Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2022; 18(3):1271-1287. doi:10.7150/ijbs.65802 This issue Cite
Research Paper
CXCR4-dependent macrophage-to-fibroblast signaling contributes to cardiac diastolic dysfunction in heart failure with preserved ejection fraction
1. Department of Cardiology, Cardiovascular Key Laboratory of Zhejiang Province, Second Affiliated Hospital, Zhejiang University College of Medicine, 88 Jiefang Rd, Hangzhou, Zhejiang Province, 310009, PR China
2. State Key Laboratory of Fluid Power and Mechatronic Systems, Department of Mechanics, Zhejiang University, Hangzhou 310027, China
# NZ and QCM contributed equally to this article.
Abstract
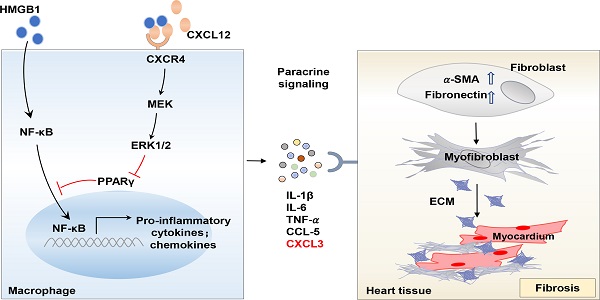
Rationale: Heart failure with preserved ejection fraction (HFpEF) can arise from hypertension‐induced cardiac remodeling. Monocyte/macrophage accumulation and inflammation are crucial elements in the pathogenesis of hypertension-induced cardiac remodeling. The C-X-C chemokine receptor 4 (CXCR4) is a critical regulator of the macrophage-mediated immune response. Nevertheless, the contribution of CXCR4 to macrophage phenotype and function during the progression of HFpEF remains unclear. Herein, we aimed to determine the role of macrophagic CXCR4 in heart failure with preserved ejection fraction (HFpEF).
Methods: As a HFpEF model, wild type mice and myeloid-specific CXCR4 deficiency mice were subjected to pressure overload for 30 days to assess the function of macrophagic CXCR4 on cardiac function. Medium from macrophages was used to treat cardiac fibroblasts to study macrophage-to-fibroblast signaling.
Results: We found circulatory CXCR4+ immune cells, mainly monocytes, markedly increased in HFpEF patients with hypertension. In the experimental HFpEF mice model, macrophages but not neutrophils represent the main infiltrating inflammatory cells in the heart, abundantly expressing CXCR4. Myeloid-specific CXCR4 deficient impeded macrophage infiltration and inflammatory response in the heart of HFpEF mice, thus ameliorating cardiac fibrosis and improving cardiac diastolic function. Furthermore, transcriptomic profiling data revealed that CXCR4 loss in macrophages exhibited a decreased transcriptional signature associated with the regulation of inflammatory response. Notably, CXCR4 significantly augmented chemokine (C‑X‑C) motif ligand (CXCL3) expression, which at least partly contributed to fibrosis by promoting myofibroblast differentiation. Mechanistically, the increased production of pro-inflammatory cytokines in CXCR4 expressed macrophages could be attributed to the suppression of the peroxisome proliferator-activated receptor γ (PPARγ) activity.
Conclusions: Collectively, our data supported that the infiltration of CXCR4+ macrophages in the heart exacerbates hypertension-induced diastolic function by promoting pro-inflammatory cytokines production and thus may serve as a potential therapeutic target for hypertension-induced HFpEF.
Keywords: CXCR4, macrophages, HFpEF, inflammation, fibrosis

 Global reach, higher impact
Global reach, higher impact