported by the similar location of the binding sites on vimentin for dengue virus DENV-2 envelope protein domain III, Clostridium botulinum C3 exoenzyme and NnPLA2-I, all of which have been proposed to bind to the rod domain of vimentin [26, 84, 87].
Nucleolin was described recently as an sPLA2-BP due to its binding to the myotoxin MT-II, a Lys49 sPLA2 from venom of a pit viper, the terciopelo (Bothrops asper) [27]. It appears most likely that nucleolin participates in the toxic mechanism of MT-II through promotion of its translocation from the outside into the perinuclear and nuclear areas of myotubes and macrophages. Nucleolin was previously reported to mediate the internalization of several other molecules from the cell surface to the nucleus through an active Ca2+-dependent transport mechanism [88], which might also be the mechanism for sPLA2 internalization. It would be interesting to investigate the potential involvement of nucleolin in GV sPLA2 translocation to the nucleus, where this sPLA2 has been shown to act on the nuclear envelope [89]. Nucleolin has been shown to act as a chaperone for histones and TDP-43 [90, 91], so it might also stabilize sPLA2s in the reducing environment of the cytosol.
Neuronal pentraxins (i.e., NP1, NP2/Narp) are sPLA2-BPs that are homologous to acute phase proteins, and these might also function in retrograde transport of sPLA2s from the cell surface [28, 92]. An sPLA2-shuttling function has also been suggested for the ubiquitously expressed eukaryotic cytosolic 14-3-3 proteins, which were discovered through their interactions with Atx [29]. The neurotoxicity of Atx also involves its binding to the 14-3-3γ and 14-3-3ε isoforms, to correctly position it at the plasma membrane to hinder the function of amphiphysin, and thus of vesicle endocytosis [69].
HSPGs such as the GPI-anchored glypican I, biglycan, syndecan and perlecan, can bind mammalian sPLA2s, e.g. GIIA, GIID and GV sPLA2s. Using transfected human embryonic kidney 293 cells, it was shown that a GPI-anchored HSPG facilitated the shuttling of GIIA sPLA2 into certain subcellular compartments where the sPLA2 then released arachidonic acid for prostaglandin synthesis [93]. Binding of human GIIA sPLA2 to HSPGs was further demonstrated in human primary T-lymphocytes [25]. Recently, it was suggested that GIIA sPLA2 can increase endothelial cell permeability after binding to HSPGs on the surface of these cells, in a process that is dependent on the sPLA2 phospholipase activity [39]. In a similar way, HSPGs might be receptors for heparin-binding snake venom sPLA2s. By binding to HSPGs, these toxins might enhance inflammation and permeability of the endothelium, to allow the venom to spread more efficiently in the tissue.
M-type sPLA2R can rapidly internalize sPLA2s and direct them to the lysosomes, where they are then degraded [94, 95]. In this way, M-type sPLA2R can down-regulate the activity of sPLA2s. M-type sPLA2R can also mediate sPLA2 signalling by transporting sPLA2s into specific intracellular compartments [96, 97]. Recent structural studies of the human M-type sPLA2R ectodomain using cryo-electron microscopy showed pH-dependent conformational changes that might have important roles in the control of the functional properties of this receptor, including its sPLA2 binding [41].
Signalling triggered by sPLA2s as ligands
As proteins that are secreted into the extracellular space, sPLA2s encounter various plasma-membrane receptors on target cells. By binding to some of these receptors, sPLA2s can induce intracellular responses that are associated with different physiological processes, such as vascular permeability, cell growth, migration and senescence, hormone release, cytokine and NO production, inflammation, cell adhesion and angiogenicity (Figure 3B).
In this context, sPLA2s have been reported to interact with different ion channels (K+, Na+ or Ca2+). β-Butx is a neurotoxic sPLA2 from venom of the many-banded krait (Bungarus multicinctus), and it has been shown to interact with voltage-dependent K+ channels. Further, MitTx from venom of the Texas coral snake (Micrurus t. tener) was shown to bind to voltage-insensitive Na+-conducting acid-sensing ion channels [98]. However, both of these toxins are heterodimers that are composed of the sPLA2 subunit and a subunit homologous to the Kunitz-type serine protease inhibitor and, with the ion channel binding attributed to the latter. Therefore, these ion channels are not sPLA2-BPs. Nonetheless, the neurotoxicity with β-Butx and the pain with MitTx did not occur in the absence of the sPLA2 subunit. Some sPLA2s can influence the conductance of Ca2+ channels, the N-methyl-D-aspartate receptor or L-type voltage-dependent Ca2+ channels. Direct binding of sPLA2s to these channels has, however, not been demonstrated to date [30, 99].
Recently, pentameric ligand-gated ion channels, nAChRs [31, 59] and their bacterial homologue GLIC from the cyanobacterium Gloeobacter violaceus [32], were reported to be sPLA2-BPs. By binding to nAChRs, sPLA2s might affect nAChR-related functions, such as neuromuscular transmission and cell proliferation. Interestingly, nAChRs have also been described for the outer mitochondrial membrane, and were associated with regulation of the formation of mitochondrial permeability transition pores, which release pro-apoptotic substances like cytochrome c and reactive oxygen species [100]. Antagonists of nAChR were shown to attenuate the release of cytochrome c. The antagonistic effect of certain sPLA2s on some nAChRs subtypes might therefore link these sPLA2s to control of cellular viability through these nAChRs [31, 101].
CFTR is a Cl- channel that was defined as an sPLA2-BP using CB, the basic sPLA2 subunit of crotoxin [33]. CB was shown to bind and allosterically potentiate the activity of CFTR. Of a very high medical significance, CB was found to bind with nanomolar affinity also to ΔF508CFTR mutant, the causative factor of cystic fibrosis, thus augmenting its activity. Importantly, by binding to ΔF508CFTR, CB acts also as a corrector, facilitating trafficking and delivery of the abnormal protein to the plasma membrane.
VEGFRs are receptor tyrosine kinases that can interact with some myotoxic snake venom sPLA2s. Although some of these toxins are potent antagonists of VEGFRs, it is still not clear whether their binding to VEGFRs is directly involved in their sPLA2 myotoxicity [22, 23]. Nevertheless, the interactions between snake venom sPLA2s and VEGFRs might be important to enhance vascular permeability, to facilitate penetration of the snake venoms into tissues.
Epidermal growth factor receptor (EGFR) is another receptor tyrosine kinase that interacts with sPLA2s. It was reported to interact with human GIIA sPLA2 [34]. However, in this case, the sPLA2 acted as an agonist, as it up-regulated HER (human EGFR)/HER2-elicited signalling in lung cancer, thus stimulating cancer cell growth.
M-type sPLA2R has already been mentioned as an sPLA2-translocator (see section 4), and it can also act as a signalling receptor to transduce sPLA2-dependent signals independent of sPLA2 catalytic activity. Through M-type sPLA2R, sPLA2s have been implicated in cell proliferation, migration and senescence, and in hormone release and cytokine and NO production. It was also suggested that human GIB sPLA2 can induce kidney glomerular podocyte apoptosis via M-type sPLA2R [102].
PA2-Vb is an acidic sPLA2 from venom of the Chinese green tree viper (Trimeresurus stejnegeri), and it was shown to induce mouse aorta contraction independent of its enzymatic activity, by acting on a protease-activated receptor (PAR-1), which is a GPCR [35]. GPCRs are the largest and most diverse class of membrane receptors in eukaryotes, and their primary function is to transduce extracellular stimuli into intracellular signals, which lead to different cell responses.
Integrins are extracellular plasma membrane (transmembrane) proteins that are responsible for cell adhesion to the extracellular matrix. They are dimers, as combinations of one of 18 α-subunits and one of 8 β-subunits. Integrins αvβ3 and α4β1 have been shown to bind mammalian GIIA sPLA2, while an acidic GIIA sPLA2 from venom of the viper Macrovipera lebetina transmediterranea was shown to interact with integrins α5β1, αvβ3 and αvβ6, to induce inflammation and inhibition of cell adhesion and migration and angiogenicity [36, 103].
CCOX is an essential constituent of the respiratory chain complex, and was characterized as an sPLA2-BP due to its binding to Atx [37]. Through binding to subunit II, Atx was shown to inhibit the oxidase activity of CCOX, independent of its phospholipase activity. This might explain the inhibition of ATP production in nerve endings poisoned by the snake venom neurotoxic sPLA2s [104]. These findings also provide novel indications for the potential functions and malfunctions of the orthologous mammalian GIIA sPLA2 in mitochondria.
The blood coagulation system consists of a strictly regulated proteolytic cascade of blood coagulation factors that convey signals downstream to fibrinogen, which is then transformed into the insoluble fibrin network, and to activate platelets. Ultimately, a blood clot is formed to prevent blood loss from the injured blood vessel [105]. Several sPLA2s can affect this process by binding to one of these coagulation factors, to thus inhibit their functions (Figure 3B). Some snake venom sPLA2s bind to FX, or to its activated form FXa, or to thrombin (FIIa); e.g., crotoxin and Atx can induce anticoagulant effects through binding to FXa, to thus prevent formation of the prothrombinase complex [40, 106]. Human GIIA sPLA2 acts on blood coagulation in exactly the same way [107]. Daboxin is an anticoagulant sPLA2 that can bind to both FXa and FX [57]. On the other hand, Nk-PLA2α and Nk-PLA2β from venom of the monocled cobra (Naja kaouthia) act as anticoagulants through their binding to thrombin and their consequent inhibition of its proteolytic activity [108].
Regulation of sPLA2 enzymatic activity by sPLA2-BPs
sPLA2s participate in many important physiological processes through their enzymatic activity [2]. These range from those dependent on the composition and flexibility of biological membranes, to those regulated by the products of the sPLA2 phospholipolytic activity, which include lysophospholipids, free fatty acids and their metabolites - the whole range of signalling hormones [109,110]. Regulation of the enzymatic activity of sPLA2s is thus extremely important for the correct functioning of an organism, and this regulation is also mediated through the binding of sPLA2s to particular sPLA2-BPs (Figure 3C).
M-type sPLA2R has been shown to regulate the activity of sPLA2s in two ways: through acting as an sPLA2 inhibitor; and through mediating sPLA2 endocytosis, which leads to sPLA2 degradation in lysosomes [111]. Soluble form of M-type sPLA2R that is detected in the blood can function only in the first way [8]. Pulmonary SP-A is structurally and functionally similar to the soluble form of M-type sPLA2R. SP-A belongs to the CRD-containing family of proteins, and it inhibits the enzymatic activity of GIIA and GX sPLA2s in pulmonary surfactant [9].
Soluble proteins that inhibit sPLA2s have been isolated from the blood of snakes and some other animals. These are ecologically connected with venomous snakes, and are known as the α-PLIs, β-PLIs and γ-PLIs [9].
sPLA2 inhibitors have also been found in plants. Withania somnifera glycoprotein (WSG) is an acidic glycoprotein that is similar to the α-chain of the γ-PLIs, and it was isolated from the medicinal plant known variously as Ashwagandha, Indian ginseng and winter cherry. WSG inhibits the enzymatic activity and toxicity of NN-XIa-PLA2, an sPLA2 myotoxin from venom of the Indian cobra [112-114].
Interestingly, sPLA2-BPs have also been shown to increase the enzymatic activity of sPLA2s. One such sPLA2-BP is the EF-hand Ca2+-binding intracellular protein CaM. When sPLA2s are in a complex with CaM, they also become more resistant to chemical denaturation [16, 17]. Two other sPLA2-BPs that can potentiate the catalytic activity of sPLA2s are vimentin [65] and GLIC [32]. The potentiating effect appears to occur because the interactions with these three sPLA2-BPs position the complexed sPLA2 on the membrane in a way that provides more efficient catalytic function.
Medical potential of sPLA2s as ligands for receptors
sPLA2-BPs have diverse physiological functions, and therefore their interactions with sPLA2s can be insightful and potentially helpful for medical applications. Important roles of M-type sPLA2R in cancers have been outlined recently, with a tumour suppressive role demonstrated [74, 115]. As ligands of M-type sPLA2R, sPLA2s can be used to study or to regulate processes in which M-type sPLA2R is involved. By binding to M-type sPLA2R, sPLA2s have already been indicated to have roles in cell proliferation, migration and senescence, hormone release, and cytokine and NO production. sPLA2s were also demonstrated to induce kidney glomerular podocyte apoptosis via M-type sPLA2R [102]. A recently generated conditional transgenic mouse that expresses human M-type sPLA2R1 will certainly facilitate future investigations into this sPLA2-BP under different pathophysiological conditions [116].
VEGFRs are sPLA2-BPs that have also been shown to be involved in cancer development. By binding to VEGFRs, sPLA2s might inhibit angiogenesis, which is an essential process for cancer metastasis formation [117]. Therefore, the use of sPLA2s in chemotherapy has been proposed. In addition, the angiogenic pathways of VEGFRs and endothelial nAChRs have been shown to have cross-talk, which suggests that as antagonists of nAChRs, sPLA2s might further inhibit angiogenesis in this way [31, 118]. In contrast to the antagonistic effects on VEGFRs and nAChRs, GIIA sPLA2 can activate EGFRs in lung cancer cells [34]. This leads to elevated HER /HER2-elicited signalling, which contributes to overexpression of GIIA sPLA2. Plasma concentration of GIIA sPLA2 might therefore serve as a biomarker for lung cancer, and GIIA sPLA2 might represent a therapeutic target to treat patients with lung cancer.
The integrin binding of snake venom sPLA2s has also been exploited for development of new anti-cancer agents that target cell proliferation and migration [36, 103]. MT-II binding to nucleolin appears to explain the higher toxicity of MT-II against cancer cells, as nucleolin is more abundant on the surface of cancer cells than of normal cells [90]. Nucleolin also participates in internalization of many viruses, which suggested that the anti-viral activity of some sPLA2s might be due to their nucleolin binding [27].
The involvement of GIIA sPLA2 in synovial inflammation through liberation of arachidonic acid for production of inflammatory eicosanoids has long been known. Efforts have been made to develop inhibitors of GIIA sPLA2 to attenuate rheumatoid arthritis and sepsis, but without substantial success so far [119, 120]. Colocalization of GIIA sPLA2 and vimentin is, however, associated with phospholipase-activity-independent mechanisms of signalling through arachidonic acid metabolism [83], which provides the way for targeted studies of GIIA sPLA2 signalling, and for the development of new therapeutic strategies based on inhibition of GIIA sPLA2. Lee et al. (2013) [83] also identified vimentin as an interesting player in some other diseases where the involvement of GIIA sPLA2 has been indicated, and particularly in cancers. GIIA sPLA2 is overexpressed in many types of cancers, while vimentin is one of the signature biomarkers of tumour dedifferentiation through epithelial-mesenchymal transition [121, 122].
Abundant expression of vimentin has also been observed in adult neurons as a response to injury, such as in Alzheimer's disease [123]. As vimentin might assist the internalization of GIIA sPLA2 into neurons, this suggests why GIIA sPLA2 is involved in the aetiology of Alzheimer's disease. GIIA sPLA2 might directly damage neurons or boost inflammation by releasing excessive arachidonic acid. Targeting of vimentin is, therefore, potentially interesting as a new therapeutic approach to treat patients with Alzheimer's disease.
A phospholipase-activity-independent mode of action is also characteristic for snake venom catalytically inactive sPLA2s. These can induce inflammation [124]; e.g., MT-II can stimulate the production and release of inflammatory mediators, such as interleukin-6 [125], interleukin-1, tumour necrosis factor α, leukotriene B4, thromboxane A2 and prostaglandins E2 and D2 [126-129]. Indeed, this activity of MT-II was recently used to establish a new experimental model of acute arthritis [130].
Mammalian GIB, GIIA, GV and GX sPLA2s induce the production of pro-inflammatory cytokines and chemokines, whereby these sPLA2s increase inflammation after binding to HSPGs or to M-type sPLA2R on macrophages, neutrophils, eosinophils, monocytes and endothelial cells [131]. This binding to HSPGs also participates in clearance of these sPLA2s and in reduction of their enzymatic activity towards low-density lipoprotein, which is an important factor in atherosclerosis [3, 132].
sPLA2s also mediate pro-inflammatory actions via integrins, as binding of GIIA sPLA2 to human integrins αvβ3, α4β1 and α5β1 trigger signalling that leads to inflammation [36, 133]. The effects of sPLA2s on haemostasis have great potential for medical applications as well. The induction of vasoconstriction by PA2-Vb binding to PAR-1 makes PA2-Vb interesting for the development of new therapeutic approaches against hypertension, atherosclerosis and diabetes-related vascular problems [35]. Moreover, snake venom sPLA2s can induce strong anticoagulant effects through competitive binding to constituents of the prothrombinase complex, which suggests their great potential for the development of therapeutic procedures to attenuate or prevent blood-clot formation [108].
As already mentioned, GIIA sPLA2 has been associated with the aetiology of some neurodegenerative diseases, such as Alzheimer's disease [134-137]. A hallmark of the induction of Alzheimer's disease is the elevated expression of GIIA sPLA2 in the affected tissue, with concomitant dysfunction of the neuronal mitochondria. As the pathological effects of presynaptically neurotoxic sPLA2s from snake venoms and GIIA sPLA2 on mitochondria are similar, a description of the mode by which neurotoxic sPLA2s encounter and affect neuronal mitochondria at the molecular level is expected to advance the study of the role of endogenous GIIA sPLA2 in this and other related destructive diseases.
These findings indicate a way to the development of original diagnostic and therapeutic solutions. An important breakthrough in this direction was the recent identification of CCOX as the mitochondrial receptor for Atx [37]. Application-wise, a very promising discovery was also the binding of crotoxin to ΔF508CFTR, responsible for cystic fibrosis. The sPLA2 subunit of crotoxin has been used as a template to develop a new line of anti-cystic fibrosis agents [33].
Conclusions and outlook
As well as participating in a very broad spectrum of biological processes through their enzymatic activity, sPLA2s participate in many physiological settings as ligands for membrane or soluble receptors. As new and more sensitive analytical tools are developed, the number of newly discovered sPLA2-BPs is growing. Further technical advances will promote the discovery of even more sPLA2-BPs.
sPLA2-BPs are structurally diverse integral membrane and soluble proteins. Despite many attempts, their common sPLA2-binding attributes remain largely obscure. The solution to this puzzle will enable targeted searches of additional sPLA2-BPs. A straightforward approach to solve this problem would be the determination of the three-dimensional structures of complexes between sPLA2s and their sPLA2-BPs. With the development of powerful new technologies, such as cryo-electron microscopy, this is becoming more and more realistic.
sPLA2s are very useful molecules from the evolutionary point of view. Particular structural features have made the sPLA2s promiscuous, and promiscuous proteins can acquire new functions more readily than other proteins. In this way, these can enable organisms to adapt more successfully to environmental changes.
Finally, due to their enzymatic activity and extensive interactomes, sPLA2s are exceptional pharmacological targets. Detailed descriptions of their actions at the molecular level will initiate the development of a plethora of original diagnostic and therapeutic approaches.
Abbreviations
Atx: sPLA2 from Vipera a. ammodytes venom; CaM: calmodulin; CB: basic sPLA2 subunit of crotoxin; CCOX: cytochrome c oxidase; CFTR: cystic fibrosis transmembrane conductance regulator; CRD: carbohydrate recognition domain; DM64: metalloproteinase inhibitor from Didelphis marsupialis blood; ECD: extracellular domain; EGF(R): epidermal growth factor (receptor); ER: endoplasmic reticulum; FX/FXa/FIIa: blood coagulation factors; GLIC: proton-gated ion channel from Gloeobacter violaceus; GPCR: G-protein-coupled receptor; HER: human EGFR; hGIIA: human group IIA sPLA2; HSPGs: heparan sulphate proteoglycans; IBS: interfacial binding surface; Ig-like: immunoglobulin-like; M-type sPLA2Rs: muscle-type sPLA2 receptors; nAChR: nicotinic acetylcholine receptor; NBD: nucleotide-binding domain; NP: neuronal pentraxin; OS1: sPLA2 from Oxyuranus s. scutellatus venom; PAR: protease-activated receptor; PDI: protein disulphide isomerase; PLIs: sPLA2 inhibitors; SP-A: pulmonary surfactant protein A; sPLA2: secreted phospholipase A2; sPLA2-BP: sPLA2-binding protein; TCBP-49: taipoxin-associated 49-kDa Ca2+-binding protein; VEGFR: vascular endothelial growth factor receptor; WSG: Withania somnifera glycoprotein.
Acknowledgements
This work was supported by grants from the Slovenian Research Agency, a programme grant P1-0207 (to I.K.) and a Young Researcher grant 1000-17-0106-6 (to A.I.).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Dennis EA, Cao J, Hsu YH, Magrioti V, Kokotos G. Phospholipase A2 enzymes: Physical structure, biological function, disease implication, chemical inhibition, and therapeutic intervention. Chemical Reviews. 2011;111:6130-85
2. Murakami M, Sato H, Taketomi Y. Updating phospholipase A2 biology. Biomolecules. 2020;10:1-33
3. Lomonte B, Križaj I. Snake Venom Phospholipase A2 Toxins. In: Mackessy S, ed. Handbook of Venoms and Toxins of Reptiles, 2nd ed. Boca Raton: CRC Press. 2021:389-412
4. Murakami M. Novel functions of phospholipase A2 s: Overview. Biochimica et Biophysica Acta - Molecular and Cell Biology of Lipids. 2019;1864:763-5
5. Rehm H, Betz H. Binding of β-bungarotoxin to synaptic membrane fractions of chick brain. Journal of Biological Chemistry. 1982;257:10015-22
6. Lambeau G, Lazdunski M. Receptors for a growing family of secreted phospholipases A2. Trends in pharmacological sciences. 1999;20:162-70
7. Valentin E, Lambeau G. Increasing molecular diversity of secreted phospholipases A2 and their receptors and binding proteins. Biochimica et Biophysica Acta - Molecular and Cell Biology of Lipids. 2000;1488:59-70
8. Hanasaki K, Arita H. Phospholipase A2 receptor: A regulator of biological functions of secretory phospholipase A2. Prostaglandins and Other Lipid Mediators. 2002;68-69:71-82
9. Šribar J, Križaj I. Secreted phospholipases A2 - Not just enzymes. Acta Chimica Slovenica. 2011;58:678-88
10. Nicolas JP, Lambeau G, Lazdunski M. Identification of the binding domain for secretory phospholipases A2 on their M-type 180-kDa membrane receptor. Journal of Biological Chemistry. 1995;270:28869-73
11. Lambeau G, Schmid-Alliana A, Lazdunski M, Barhanin J. Identification and purification of a very high affinity binding protein for toxic phospholipases A2 in skeletal muscle. The Journal of biological chemistry. 1990;265:9526-32
12. Santos-Filho NA, Santos CT. Alpha-type phospholipase A2 inhibitors from snake blood. Journal of Venomous Animals and Toxins including Tropical Diseases. 2017;23:19
13. Dy ABC, Tanyaratsrisakul S, Voelker DR, Ledford JG. The emerging roles of surfactant protein-A in asthma. Journal of clinical & cellular immunology. 2018;9:553
14. Campos PC, de Melo LA, Dias GLF, Fortes-Dias CL. Endogenous phospholipase A2 inhibitors in snakes: a brief overview. The journal of venomous animals and toxins including tropical diseases. 2016;22:37
15. Fortes-Dias CL, Ortolani PL, Fernandes CAH, Lobo KR, Melo LA De, Borges MH. et al. Insights on the structure of native CNF, an endogenous phospholipase A2 inhibitor from Crotalus durissus terrificus, the South American rattlesnake. Biochimica et Biophysica Acta - Proteins and Proteomics. 2014;1844:1569-79
16. Kovačič L, Novinec M, Petan T, Križaj I. Structural basis of the significant calmodulin-induced increase in the enzymatic activity of secreted phospholipases A2. Protein Engineering, Design and Selection. 2010;23:479-87
17. Kovačič L, Novinec M, Petan T, Baici A, Križaj I. Calmodulin is a nonessential activator of secretory phospholipase A2. Biochemistry. 2009;48:11319-28
18. Dodds D, Schlimgen AK, Lu S -Y, Perin MS. Novel reticular calcium binding protein is purified on taipoxin columns. Journal of Neurochemistry. 1995;64:2339-44
19. Hseu MJ, Yen CH, Tzeng MC. Crocalbin: A new calcium-binding protein that is also a binding protein for crotoxin, a neurotoxic phospholipase A2. FEBS Letters. 1999;445:440-4
20. Oberčkal J, Kovačič L, Šribar J, Leonardi A, Dolinar K, Janež AP. et al. On the role of protein disulfide isomerase in the retrograde cell transport of secreted phospholipases A2. PLoS ONE. 2015;10:e0120692
21. Šribar J, Anderluh G, Fox JW, Križaj I. Protein disulphide isomerase binds ammodytoxin strongly: possible implications for toxin trafficking. Biochemical and biophysical research communications. 2005;329:733-7
22. Fujisawa D, Yamazaki Y, Lomonte B, Morita T. Catalytically inactive phospholipase A2 homologue binds to vascular endothelial growth factor receptor-2 via a C-terminal loop region. Biochemical Journal. 2008;411:515-22
23. Yamazaki Y, Matsunaga Y, Nakano Y, Morita T. Identification of vascular endothelial growth factor receptor-binding protein in the venom of eastern cottonmouth: A new role of snake venom myotoxic LYS49-phospholipase A2. Journal of Biological Chemistry. 2005;280:29989-92
24. Rocha SLG, Lomonte B, Neves-Ferreira AGC, Trugilho MRO, Junqueira-de-Azevedo IDLM, Ho PL. et al. Functional analysis of DM64, an antimyotoxic protein with immunoglobulin-like structure from Didelphis marsupialis serum. European Journal of Biochemistry. 2002;269:6052-62
25. Boilard E, Bourgoin SG, Bernatchez C, Poubelle PE, Surette ME. Interaction of low molecular weight group IIA phospholipase A2 with apoptotic human T cells: Role of heparan sulfate proteoglycans. The FASEB Journal. 2003;17:1068-80
26. Dutta S, Sinha A, Dasgupta S, Mukherjee AK. Binding of a Naja naja venom acidic phospholipase A2 cognate complex to membrane-bound vimentin of rat L6 cells: Implications in cobra venom-induced cytotoxicity. Biochimica et Biophysica Acta - Biomembranes. 2019;1861:958-77
27. Massimino ML, Simonato M, Spolaore B, Franchin C, Arrigoni G, Marin O. et al. Cell surface nucleolin interacts with and internalizes Bothrops asper Lys49 phospholipase A2 and mediates its toxic activity. Scientific Reports. 2018;8:10619
28. Kirkpatrick LL, Matzuk MM, Dodds DC, Perin MS. Biochemical interactions of the neuronal pentraxins. Neuronal pentraxin (NP) receptor binds to taipoxin and taipoxin-associated calcium-binding protein 49 via NP1 and NP2. Journal of Biological Chemistry. 2000;275:17786-92
29. Šribar J, Sherman NE, Prijatelj P, Faure G, Gubenšek F, Fox JW. et al. The neurotoxic phospholipase A2 associates, through a non-phosphorylated binding motif, with 14-3-3 protein γ and ε isoforms. Biochemical and Biophysical Research Communications. 2003;302:691-6
30. Yagami T, Yamamoto Y, Kohma H, Nakamura T, Takasu N, Okamura N. L-type voltage-dependent calcium channel is involved in the snake venom group IA secretory phospholipase A2-induced neuronal apoptosis. NeuroToxicology. 2013;35:146-53
31. Vulfius CA, Kasheverov IE, Kryukova E V, Spirova EN, Shelukhina I V, Starkov VG. et al. Pancreatic and snake venom presynaptically active phospholipases A2 inhibit nicotinic acetylcholine receptors. PLoS ONE. 2017;12:e0186206
32. Ostrowski M, Porowinska D, Prochnicki T, Prevost M, Raynal B, Baron B. et al. Neurotoxic phospholipase A2 from rattlesnake as a new ligand and new regulator of prokaryotic receptor GLIC (proton-gated ion channel from G. violaceus). Toxicon. 2016;116:63-71
33. Faure G, Bakouh N, Lourdel S, Odolczyk N, Premchandar A, Servel N. et al. Rattlesnake phospholipase A2 increases CFTR-chloride channel current and corrects ∆F508CFTR dysfunction: Impact in cystic fibrosis. Journal of Molecular Biology. 2016;428:2898-915
34. Dong Z, Meller J, Succop P, Wang J, Wikenheiser-Brokamp K, Starnes S. et al. Secretory phospholipase A2-IIA upregulates HER/HER2-elicited signaling in lung cancer cells. International Journal of Oncology. 2014;45:978-84
35. Zeng F, Zhang W, Xue N, Teng M, Li X, Shen B. Crystal structure of phospholipase PA2-Vb, a protease-activated receptor agonist from the Trimeresurus stejnegeri snake venom. FEBS Letters. 2014;588:4604-12
36. Fujita M, Zhu K, Fujita CK, Zhao M, Lam KS, Kurth MJ. et al. Proinflammatory secreted phospholipase A2 type IIA (sPLA-IIA) induces integrin activation through direct binding to a newly identified binding site (site 2) in integrins αvβ3, α4β1, and α5β1. Journal of Biological Chemistry. 2015;290:259-71
37. Šribar J, Kovačič L, Oberčkal J, Ivanušec A, Petan T, Fox JW. et al. The neurotoxic secreted phospholipase A2 from the Vipera a. ammodytes venom targets cytochrome c oxidase in neuronal mitochondria. Scientific Reports. 2019;9:283
38. Vučemilo N, Čopič A, Gubenšek F, Križaj I. Identification of a new high-affinity binding protein for neurotoxic phospholipases A2. Biochemical and biophysical research communications. 1998;251:209-12
39. Loffredo S, Ferrara AL, Bova M, Borriello F, Suffritti C, Veszeli N. et al. Secreted phospholipases A2 in hereditary angioedema with C1-inhibitor deficiency. Frontiers in Immunology. 2018;9:1-9
40. Faure G, Gowda VT, Maroun RC. Characterization of a human coagulation factor Xa-binding site on Viperidae snake venom phospholipases A2 by affinity binding studies and molecular bioinformatics. BMC Structural Biology. 2007;7:82
41. Dong Y, Cao L, Tang H, Shi X, He Y. Structure of human M-type phospholipase A2 receptor revealed by cryo-electron microscopy. Journal of Molecular Biology. 2017;429:3825-35
42. Lambeau G, Ancian P, Nicolas JP, Beiboer SHW, Moinier D, Verheij H. et al. Structural elements of secretory phospholipases A2 involved in the binding to M-type receptors. Journal of Biological Chemistry. 1995;270:5534-40
43. Estevão-Costa MI, Fernandes CAH, Mudadu MDA, Franco GR, Fontes MRM, Fortes-Dias CL. Structural and evolutionary insights into endogenous alpha-phospholipase A2 inhibitors of Latin American pit vipers. Toxicon. 2016;112:35-44
44. Shin D, Choi W, Bae H. Bee venom phospholipase A2 alleviate house dust mite-induced atopic dermatitis-like skin lesions by the CD206 mannose receptor. Toxins. 2018;10:146
45. West AP, Herr AB, Bjorkman PJ. The chicken yolk sac IgY receptor, a functional equivalent of the mammalian MHC-related Fc receptor, is a phospholipase A2 receptor homolog. Immunity. 2004;20:601-10
46. Kini RM, Evans HJ. A model to explain the pharmacological effects of snake venom phospholipases A2. Toxicon. 1989;27:613-35
47. Rouault M, Le Calvez C, Boilard E, Surrel F, Singer A, Ghomashchi F. et al. Recombinant production and properties of binding of the full set of mouse secreted phospholipases A2 to the mouse M-type receptor. Biochemistry. 2007;46:1647-62
48. Lomonte B, Moreno E, Tarkowski A, Hanson LA, Maccarana M. Neutralizing interaction between heparins and myotoxin II, a lysine 49 phospholipase A2 from Bothrops asper snake venom. Identification of a heparin-binding and cytolytic toxin region by the use of synthetic peptides and molecular modeling. Journal of Biological Chemistry. 1994;269:29867-73
49. Núñez CE, Angulo Y, Lomonte B. Identification of the myotoxic site of the Lys49 phospholipase A2 from Agkistrodon piscivorus piscivorus snake venom: synthetic C-terminal peptides from Lys49, but not from Asp49 myotoxins, exert membrane-damaging activities. Toxicon. 2001;39:1587-94
50. Ward RJ, Chioato L, de Oliveira AHC, Ruller R, Sá JM. Active-site mutagenesis of a Lys49-phospholipase A2: biological and membrane-disrupting activities in the absence of catalysis. The Biochemical journal. 2002;362:89-96
51. Fernandes CAH, Borges RJ, Lomonte B, Fontes MRM. A structure-based proposal for a comprehensive myotoxic mechanism of phospholipase A2-like proteins from viperid snake venoms. Biochimica et Biophysica Acta - Proteins and Proteomics. 2014;1844:2265-76
52. Gutiérrez JM, Rucavado A, Escalante T, Herrera C, Fernández J, Lomonte B. et al. Unresolved issues in the understanding of the pathogenesis of local tissue damage induced by snake venoms. Toxicon. 2018;148:123-31
53. Peggion C, Tonello F. Short linear motifs characterizing snake venom and mammalian phospholipases A2. Toxins. 2021 13
54. Zheng M, Cheng H, Banerjee I, Chen J. ALP/Enigma PDZ-LIM domain proteins in the heart. Journal of Molecular Cell Biology. 2010;2:96-102
55. Bhat HF, Adams ME, Khanday FA. Syntrophin proteins as Santa Claus: Role(s) in cell signal transduction. Cellular and Molecular Life Sciences. 2013;70:2533-54
56. Faure G, Saul F. Structural and functional characterization of anticoagulant, FXa-binding Viperidae snake venom phospholipases A2. Acta Chimica Slovenica. 2011;58:671-7
57. Sharma M, Iyer JK, Shih N, Majumder M, Mattaparthi VSK, Mukhopadhyay R. et al. Daboxin P, a major phospholipase A2 enzyme from the Indian Daboia russelii russelii venom targets factor x and factor Xa for its anticoagulant activity. PLoS ONE. 2016;11:e0153770
58. Nemecz D, Ostrowski M, Ravatin M, Saul F, Faure G. Crystal structure of isoform CBd of the basic phospholipase A2 subunit of crotoxin: Description of the structural framework of CB for interaction with protein targets. Molecules. 2020;25:5290
59. Vulfius CA, Kasheverov IE, Starkov VG, Osipov A V, Andreeva T V, Filkin SY. et al. Inhibition of nicotinic acetylcholine receptors, a novel facet in the pleiotropic activities of snake venom phospholipases A2. PLoS ONE. 2014;9:e115428
60. Bhat V, Olenick MB, Schuchardt BJ, Mikles DC, McDonald CB, Farooq A. Biophysical basis of the promiscuous binding of B-cell lymphoma protein 2 apoptotic repressor to BH3 ligands. Journal of Molecular Recognition. 2013;26:501-13
61. Sayou C, Monniaux M, Nanao MH, Moyroud E, Brockington SF, Thévenon E. et al. A promiscuous intermediate underlies the evolution of LEAFY DNA binding specificity. Science. 2014;343:645-8
62. Saul FA, Prijatelj-Žnidaršič P, Vulliez-le Normand B, Villette B, Raynal B, Pungerčar J. et al. Comparative structural studies of two natural isoforms of ammodytoxin, phospholipases A2 from Vipera ammodytes ammodytes which differ in neurotoxicity and anticoagulant activity. Journal of Structural Biology. 2010;169:360-9
63. Fortes-Dias CL, Santos RMM dos, Magro AJ, Fontes MR de M, Chávez-Olórtegui C, Granier C. Identification of continuous interaction sites in PLA2-based protein complexes by peptide arrays. Biochimie. 2009;91:1482-92
64. Silliman CC, Moore EE, Zallen G, Gonzalez R, Johnson JL, Elzi DJ. et al. Presence of the M-type sPLA2 receptor on neutrophils and its role in elastase release and adhesion. American Journal of Physiology - Cell Physiology. 2002;283:C1102-13
65. Boilard E, Bourgoin SG, Bernatchez C, Surette ME. Identification of an autoantigen on the surface of apoptotic human T cells as a new protein interacting with inflammatory group IIA phospholipase A2. Blood. 2003;102:2901-9
66. Nomura K, Fujita H, Arita H. Gene expression of pancreatic-type phospholipase-A2 in rat ovaries: Stimulatory action on progesterone release. Endocrinology. 1994;135:603-9
67. Ramanadham S, Ma Z, Arita H, Zhang S, Turk J. Type IB secretory phospholipase A2 is contained in insulin secretory granules of pancreatic islet β-cells and is co-secreted with insulin from glucose-stimulated islets. Biochimica et Biophysica Acta - Lipids and Lipid Metabolism. 1998;1390:301-12
68. Prijatelj P, Križaj I, Kralj B, Gubenšek F, Pungerčar J. The C-terminal region of ammodytoxins is important but not sufficient for neurotoxicity. European Journal of Biochemistry. 2002;269:5759-64
69. Mattiazzi M, Sun Y, Wolinski H, Bavdek A, Petan T, Anderluh G. et al. A neurotoxic phospholipase A2 impairs yeast amphiphysin activity and reduces endocytosis. PLoS ONE. 2012;7:e40931
70. Kim RR, Chen Z, Mann TJ, Bastard K, Scott KF, Bret Church W. Structural and functional aspects of targeting the secreted human group IIA phospholipase A2. Molecules. 2020;25:4459
71. Petrovič U, Šribar J, Matis M, Anderluh G, Peter-Katalinić J, Križaj I. et al. Ammodytoxin, a secretory phospholipase A2, inhibits G2 cell-cycle arrest in the yeast Saccharomyces cerevisiae. Biochemical Journal. 2005;391:383-8
72. Saegusa J, Akakura N, Wu CY, Hoogland C, Ma Z, Lam KS. et al. Pro-inflammatory secretory phospholipase A2 type IIA binds to integrins αvβ3 and α4β1 and induces proliferation of monocytic cells in an integrin-dependent manner. Journal of Biological Chemistry. 2008;283:26107-15
73. Lomonte B, Angulo Y, Sasa M, Gutierrez J. The phospholipase A2 homologues of snake venoms: Biological activities and their possible adaptive roles. Protein & Peptide Letters. 2009;16:860-76
74. Sukocheva O, Menschikowski M, Hagelgans A, Yarla NS, Siegert G, Reddanna P. et al. Current insights into functions of phospholipase A2 receptor in normal and cancer cells: More questions than answers. Seminars in Cancer Biology. 2019;56:116-27
75. Hurley BP, McCormick BA. Multiple roles of phospholipase A2 during lung infection and inflammation. Infection and Immunity. 2008;76:2259-72
76. Nobeli I, Favia AD, Thornton JM. Protein promiscuity and its implications for biotechnology. Nature Biotechnology. 2009;27:157-67
77. Copley SD. An evolutionary biochemist's perspective on promiscuity. Trends in Biochemical Sciences. 2015;40:72-8
78. Rigoni M, Paoli M, Milanesi E, Caccin P, Rasola A, Bernardi P. et al. Snake phospholipase A2 neurotoxins enter neurons, bind specifically to mitochondria, and open their transition pores. Journal of Biological Chemistry. 2008;283:34013-20
79. Logonder U, Jenko-Pražnikar Z, Scott-Davey T, Pungerčar J, Križaj I, Harris JB. Ultrastructural evidence for the uptake of a neurotoxic snake venom phospholipase A2 into mammalian motor nerve terminals. Experimental Neurology. 2009;219:591-4
80. Macchioni L, Corazzi L, Nardicchi V, Mannucci R, Arcuri C, Porcellati S. et al. Rat brain cortex mitochondria release group II secretory phospholipase A2 under reduced membrane potential. The Journal of biological chemistry. 2004;279:37860-9
81. Kruppa AJ, Buss F. Motor proteins at the mitochondria-cytoskeleton interface. Journal of Cell Science. 2021 134
82. Eriksson JE, Dechat T, Grin B, Helfand B, Mendez M, Pallari HM. et al. Introducing intermediate filaments: From discovery to disease. Journal of Clinical Investigation. 2009;119:1763-71
83. Lee LK, Bryant KJ, Bouveret R, Lei PW, Duff AP, Harrop SJ. et al. Selective inhibition of human group IIA-secreted phospholipase A2 (hGIIA) signaling reveals arachidonic acid metabolism is associated with colocalization of hGIIA to vimentin in rheumatoid synoviocytes. Journal of Biological Chemistry. 2013;288:15269-79
84. Rohrbeck A, Schröder A, Hagemann S, Pich A, Höltje M, Ahnert-Hilger G. et al. Vimentin mediates uptake of C3 exoenzyme. PLoS ONE. 2014;9:e101071
85. Adolf A, Leondaritis G, Rohrbeck A, Eickholt BJ, Just I, Ahnert-Hilger G. et al. The intermediate filament protein vimentin is essential for axonotrophic effects of Clostridium botulinum C3 exoenzyme. Journal of Neurochemistry. 2016;139:234-44
86. Zhang Y, Wen Z, Shi X, Liu YJ, Eriksson JE, Jiu Y. The diverse roles and dynamic rearrangement of vimentin during viral infection. Journal of Cell Science. 2021 134
87. Yang J, Zou L, Yang Y, Yuan J, Hu Z, Liu H. et al. Superficial vimentin mediates DENV-2 infection of vascular endothelial cells. Scientific Reports. 2016;6:38372
88. Hovanessian AG, Soundaramourty C, El Khoury D, Nondier I, Svab J, Krust B. Surface expressed nucleolin is constantly induced in tumor cells to mediate calcium-dependent ligand internalization. PLoS ONE. 2010;5:e15787
89. Kim YJ, Kim KP, Rhee HJ, Das S, Rafter JD, Oh YS. et al. Internalized group V secretory phospholipase A2 acts on the perinuclear membranes. Journal of Biological Chemistry. 2002;277:9358-65
90. Jia W, Yao Z, Zhao J, Guan Q, Gao L. New perspectives of physiological and pathological functions of nucleolin (NCL). Life Sciences. 2017;186:1-10
91. Peggion C, Massimino ML, Stella R, Bortolotto R, Agostini J, Maldi A. et al. Nucleolin rescues TDP-43 toxicity in yeast and human cell models. Frontiers in Cellular Neuroscience. 2021 15
92. Schlimgen AK, Helms JA, Vogel H, Perin MS. Neuronal pentraxin, a secreted protein with homology to acute phase proteins of the immune system. Neuron. 1995;14:519-26
93. Murakami M, Kambe T, Shimbara S, Yamamoto S, Kuwata H, Kudo I. Functional association of type IIA secretory phospholipase A2 with the glycosylphosphatidylinositol-anchored heparan sulfate proteoglycan in the cyclooxygenase-2-mediated delayed prostanoid-biosynthetic pathway. Journal of Biological Chemistry. 1999;274:29927-36
94. Zvaritch E, Lambeau G, Lazdunski M. Endocytic properties of the M-type 180-kDa receptor for secretory phospholipases A2. Journal of Biological Chemistry. 1996;271:250-7
95. Yokota Y, Notoya M, Higashino K ichi, Ishimoto Y, Nakano K, Arita H. et al. Clearance of group X secretory phospholipase A2 via mouse phospholipase A2 receptor. FEBS Letters. 2001;509:250-4
96. Kinoshita E, Handa N, Hanada K, Kajiyama G, Sugiyama M. Activation of MAP kinase cascade induced by human pancreatic phospholipase A2 in a human pancreatic cancer cell line. FEBS Letters. 1997;407:343-6
97. Fayard JM, Tessier C, Pageaux JF, Lagarde M, Laugier C. Nuclear location of PLA2-I in proliferative cells. Journal of Cell Science. 1998;111:985-94
98. Bohlen CJ, Chesler AT, Sharif-Naeini R, Medzihradszky KF, Zhou S, King D. et al. A heteromeric Texas coral snake toxin targets acid-sensing ion channels to produce pain. Nature. 2011;479:410-4
99. Chiricozzi E, Fernandez-Fernandez S, Nardicchi V, Almeida A, Bolaños JP, Goracci G. Group IIA secretory phospholipase A2 (GIIA) mediates apoptotic death during NMDA receptor activation in rat primary cortical neurons. Journal of Neurochemistry. 2010;112:1574-83
100. Lykhmus O, Gergalova G, Koval L, Zhmak M, Komisarenko S, Skok M. Mitochondria express several nicotinic acetylcholine receptor subtypes to control various pathways of apoptosis induction. International Journal of Biochemistry and Cell Biology. 2014;53:246-52
101. Gergalova G, Lykhmus O, Komisarenko S, Skok M. α7 nicotinic acetylcholine receptors control cytochrome c release from isolated mitochondria through kinase-mediated pathways. International Journal of Biochemistry and Cell Biology. 2014;49:26-31
102. Pan Y, Wan J, Liu Y, Yang Q, Liang W, Singhal PC. et al. sPLA2 IB induces human podocyte apoptosis via the M-type phospholipase A2 receptor. Scientific Reports. 2014;4:6660
103. Ye L, Dickerson T, Kaur H, Takada YK, Fujita M, Liu R. et al. Identification of inhibitors against interaction between pro-inflammatory sPLA2-IIA protein and integrin αvβ3. Bioorganic and Medicinal Chemistry Letters. 2013;23:340-5
104. Šribar J, Oberčkal J, Križaj I. Understanding the molecular mechanism underlying the presynaptic toxicity of secreted phospholipases A2: An update. Toxicon. 2014;89:9-16
105. Kini RM, Koh CY. Metalloproteases affecting blood coagulation, fibrinolysis and platelet aggregation from snake venoms: Definition and nomenclature of interaction sites. Toxins. 2016;8:284
106. Prijatelj P, Charnay M, Ivanovski G, Jenko Z, Pungerčar J, Križaj I. et al. The C-terminal and β-wing regions of ammodytoxin A, a neurotoxic phospholipase A2 from Vipera ammodytes ammodytes, are critical for binding to factor Xa and for anticoagulant effect. Biochimie. 2006;88:69-76
107. Mounier CM, Luchetta P, Lecut C, Koduri RS, Faure G, Lambeau G. et al. Basic residues of human group IIA phospholipase A2 are important for binding to factor Xa and prothrombinase inhibition: Comparison with other mammalian secreted phospholipases A2. European Journal of Biochemistry. 2000;267:4960-9
108. Mukherjee AK, Kalita B, Thakur R. Two acidic, anticoagulant PLA2 isoenzymes purified from the venom of monocled cobra Naja kaouthia exhibit different potency to inhibit thrombin and factor Xa via phospholipids independent, non-enzymatic mechanism. PLoS ONE. 2014;9:e101334
109. Dennis EA, Norris PC. Eicosanoid storm in infection and inflammation. Nature reviews Immunology. 2015;15:511-23
110. Knuplez E, Sturm EM, Marsche G. Emerging role of phospholipase-derived cleavage products in regulating eosinophil activity: Focus on lysophospholipids, polyunsaturated fatty acids and eicosanoids. International Journal of Molecular Sciences. 2021;22:4356
111. Murakami M, Taketomi Y, Miki Y, Sato H, Yamamoto K, Lambeau G. Emerging roles of secreted phospholipase A2 enzymes: The 3rd edition. Biochimie. 2014;107:105-13
112. Deepa M, Veerabasappa Gowda T. Purification and characterization of a glycoprotein inhibitor of toxic phospholipase from Withania somnifera. Archives of Biochemistry and Biophysics. 2002;408:42-50
113. Machiah DK, Gowda TV. Purification of a post-synaptic neurotoxic phospholipase A2 from Naja naja venom and its inhibition by a glycoprotein from Withania somnifera. Biochimie. 2006;88:701-10
114. Gupta P, Dash PK. Molecular details of secretory phospholipase A2 from flax (Linum usitatissimum L.) provide insight into its structure and function. Scientific Reports. 2017;7:11080
115. Bernard D, Vindrieux D. PLA2R1: Expression and function in cancer. Biochimica et Biophysica Acta - Reviews on Cancer. 2014;1846:40-4
116. Jaber S, Goehrig D, Bertolino P, Massemin A, Bihl F, Chabry J. et al. Generation of a conditional transgenic mouse model expressing human Phospholipase A2 Receptor 1. Scientific Reports. 2020;10:8190
117. Hiu JJ, Yap MKK. Cytotoxicity of snake venom enzymatic toxins: Phospholipase A2 and L-amino acid oxidase. Biochemical Society Transactions. 2020;48:719-31
118. Ng MKC, Wu J, Chang E, Wang BY, Katzenberg-Clark R, Ishii-Watabe A. et al. A central role for nicotinic cholinergic regulation of growth factor-induced endothelial cell migration. Arteriosclerosis, Thrombosis, and Vascular Biology. 2007;27:106-12
119. Bradley JD, Dmitrienko AA, Kivitz AJ, Gluck OS, Weaver AL, Wiesenhutter C. et al. A randomized, double-blinded, placebo-controlled clinical trial of LY333013, a selective inhibitor of group II secretory phospholipase A2, in the treatment of rheumatoid arthritis. Journal of Rheumatology. 2005;32:417-23
120. Zeiher BG, Steingrub J, Laterre PF, Dmitrienko A, Fukiishi Y, Abraham E. LY315920NA/S-5920, a selective inhibitor of group IIA secretory phospholipase A2, fails to improve clinical outcome for patients with severe sepsis. Critical Care Medicine. 2005;33:1741-8
121. Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions. Journal of Clinical Investigation. 2009;119:1429-37
122. Scott KF, Graham GG, Bryant KJ. Secreted phospholipase A2 enzymes as therapeutic targets. Expert Opinion on Therapeutic Targets. 2003;7:427-40
123. Levin EC, Acharya NK, Sedeyn JC, Venkataraman V, D'Andrea MR, Wang HY. et al. Neuronal expression of vimentin in the Alzheimer's disease brain may be part of a generalized dendritic damage-response mechanism. Brain Research. 2009;1298:194-207
124. Costa SK, Camargo EA, Antunes E. Inflammatory action of secretory PLA2 from snake venoms. Toxins and Drug Discovery. Dordrecht, The Netherlands: Springer. 2015
125. Lomonte B, Tarkowski A, Hanson LÅ. Host response to Bothrops asper snake venom - Analysis of edema formation, inflammatory cells, and cytokine release in a mouse model. Inflammation. 1993;17:93-105
126. Zuliani JP, Fernandes CM, Zamuner SR, Gutiérrez JM, Teixeira CFP. Inflammatory events induced by Lys-49 and Asp-49 phospholipases A2 isolated from Bothrops asper snake venom: role of catalytic activity. Toxicon. 2005;45:335-46
127. Moreira V, Gutiérrez JM, Soares AM, Zamunér SR, Purgatto E, Teixeira C de FP. Secretory phospholipases A2 isolated from Bothrops asper and from Crotalus durissus terrificus snake venoms induce distinct mechanisms for biosynthesis of prostaglandins E2 and D2 and expression of cyclooxygenases. Toxicon. 2008;52:428-39
128. Moreira V, de Castro Souto PCM, Ramirez Vinolo MA, Lomonte B, María Gutiérrez J, Curi R. et al. A catalytically-inactive snake venom Lys49 phospholipase A₂ homolog induces expression of cyclooxygenase-2 and production of prostaglandins through selected signaling pathways in macrophages. European journal of pharmacology. 2013;708:68-79
129. Teixeira CFP, Landucci ECT, Antunes E, Chacur M, Cury Y. Inflammatory effects of snake venom myotoxic phospholipases A2. 2003; 42: 947-62.
130. Dias RG, Sampaio SC, Sant'Anna MB, Cunha FQ, Gutiérrez JM, Lomonte B. et al. Articular inflammation induced by an enzymatically-inactive Lys49 phospholipase A2: activation of endogenous phospholipases contributes to the pronociceptive effect. Journal of Venomous Animals and Toxins including Tropical Diseases. 2017;23:18
131. Križaj I. Roles of secreted phospholipases A₂ in the mammalian immune system. Protein and peptide letters. 2014;21:1201-8
132. Rosenson RS, Hurt-Camejo E. Phospholipase A2 enzymes and the risk of atherosclerosis. European Heart Journal. 2012;33:2899-909
133. Takada Y, Fujita M. Secreted Phospholipase A2 Type IIA (sPLA2-IIA) Activates Integrins in an Allosteric Manner. In: Atassi M, ed. Protein Reviews. Advances in Experimental Medicine and Biology, vol 925. Singapore: Springer. 2016:103-15
134. Moses GSD, Jensen MD, Lue LF, Walker DG, Sun AY, Simonyi A. et al. Secretory PLA2-IIA: A new inflammatory factor for Alzheimer's disease. Journal of Neuroinflammation. 2006;3:28
135. Mathisen GH, Thorkildsen IH, Paulsen RE. Secretory PLA2-IIA and ROS generation in peripheral mitochondria are critical for neuronal death. Brain Research. 2007;1153:43-51
136. Villanueva EB, Little JP, Lambeau G, Klegeris A. Secreted phospholipase A2 group IIA is a neurotoxin released by stimulated human glial cells. Molecular and Cellular Neuroscience. 2012;49:430-8
137. Yagami T, Yamamoto Y, Koma H. The role of secretory phospholipase A2 in the central nervous system and neurological diseases. Molecular Neurobiology. 2014;49:863-76
Author contact
![]() Corresponding author: igor.krizajsi
Corresponding author: igor.krizajsi

 Global reach, higher impact
Global reach, higher impact