10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2022; 18(4):1612-1626. doi:10.7150/ijbs.67852 This issue Cite
Research Paper
Hepatocyte-specific deletion of cellular repressor of E1A-stimulated genes 1 exacerbates alcohol-induced liver injury by activating stress kinases
1. School of Pharmacy, Anhui Medical University, Hefei, 230032, China.
2. Department of Oncology, the First Affiliated Hospital of Anhui Medical University, Hefei, 230022, China.
3. Inflammation and Immune Mediated Diseases Laboratory of Anhui Province, Anhui Medical University, Hefei, 230032, China.
4. School of Life Sciences, Anhui Medical University, Hefei, Anhui 230032, China.
#These authors contributed equally to this work.
Abstract
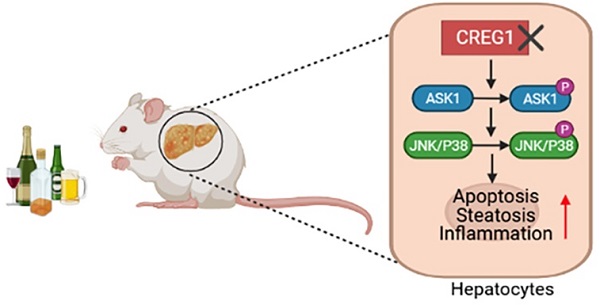
Alcohol-associated liver disease (ALD) encompasses a wide range of pathologies from simple steatosis to cirrhosis and hepatocellular carcinoma and is a global health problem. Currently, there are no effective pharmacological treatments for ALD. We have previously demonstrated that aging exacerbates the pathogenesis of ALD, but the underlying mechanisms are still poorly understood. Cellular repressor of E1A-stimulated genes 1 protein (CREG1) is a recently identified small glycoprotein that has been implicated in aging process by promoting cellular senescence and activating stress kinases. Thus, the current study aimed to explore the role of aging associated CREG1 in ALD pathogenesis and CREG1 as a potential therapeutic target. Hepatic and serum CREG1 protein levels were elevated in ALD patients. Elevation of hepatic CREG1 protein and mRNA was also observed in a mouse model of Gao-binge alcohol feeding. Genetic deletion of the Creg1 gene in hepatocytes (Creg1∆hep) markedly exacerbated ethanol-induced liver injury, apoptosis, steatosis and inflammation. Compared to wild-type mice, Creg1∆hep mice had increased phosphorylation of hepatic stress kinases such as apoptosis signal-regulating kinase 1 (ASK1), c-Jun N-terminal kinase (JNK) and p38 but not TGF-β-activated kinase 1 (TAK1) or extracellular signal-regulated kinase (ERK) after alcohol feeding. In vitro, ethanol treatment elevated the phosphorylation of ASK1, JNK, and p38 in mouse hepatocyte AML-12 cells. This elevation was further enhanced by CREG1 knockdown but alleviated by CREG1 overexpression. Last, treatment with an ASK1 inhibitor abolished ethanol-induced liver injury and upregulated hepatic lipogenesis, proinflammatory genes and stress kinases in Creg1∆hep mice. Taken together, our data suggest that CREG1 protects against alcoholic liver injury and inflammation by inhibiting the ASK1-JNK/p38 stress kinase pathway and that CREG1 is a potential therapeutic target for ALD.
Keywords: CREG1, ALD, Apoptosis, Steatosis, Inflammation

 Global reach, higher impact
Global reach, higher impact