10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2022; 18(5):1961-1973. doi:10.7150/ijbs.68224 This issue Cite
Research Paper
SCM-198 Alleviates Endometriosis by Suppressing Estrogen-ERα mediated Differentiation and Function of CD4+CD25+ Regulatory T Cells
1. NHC Key Lab of Reproduction Regulation (Shanghai Institute of Planned Parenthood Research), Shanghai Key Laboratory of Female Reproductive Endocrine Related Diseases, Hospital of Obstetrics and Gynecology, Fudan University Shanghai Medical College, Shanghai, China.
2. State Key Laboratory of Quality Research in Chinese Medicine and School of Pharmacy, Macau University of Science and Technology, Macau SAR, China.
3. Shanghai Key Laboratory of Bioactive Small Molecules, Department of Pharmacology, School of Pharmacy, Fudan University, Shanghai, China.
4. Shanghai JIAI Genetics & IVF Institute, Obstetrics & Gynecology Hospital of Fudan University, Shanghai, China.
5. Department of Obstetrics and Gynecology, Guangzhou First People's Hospital, School of Medicine, South China University of Technology, Guangzhou, China.
#These authors contributed equally to this work.
Received 2021-10-19; Accepted 2022-1-21; Published 2022-2-21
Abstract
Background: Endometriosis (EMS), a typical endocrine immune disorder, associates with dramatically increased estrogen production and disorganized immune response in ectopic focus. Peritoneal regulatory T cells (Tregs) expansion in women with EMS and their pathogenic role attributable to endometriotic immunotolerance has been reported. Whether local high estrogen promotes EMS by discipling Tregs needs to be further explored. Up to date, there is no effective medicine for the treatment of EMS. SCM-198 is a synthetic leonurine with multiple physiological activities. Whether SCM-198 could regulate Tregs via estrogen and facilitate the radical cure of EMS has not yet been reported.
Methods: Proportion of Tregs in peritoneal fluid of patients with EMS was firstly analyzed via flow cytometry. Peritoneal estrogen concentration and the mRNA levels of estrogen receptor α (ERα) and estrogen receptor β (ERβ) of Tregs were detected by ELISA and RT-PCR, respectively. Grouped in vitro induction assays were performed to explore the effects of SCM-198 and estrogen signaling on Tregs. Cell invasion and viability assays were utilized to detect the crosstalk between Tregs and ectopic endometrial stromal cells (eESCs), with or without SCM-198 treatment. Furthermore, EMS mice models were established to verify the therapeutic effects of SCM-198.
Results: Increased Tregs were found in peritoneal fluid of EMS patients, accompanied with estrogen-ERα overactivation. Estrogen-ERα triggered the expansion of Tregs and their cytokine production (IL-10 and TGF-β1), which could be reversed by SCM-198 treatment. Moreover, SCM-198 abated the invasion and viability of eESCs enhanced by Tregs. In vivo experiments confirmed that SCM-198 obviously retarded the growth of ectopic lesions and downregulated the functions of Tregs via estrogen-ERα inactivation.
Conclusions: These data suggest that SCM-198 attenuates Tregs expansion via the inhibition of estrogen-ERα signaling in EMS and offer a promising therapy for such a refractory disease.
Keywords: SCM-198, EMS, Tregs, estrogen, ERα
Introduction
Endometriosis (EMS), a refractory estrogen-dependent disease, is defined as the growth of endometrial tissue outside the uterine cavity [1, 2]. As a common gynecological disease, the prevalence of EMS dramatically reaches 10% in 2020 with 50% recurrent rate [3-5]. Ectopic endometrial tissue growth and its periodic bleeding leads to inflammation and fibrosis, which eventually result in chronic pelvic pain and dysmenorrhea. The long-term presence of EMS give rise to estimated 30%-50% incurable infertility, which deserving more attention in patients of reproductive age [6, 7]. Although, extensive research has been carried out to verify the role of estrogen dependence and progesterone resistance in EMS, mechanisms require further deepening to elucidate the occurrence and development of EMS. Furthermore, a new therapeutic strategy is urgently needed to alleviate suffering and tackle such an incurable disease.
Actually, immune disorder cannot shirk the responsibility in the progress of EMS [8, 9]. Weakened phagocytosis ability of macrophages and reduced cytotoxicity of natural killer (NK) cells were found in peritoneal fluid of EMS, which were tightly linked with ectopic lesion formation [10-12]. Regulatory T cells (Tregs) have been extensively studied in the region of oncology for their important and irreplaceable role on immune tolerance [13, 14]. Based on the tumor-like immune suppressive microenvironment, an emerging focus in the pathogenesis of EMS has been put on the role of Tregs. Several studies have pointed out Tregs are more abundant in peritoneal fluid of women with EMS [15-17], implying the important role of Tregs on the development of EMS. Moreover, higher production of IL-10 and TGF-β1 in Tregs potentiate ectopic lesions' attachment, survival and growth [18]. Published researches are biased towards the changes in the proportion of peritoneal Tregs in EMS, while few studies have explored the reasons for the accumulation of Tregs and their influence on the growth of EMS. Meanwhile, whether Tregs could consider as a therapeutic target of EMS remains unknown.
Leonurus japonicus Houtt has long been used to treat obstetric and gynecological diseases, such as irregular menstruation, dysmenorrhea. SCM-198 is a synthetic form of leonurine, which is an active alkali component purified from Leonurus japonicus Houtt [19, 20]. Our previous study found that SCM-198 relieved the oxidative stress of endometrial stromal cells (ESCs) [21] and had a therapeutic effect on LPS-induced endometritis [22]. Moreover, Liu et al found that leonurine attenuated the hyperalgesia in mice with induced adenomyosis [23]. However, study focused on the therapeutic effects of SCM-198 on EMS has not been reported.
Herein, we focused on the regulatory impact of SCM-198 on peritoneal Tregs and estrogen signaling. We found that SCM-198 remarkably attenuated estrogen secretion of ectopic lesions and abated estrogen receptor α (ERα) activation of Tregs. Then, we observed a dramatical reduction of expansion in peritoneal Tregs induced by estrogen-ERα after SCM-198 treatment. More important, we demonstrated that SCM-198 administration effectively lessened the ectopic lesions in EMS mice models, with a significant decrease of peritoneal Tregs. Accordingly, our data suggest that SCM-198 might be a promising therapy for EMS.
Materials and Methods
Human sample collections
We recruited 76 women undergoing laparoscopic surgery for ovarian endometriosis, uterine leiomyoma or infertility, etc., at Obstetrics and Gynecology Hospital of Fudan University. Inclusion criteria for EMS utilized in this study were patients diagnosed with EMS by laparoscopy or histology without the combination of adenomyosis or uterine leiomyoma or polycystic ovary syndrome (PCOS) (age < 48 years). Inclusion criteria for non-EMS were patients diagnosed with non-EMS diseases, such as adenomyosis, uterine leiomyoma, benign teratoma or infertility (age < 48 years). Patients who had received medical/hormonal supplementation during the last 3 months or diagnosed with malignant tumor or pelvic inflammatory disease were excluded from both EMS and non-EMS patients' groups. Among them, 46 EMS peritoneal fluid (ePF) and 30 non-EMS peritoneal fluid (nPF) were collected. Ectopic endometrial tissues were obtained from EMS patients (aged 22-45 years) via laparoscopy. The collection and use of these samples were approved by the Human Research Ethics Committee of the Obstetrics and Gynecology Hospital of Fudan University (No. Kyy2016-4) (Shanghai, China).
Isolation and culture of human ectopic endometrial stromal cells
Briefly, the ectopic endometrial tissues were minced (2-3-mm pieces) and digested in DMEM/F-12 containing collagenase type IV (0.1%, Sigma, USA) for 30 min at 37 °C. The dispersed cells were then filtered through a 400-mesh wire sieve to remove the undigested tissue pieces containing the glandular epithelium. After gentle centrifugation, the supernatant was discarded, and the cells were resuspended in DMEM/F-12 containing 10% fetal bovine serum (FBS) (Gemini, USA), 100 IU/ml penicillin (Sigma, USA), 100 μg/ml streptomycin (Sigma, USA), and 1 μg/ml amphotericin B (Sangon, China) at 37 °C in 5% CO2.
Flow Cytometry
Cell surface and intracellular molecular expressions were evaluated by flow cytometry using CytoFLEX (Beckman Coulter, USA). Fluorescein-conjugated anti-mouse antibodies, including CD3-BV785/BV510/AF700, CD4-BV510/BV605, CD25-FITC, FOXP3-PE, IL-10-APC, TGF-β1-BV421 (Biolegend, UK), and anti-human antibodies, including CD3-BV510, CD4-BV650, CD25-APC, FOXP3-PE, IL-10-BV421, TGF-β1-PE/CY7 (Biolegend, UK), were used according to the manufacturer's procedures. For cell-surface staining, single-cell suspensions were stained on ice for 30min in PBS with 1% FBS. For intracellular staining, cells were fixed and permeabilized using the Fix/Perm kit (Biolegend, UK). To detect intracellular cytokines, cells were stimulated for 4 h with Cell Activation Cocktail (with Brefeldin A) (Biolegend, UK). Thereafter, cells were harvested, stained for surface expression, and then fixed and permeabilized for intracellular staining. Flow cytometry data were analyzed using FlowJo software (BD, UK).
ELISA
Collect cell supernatant of nPF, ePF and ectopic endometrial stromal cells (eESCs) to evaluate the levels of estrogen. The concentration of estrogen was assayed by ELISA according to the manufacturer's instructions (estrogen, #CSB-E07286h, China).
Quantitative real-time PCR
Total RNA was extracted using TRIzol reagent (Invitrogen, USA) and then reverse-transcribed into first-strand complementary DNA (cDNA) (Takara, Japan) by using PrimeScript RT Master Mix (#RR036A, TaKaRa, Japan) according to the manufacturer's instructions. The synthesized cDNA was amplified with specific primers and SYBR Green (Takara, Japan) using an ABI PRISM 7900 Sequence Detection System (Applied Biosystems, USA). Triplicate samples were examined for each condition. A comparative threshold cycle value was normalized for each sample using the 2-ΔΔCt method.
Isolation of CD4+CD25- T cells and CD4+CD25+ Tregs
Peripheral blood mononuclear cells (PBMCs) isolated from healthy women were separated by use of lymphoprep (#07851, STEMCELL, Canada) based on gradient centrifugation. CD4+ T cells are obtained by negative selection, and CD25+ cells are obtained by positive selection according to the magnetic beads sorting instructions (#130-091-301, Miltenyi, Germany).
In vitro generation of CD4+CD25+ Tregs
CD4+CD25+ Tregs were generated as previously described [24]. Coat a 24-well plate with 10 µg/ml of anti-CD3 mAb (Biolegend, UK) at 37 °C for 2 h and then wash the plate once with PBS before cell plating. Plate 1 × 106 CD4+CD25- T cells into the precoated well and add anti-CD28 mAb (Biolegend, UK) (2 µg/ml), TGF-β1 (5 ng/ml, PeproTech, USA) and rhIL-2 (20 ng/ml, Biolegend, UK) for 72h incubation. At 24th hour induction, add 17β-estradiol (E2) (#E2758, Sigma, USA) (100 nM), ERα-specific agonist propyl pyrazole triol (PPT) (#HY-100689, MCE, USA) (5 nM), SCM-198 (200 µM), E2+SCM-198 (100 nM, 200 µM) or PPT+SCM-198 (5 nM, 200 µM) to the cells for the rest 48h.
Cell invasion assay
CD4+CD25+ Tregs were collected and washed with PBS for three times after the pretreatment with E2 (100 nM), E2+SCM-198 (100 nM, 200 µM), PPT (5 nM), or PPT+SCM-198 (5 nM, 200 µM) for 48 h. Then, eESCs were cocultured with the pretreated Tregs or SCM-198 (0, 50, 100, 200 µM) for 48h.
Spread 20 µl matrigel (BD, USA) on the upper layer of the chamber (Corning, USA) and then culture it at 37 °C for 2 h. Add 5× 104 eESCs (200 µl) to the upper chamber, which containing 10% FBS. Add 800 µl medium, which containing 20% FBS, to the lower chamber. Incubate cells at 37 °C for 48 h. The cells were fixed with 4% formaldehyde solution for 30min, and then stained with crystal violet (Beyotime, China) for 30 min. Observe the invasion with upright microscope (Olympus, Japan).
Cell viability assay
eESCs were cocultured with SCM-198 (0, 50, 100, 200, 400 µM) or CD4+CD25+ Tregs, which were washed with PBS for three times after the pretreatment with E2 (100 nM), E2+SCM-198 (100 nM, 200 µM), PPT (5 nM), or PPT+SCM-198 (5 nM, 200 µM) for 48 h.
According to the instructions, add 10 µl CCK8 detection reagent (Dojindo, Japan) and 90 µl fresh culture medium to each well. Measure the absorbance at 450 nm wavelength to detect cell viability.
Mouse EMS model
Female C57BL/6 mice (6 weeks old) were purchased from Shanghai JieSiJie Laboratory Animal Co., Ltd. SCM-198 was chemically synthesized by Dr Zhu Yizhun's lab. Permission for animal experimental was provided by the Research Ethics Committee of Obstetrics and Gynecology Hospital of Fudan University.
After two weeks adaptation, mice were randomly selected as the donors of EMS models. Donor mice were intraperitoneally injected with E2 (0.2 µg/g bodyweight) three times for one week. Vaginal smears were utilized to select estrus mice as the recipients of EMS mice models. Mixture of the donor mouse uterine fragments and PBS were intraperitoneally injected to recipient mouse. Recipient mice were allowed to rest for one week.
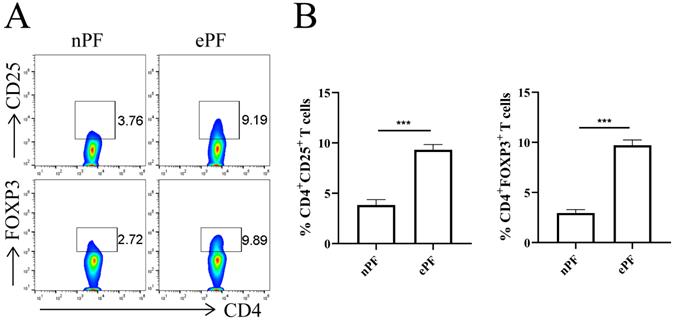
Tregs were accumulated in peritoneal fluid of EMS patients. (A) Representative pictures showing the analysis of CD25 and FOXP3 expression in CD4+T cells of nPF and ePF. (B) Quantitative flow cytometry results for CD25 and FOXP3 expression in CD4+T cells of nPF and ePF (n=10). Data are presented as the mean ± SEM. ***P < 0.001.
To investigate the effects of SCM-198 on EMS, recipient mice were randomly divided into three groups: EMS group, EMS + low-dose SCM-198 group (EMS+SCM-198 L, 7.5 mg/kg) and EMS + high-dose SCM-198 group (EMS+SCM-198 H, 15 mg/kg). Equal number mice were taken as control group. To investigate the effects of SCM-198 on estrogen treated EMS, recipient mice were randomly divided into four groups: EMS group, EMS + E2 group (150 µg/kg), EMS + SCM-198 group (15 mg/kg), EMS + E2 + SCM-198 group (150 µg/kg, 15 mg/kg). Intraperitoneally inject 200 µl of E2 or SCM-198 to the recipient according to the corresponding dose (once a day for total one week). EMS group were given the same dose and frequency of PBS and the control group received no treatment. One week later, the mice were sacrificed. Collect EMS ectopic tissues, uteri and peritoneal fluid for subsequent treatment.
Western blotting analysis
The total proteins of ectopic lesions in EMS mice were extracted using radio-immunoprecipitation assay (RIPA) buffer (Beyotime, China) supplemented with protease and phosphatase inhibitors (Sigma, USA). The protein concentration was measured using a BCA protein assay kit (Beyotime, China). After denaturation, equal amounts of proteins were separated via SDS-polyacrylamide gel electrophoresis (PAGE) before wet transfer onto polyvinylidene difluoride membranes. Nonspecific binding sites were blocked by incubating the membranes with 5% skim milk in Tris-buffered saline with 0.1% Tween 20 (TBS-T) for 1 h. Then, the membranes were incubated overnight at 4 °C with primary antibodies (1:1000) against MMP9 (#ab228402, Abcam, UK), MMP2 (#87809, CST, USA), BCL2 (#2870, CST, USA) and GAPDH (#10112, Arigo, China). Subsequently, membranes were incubated with appropriate horseradish peroxidase-conjugated anti-rabbit (#65351, Arigo, China) or anti-mouse (#65350, Arigo, China) IgG secondary antibodies for 1h at room temperature. After three washes with TBS-T, immunopositive bands on the blots were visualized using chemiluminescent HRP substrate (#WBKLS0100, Millipore, USA) on the enhanced chemiluminescence detection system (Merck Millipore, USA).
Statistical analysis
Flow cytometry data were analyzed using FlowJo software (BD, UK). Prism 8 software (GraphPad, USA) was used for data analysis. Statistical significance was determined using Student's t-test for 2-group or one-way ANOVA for multiple group comparisons. The data are presented as mean±SEM. Statistical significance was attained when P < 0.05.
Results
Tregs accumulates in peritoneal fluid of EMS patients
The demographic and obstetrical characteristics of enrolled participants were summarized in Table 1. Flow cytometry experiments were conducted to evaluate the change of peritoneal Tregs in EMS. Tregs were labeled with surface marker CD25 and transcription factor FOXP3. The results showed that approximately 3.8% of CD4+T cells were CD25 positive and 3.0% of CD4+T cells were FOXP3 positive in nPF (Fig. 1A and 1B). Whereas, the percentage of CD25+ and FOXP3+ in CD4+T cells increased to 9.3% and 9.7% in ePF (Fig. 1A and 1B). Compared with control groups, peritoneal Tregs remarkably increased in EMS, suggesting the possible roles of Tregs in the development of EMS.
Characteristics of recruited participants
| Subjects | Non-EMS | EMS | p |
|---|---|---|---|
| Number | 30 | 46 | - |
| Age range (years) | 23-46 | 22-45 | - |
| Age meana | 35.6±0.99 | 34.98±0.87 | ns |
| Cyst diameter size (cm)b | - | 5.79±0.91 | - |
| rAFS stage (n(%)) | |||
| I | NA | 0 | - |
| II | NA | 0 | - |
| III | NA | 18(39.1%) | - |
| IV | NA | 28(60.9%) | - |
| Menstrual cycle (n (%)) | |||
| Proliferative phase | 12(40%) | 16(34.78%) | - |
| Secretory phase | 18(60%) | 30(65.22%) | - |
| Treatment history | - | - | - |
aMean ± standard error of the mean (SEM); bMean ± standard deviation (SD); rAFS: revised American Fertility Society.
Estrogen-ERα signaling potentiates the expansion of Tregs
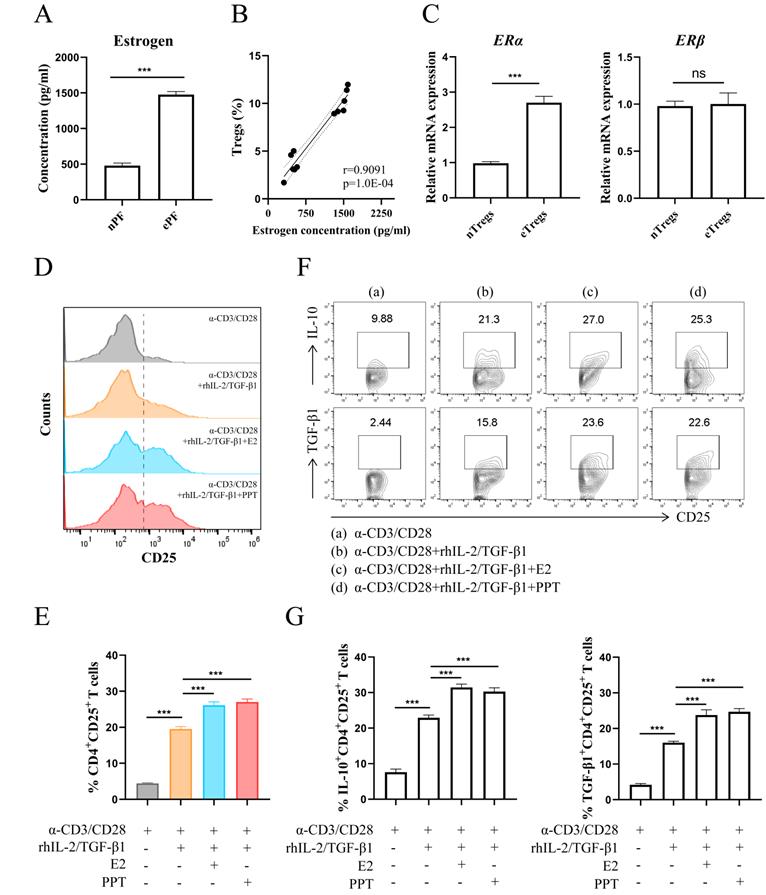
The results in Fig. 2A showed a predominance of estrogen in EMS via ELISA assay. There was a highly positive correlation between the concentration of estrogen and the proportion of Tregs (Fig. 2B). The transcriptional levels of estrogen receptors in Tregs were assessed via RT-PCR and higher ERα mRNA level was observed in Tregs from ePF (Fig. 2C). While no significant change of estrogen receptor β (ERβ) mRNA was detected in Tregs (Fig. 2C). Therefore, we choose ERα as the follow-up research object. To assess whether there is a correlation between Tregs and estrogen-ERα signaling, CD4+CD25- T cells purified from PBMCs were cultured with α-CD3, α-CD28, rhIL-2 and TGF-β1 to mimic the process of Tregs differentiation in vitro, with or without E2 or PPT. The results revealed that E2 and PPT both promoted expansion of Tregs (Fig. 2D and 2E). In addition, E2 and PPT notably increased the production of IL-10 and TGF-β1 in Tregs (Fig. 2F and 2G).
Collectively, these data indicate that estrogen-ERα signaling promotes the differentiation of Tregs and their production of immunosuppressive cytokines (IL-10 and TGF-β1).
SCM-198 abates the differentiation and cytokine production of Tregs by inhibiting estrogen-ERα signaling
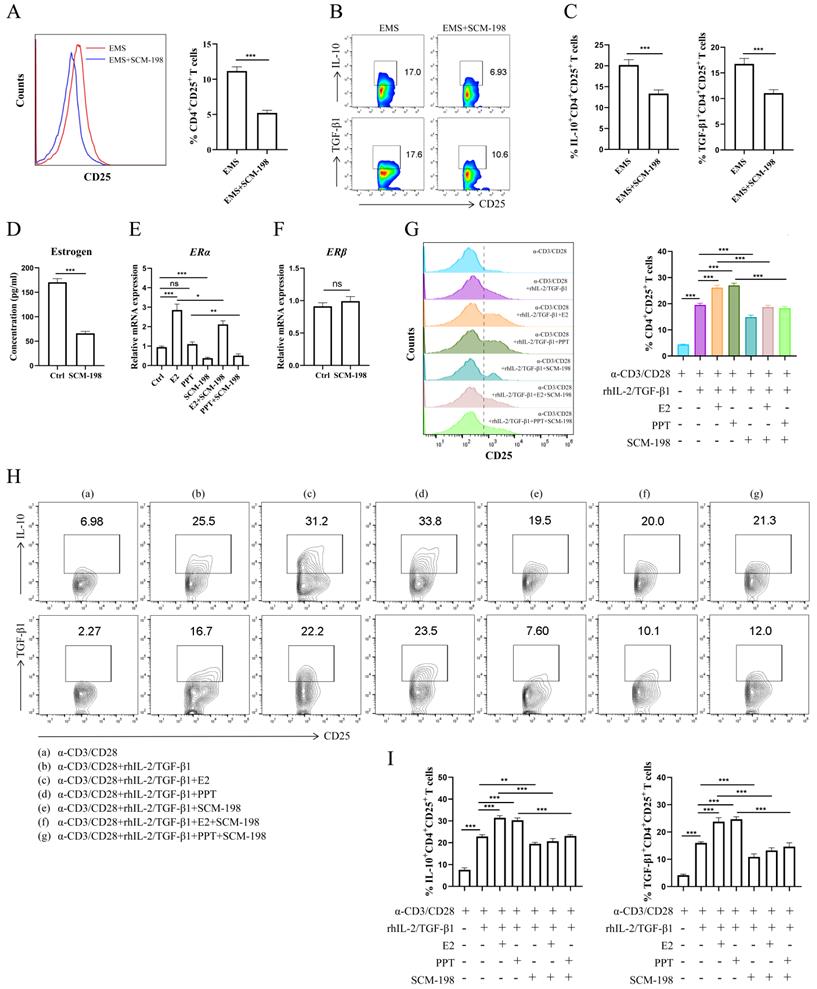
To explore the regulatory effects of SCM-198 on Tregs, we cocultured the total peritoneal immune cells from ePF with SCM-198. The data showed that SCM-198 obviously downregulated the proportion of Tregs (Fig. 3A). Lowered IL-10 and TGF-β1 levels of Tregs were also detected after SCM-198 treatment (Fig. 3B and 3C). To assess whether SCM-198 regulated estrogen production, we treated eESCs, the main source of estrogen in ePF, with SCM-198. The results revealed that SCM-198 notably suppressed estrogen production in eESCs (Fig. 3D). Moreover, SCM-198 suppressed the mRNA level of ERα in Tregs (Fig. 3E). While, no significant change was detected in ERβ with SCM-198 treatment (Fig. 3F). To confirm whether SCM-198 inhibited Tregs by suppressing estrogen-ERα signaling, we conducted in vitro differentiation assay of Tregs under the treatment of SCM-198. As shown in Fig. 3G, SCM-198 dramatically decreased the expansion of Tregs induced by E2 or PPT. Furthermore, the production of IL-10 and TGF-β1, which were reinforced by E2 or PPT, can also be reversed by SCM-198 (Fig. 3H and 3I). These findings demonstrate that SCM-198 can suppress Tregs accumulation and their immunosuppressive cytokines production via estrogen-ERα signaling inhibition.
SCM-198 ameliorates the invasion and viability of eESCs enhanced by Tregs
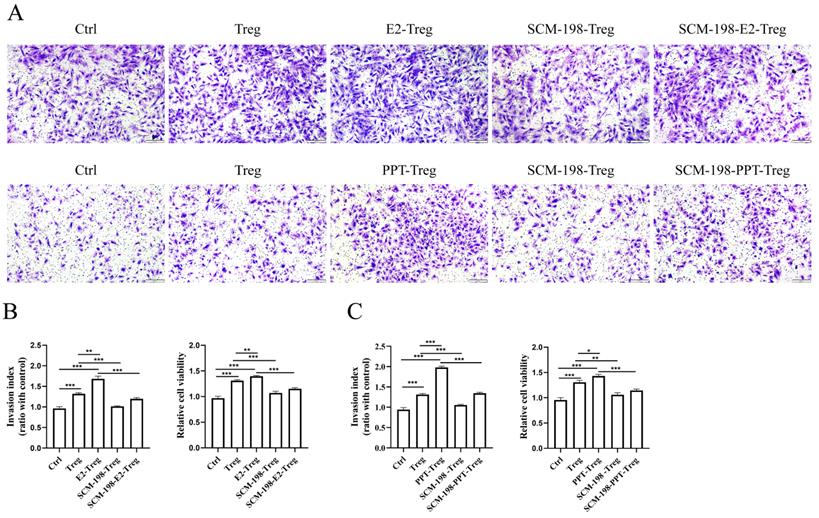
The occurrence and development of EMS mainly depends on the augmented invasion and viability of eESCs. We first explored the direct effects of SCM-198 on the biological behavior of eESCs. The results revealed that SCM-198 had little effect on the invasion, but could restrain the growth of eESCs (Fig. S1A-S1C). Studies have shown that Tregs could stimulate the invasion and viability of eESCs [18]. Therefore, we further explored whether SCM-198 could attenuate the abnormal interaction between Tregs and eESCs. The results showed that Tregs facilitated the invasion and viability of eESCs, which could be enhanced by the pretreatment of E2 and PPT (Fig. 4A-4C). Reversely, SCM-198 significantly diminished the promotive effects induced by E2 or PPT (Fig. 4A-4C). These results suggest that SCM-198 alleviates the promotion of Tregs on eESCs' invasiveness and growth induced by estrogen-ERα signaling.
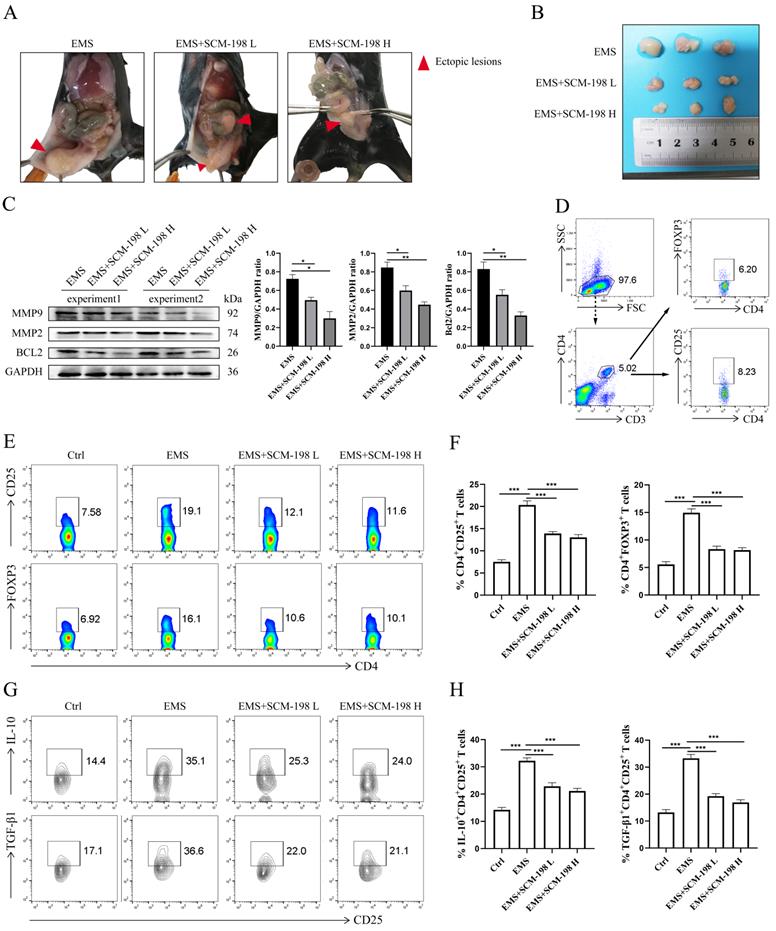
SCM-198 suppresses the growth of EMS and downregulates the accumulation of Tregs in EMS mice
Next, we investigated the therapeutic effects of SCM-198 on in vivo EMS mice models. Fig. 5A exhibited the overall condition of ectopic lesions in the abdominal cavity of EMS mice models under SCM-198 administration. As shown in Fig. 5B, SCM-198 significantly suppressed the growth of ectopic lesions at both low and high dose. SCM-198 appreciably constrained the invasion and viability of ectopic lesions by downregulating the protein levels of MMP9, MMP2 and BCL2 (Fig. 5C). Consistent with the above results observed in human ePF, the proportion of Tregs was obviously upregulated in mice ePF, compared with that from nPF (Fig. 5D-5F). The production levels of IL-10 and TGF-β1 in Tregs were also increased in EMS groups (Fig. 5G and 5H). Similarly, SCM-198 administration significantly reduced the proportion of Tregs (Fig. 5E and 5F) and decreased the production of IL-10 and TGF-β1 in Tregs (Fig. 5G and 5H). These results indicate that SCM-198 might suppresses the EMS progress by downregulating peritoneal Tregs.
Estrogen-ERα signaling potentiated the expansion of Tregs. (A) The concentrations of estrogen in nPF and ePF were measured by ELISA (n=6). (B) Spearman correlation of estrogen concentration and Tregs' proportion. (C) The mRNA expression of ERα and ERβ in CD4+CD25+ Tregs from nPF and ePF (n=5). (D-G) CD4+ CD25- T cells from PBMCs of healthy women were cultured in the presence of anti-CD3 (10 µg/ml) mAb+ anti-CD28 (2 µg/ml) mAb + TGF-β1 (5 ng/ml) + rhIL-2 (20 ng/ml) for total 72 h and at the 24th hour, treated with or without E2 (100 nM) or PPT (5 nM). The cells were harvested and evaluated by flow cytometry for CD25 expression in CD4+T cells (D, E), and for IL-10, TGF-β1 expression in CD4+CD25+ Tregs (F, G) (n=9). Data are presented as the mean ± SEM. ***P < 0.001; ns, not significant.
SCM-198 abated the differentiation and cytokine production of Tregs by inhibiting estrogen-ERα signaling. (A-C) The total peritoneal immune cells from ePF were cultured with SCM-198 (200 µM) for 48 h. The proportions of CD4+CD25+ Tregs were detected by flow cytometry (A) (n=10). The expression of IL-10 and TGF-β1 in CD4+CD25+ Tregs were analyzed by flow cytometry (B, C) (n=10). (D) eESCs were treated with SCM-198 (200 µM) for 48h. The concentration of estrogen produced by eESCs were measured by ELISA (n=4). (E) The mRNA expression of ERα in Tregs treated with or without E2 (100 nM), PPT (5 nM), SCM-198 (200 µM), E2+SCM-198 (100 nM, 200 µM) or PPT+SCM-198 (5 nM, 200 µM) for 48 h were measured by RT-PCR (n=5). (F) The mRNA expression of ERβ in Tregs treated with or without SCM-198 (200 µM) for 48 h were measured by RT-PCR (n=5). (G-I) CD4+ CD25- T cells from PBMCs were cultured in the presence of anti-CD3 (10 µg/ml) mAb + anti-CD28 (2 µg/ml) mAb + TGF-β1 (5 ng/ml) + rhIL-2 (20 ng/ml) for total 72h and at the 24th hour, treated with or without E2 (100 nM), PPT (5 nM), SCM-198 (200 µM), E2+SCM-198 (100 nM, 200 µM) or PPT+SCM-198 (5 nM, 200 µM). The cells were harvested and evaluated by flow cytometry for CD25 expression in CD4+T cells (G), and for IL-10, TGF-β1 expression in CD4+CD25+ Tregs (H, I) (n=9). The Data are presented as the mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001; ns, not significant.
SCM-198 ameliorated the invasion and viability of eESCs enhanced by Tregs. CD4+ CD25+Tregs from ePF were cultured with or without E2 (100 nM), E2+SCM-198 (100 nM, 200 µM), PPT (5 nM), or PPT+SCM-198 (5 nM, 200 µM) for 48 h. Then, the pretreated Tregs were cocultured with eESCs for another 48 h. (A) Representative pictures for the invasion ability of eESCs treated as indicated. Scale bar, 200 µm. (B, C) Data depicted the invasion index and cell viability of eESCs treated as indicated. The invasion index of eESCs was normalized to that of the control (n=4). The viability of eESCs was measured by CCK8 and cell viability was normalized to that of the control (n=6). The Data are presented as the mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001.
SCM-198 restrains the expansion and function of Tregs induced by estrogen in EMS mice
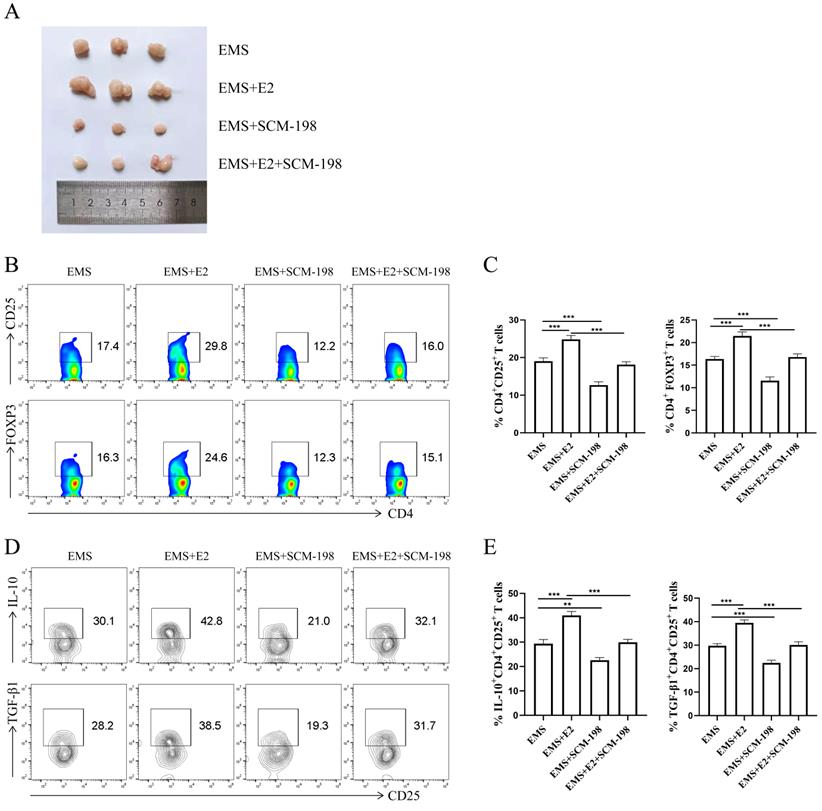
To further verify SCM-198 alleviated the development of EMS by inhibiting Tregs accumulation, we administrated EMS mice with E2. As shown in Fig. 6A, E2 aggravated the development of EMS, which could be attenuated by SCM-198. In addition, the effects of E2 on Tregs could be reversed by SCM-198 (Fig. 6B-6E).
These data suggest that estrogen contributes to the development of EMS and is favorable for peritoneal Tregs accumulation. SCM-198 administration can abate the progress of ectopic lesions induced by estrogen. Moreover, the influence of estrogen on Tregs expansion and cytokine production (IL-10 and TGF-β1) can also be ameliorated by SCM-198.
Discussion
The occurrence and development of EMS are closely related to high estrogen and immune disorders in peritoneal environment [25, 26]. Assorted immune cells, including NK cells, macrophages and T cells are reported to be dysregulated in patients with EMS. Impaired cytotoxicity of NK cells and reduced phagocytic capacity of macrophages permitted the ectopic endometrium to escape immune surveillance and clearance [10-12, 27].
Tregs, one subpopulation of T cells, has been widely studied in oncology for its immunosuppressive function [28, 29]. The researchers also focused on Tregs in EMS for its similar characteristics of benign tumors, such as increased invasion and viability. Several experiments have shown that Tregs are more abundant in peritoneal fluid of EMS patients [17, 30, 31]. Besides, accumulated Tregs induced a type 2 immune microenvironment, which accelerated the progress and fibrosis of ectopic lesions [16].
The crosstalk between endocrine and immune systems can affect both physiological and pathological processes [32-34]. Plenty of studies have revealed that estrogen possesses immunomodulatory functions in Tregs [35-37]. Impaired estrogen signaling impeded the immunosuppressive function of Tregs, which further aggravated inflammatory bowel disease (IBD) [36]. Estrogen signaling suppressed bone resorption by facilitating the production of IL-10 and TGF-β1 in Tregs [37]. Studies have uncovered that increased concentration of estrogen and upregulated accumulation of Tregs both aggravated the development of EMS. However, it has not been explored whether the expansion of Tregs are resulted from local high estrogen. Exploring the regulation of estrogen signaling on Tregs is important to clarify the pathogenesis of EMS. In the present study, we found Tregs accumulated and estrogen increased in peritoneal fluid of EMS patients. The higher mRNA expression of ERα in Tregs was observed. To investigate whether the enhanced estrogen signaling leaded to the accumulation of Tregs, in vitro Tregs differentiation assays were conducted under the treatment of E2 or PPT. The results revealed that activated estrogen signaling upregulated Tregs in EMS.
SCM-198 suppressed the growth of EMS and downregulated the accumulation of Tregs in EMS mice. (A) Representative images of the overall condition of ectopic lesions in the abdominal cavity of EMS mice models treated with or without SCM-198 at low (EMS+SCM-198 L, 7.5 mg/kg) or high (EMS+SCM-198 H, 15 mg/kg) dose once a day for one week. (B) Representative image of ectopic lesions from EMS mice under SCM-198 administration. (C) The protein levels of MMP9, MMP2 and BCL2 of ectopic lesions were detected by western blotting (n=4). (D) Representative images showing the gating strategy for Tregs in peritoneal fluid of EMS mouse. (E, F) Representative (E) and quantitative (F) flow cytometry results for CD25 and FOXP3 expression in CD4+T cells from peritoneal fluid of control and EMS mice (n=10). (G, H) Representative (G) and quantitative (H) results showing the analysis of IL-10 and TGF-β1 expression in CD4+ CD25+ Tregs from control and EMS mice (n=10). Data are presented as the mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001.
SCM-198 restrained the expansion and function of Tregs induced by estrogen in EMS mice. (A) Representative picture of ectopic lesions from EMS mice treated with or without E2 (150 µg/kg), SCM-198 (15 mg/kg) or E2+SCM-198 (150 µg/kg, 15 mg/kg) once a day for one week. (B, C) Representative (B) and quantitative (C) flow cytometry results for CD25 and FOXP3 expression in CD4+T cells from peritoneal fluid of EMS mice (n=10). (D, E) Representative (D) and quantitative (E) results showing the analysis of IL-10 and TGF-β1 expression in CD4+ CD25+ Tregs from EMS mice (n=10). Data are presented as the mean ± SEM. **P < 0.01, ***P < 0.001.
Except for clarifying the pathogenesis, its urgent to find effective drugs to treat EMS for its high prevalence and recurrence [3-5]. SCM-198 is the chemically synthesized leonurine, exerts beneficial roles in various clinical diseases, such as cardio-cerebrovascular diseases and hypercholesterolemia disorders [38, 39]. Our previous studies have demonstrated that SCM-198 has protective effects on endometrium oxidative stress and inflammation [21, 22].
Here, we explored the therapeutic effects and the related mechanisms of SCM-198 on EMS. The results showed that SCM-198 obviously downregulated the expansion of Tregs and notably inhibited the activation of estrogen signaling, including decreasing estrogen production in eESCs and suppressing the mRNA level of ERα in Tregs. In vitro Tregs differentiation assays revealed that SCM-198 abated the differentiation and cytokine production of Tregs by inhibiting estrogen-ERα signaling. The abnormal conversation between Tregs and eESCs facilitated the invasion and viability of eESCs, which allows EMS to occur and develop rapidly [18, 40]. We demonstrated that SCM-198 ameliorated the invasion and decreased the viability of eESCs enhanced by Tregs.
Furthermore, the therapeutic effects of SCM-198 on EMS mice were analyzed. SCM-198 obviously suppressed the growth of ectopic lesions. In consistent with in vitro experiments, SCM-198 dramatically downregulated the expansion of Tregs and cytokine production in Tregs (IL-10 and TGF-β1). The therapeutic effects of SCM-198 are based on the repair of endocrine-immune regulation.
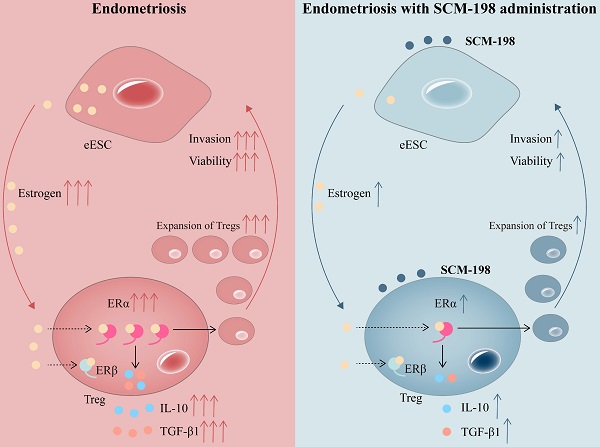
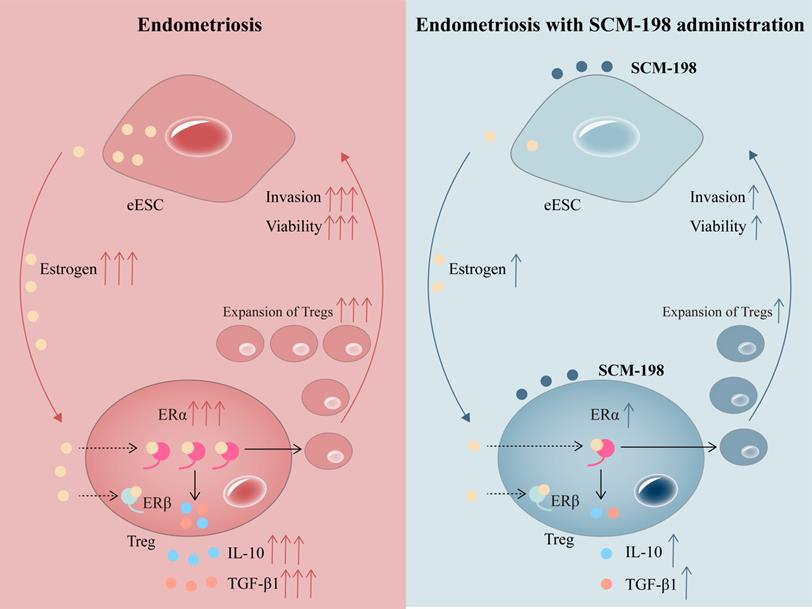
Schematic diagram showing the therapeutic mechanism of SCM-198 on EMS. In the peritoneal fluid of EMS patients, higher concentration of estrogen and accumulated Tregs are presented. Activated estrogen-ERα signaling potentiates the expansion of Tregs and their cytokine production (IL-10 and TGF-β1). In turn, cumulative Tregs facilitated the invasion and viability of eESCs. SCM-198 reduces the accumulation of Tregs by inhibiting estrogen-ERα signaling activation through inhibiting estrogen secretion of eESCs and suppressing ERα expression of Tregs. What's more, SCM-198 restrains the invasion and viability of eESCs reinforced by the accumulated Tregs.
It has been reported that estrogen-estrogen receptor (ER) signaling contributed to the survival and apoptotic resistance of eESCs [41-43]. Whether the direct inhibitory effect of SCM-198 on the viability of eESCs was achieved by estrogen-ER signaling needs further research. In addition, estrogen itself could also discipline the immune system in EMS. For example, estrogen impaired phagocytic function of macrophages [10] and reduced the cytotoxicity of NK cells [43]. Whether SCM-198 could relieve the inhibitory effects of estrogen on the immune clearance function of macrophages, NK cells and other immune cells is worthy of further study.
In conclusion, our research shows that higher concentration of estrogen and accumulated Tregs are presented in the peritoneal fluid of EMS patients. The overactivation of estrogen-ERα signaling potentiates the expansion of Tregs. SCM-198 abates the accumulation of Tregs by inhibiting estrogen-ERα signaling activation through decreasing estrogen secretion of eESCs and downregulating ERα expression of Tregs. What's more, SCM-198 restrains the growth of ectopic lesions and downregulates the accumulation of Tregs in EMS mice (Fig. 7). To sum up, this study provides a theoretical basis for SCM-198 on the treatment of EMS.
Abbreviations
EMS: endometriosis; Tregs: regulatory T cells; ESCs: endometrial stromal cells; ERα: estrogen receptor α; PCOS: polycystic ovary syndrome; ePF: EMS peritoneal fluid; nPF: non-EMS peritoneal fluid; FBS: fetal bovine serum; eESCs: endometrial stromal cells; cDNA: complementary DNA; PBMCs: peripheral blood mononuclear cells; E2: 17β-estradiol; PPT: propyl pyrazole triol; RIPA: radio-immunoprecipitation assay; IBD: inflammatory bowel disease; ERβ: estrogen receptor β; ER: estrogen receptor.
Supplementary Material
Supplementary figure.
Acknowledgements
Funding
This work was supported by the grants from the National Basic Research Program of China (2021YFE0206500), the National Natural Science Foundation of China (31970859, 81630036, 91542116), the international cooperation project between Macau and Shanghai (20410760300), Strategic Collaborative Research Program of the Ferring Institute of Reproductive Medicine (FIRMA200504), Innovation-oriented Science and Technology Grant from NHC Key Laboratory of Reproduction Regulation (CX2017-2), and Innovative research team of high-level local universities in Shanghai and a key laboratory program of the Education Commission of Shanghai Municipality (ZDSYS14005).
Author Contributions
Yun-yun Li and Yi-kong Li conducted experimental design, performance of experiments, data analysis, and drafted the manuscript; Yue Li performed literature searches; Li Wang, Da-jin Li, Xiao-lin Wang performed data interpretation and revised the manuscript. Mei-rong Du, Min Yu, and Yi-zhun Zhu conceived the project, data interpretation and revised the manuscript. All authors reviewed the manuscript. All authors read and approved the final manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Vallvé-Juanico J, Houshdaran S, Giudice LC. The endometrial immune environment of women with endometriosis. Hum Reprod Update. 2019;25:564-91
2. Lai ZZ, Yang HL, Shi JW, Shen HH, Wang Y, Chang KK. et al. Protopanaxadiol improves endometriosis associated infertility and miscarriage in sex hormones receptors-dependent and independent manners. Int J Biol Sci. 2021;17:1878-94
3. Shafrir AL, Farland LV, Shah DK, Harris HR, Kvaskoff M, Zondervan K. et al. Risk for and consequences of endometriosis: A critical epidemiologic review. Best Pract Res Clin Obstet Gynaecol. 2018;51:1-15
4. Becker CM, Gattrell WT, Gude K, Singh SS. Reevaluating response and failure of medical treatment of endometriosis: a systematic review. Fertil Steril. 2017;108:125-36
5. Zondervan KT, Becker CM, Missmer SA. Endometriosis. N Engl J Med. 2020;382:1244-56
6. Missmer SA, Hankinson SE, Spiegelman D, Barbieri RL, Marshall LM, Hunter DJ. Incidence of laparoscopically confirmed endometriosis by demographic, anthropometric, and lifestyle factors. Am J Epidemiol. 2004;160:784-96
7. Hodgson RM, Lee HL, Wang R, Mol BW, Johnson N. Interventions for endometriosis-related infertility: a systematic review and network meta-analysis. Fertil Steril. 2020;113:374-82.e2
8. Wang L, Li L, Li Y, Huang C, Lian R, Wu T. et al. A History of Endometriosis Is Associated With Decreased Peripheral NK Cytotoxicity and Increased Infiltration of Uterine CD68(+) Macrophages. Front Immunol. 2021;12:711231
9. Jeljeli M, Riccio LGC, Chouzenoux S, Moresi F, Toullec L, Doridot L. et al. Macrophage Immune Memory Controls Endometriosis in Mice and Humans. Cell Rep. 2020;33:108325
10. Weng LC, Hou SH, Lei ST, Peng HY, Li MQ, Zhao D. Estrogen-regulated CD200 inhibits macrophage phagocytosis in endometriosis. J Reprod Immunol. 2020;138:103090
11. Liu YY, Liu YK, Hu WT, Tang LL, Sheng YR, Wei CY. et al. Elevated heme impairs macrophage phagocytosis in endometriosis. Reproduction. 2019;158:257-66
12. Du Y, Liu X, Guo SW. Platelets impair natural killer cell reactivity and function in endometriosis through multiple mechanisms. Hum Reprod. 2017;32:794-810
13. Núñez NG, Tosello Boari J, Ramos RN, Richer W, Cagnard N, Anderfuhren CD. et al. Tumor invasion in draining lymph nodes is associated with Treg accumulation in breast cancer patients. Nat Commun. 2020;11:3272
14. De Matteis S, Molinari C, Abbati G, Rossi T, Napolitano R, Ghetti M. et al. Immunosuppressive Treg cells acquire the phenotype of effector-T cells in chronic lymphocytic leukemia patients. J Transl Med. 2018;16:172
15. Podgaec S, Rizzo LV, Fernandes LF, Baracat EC, Abrao MS. CD4(+) CD25(high) Foxp3(+) cells increased in the peritoneal fluid of patients with endometriosis. Am J Reprod Immunol. 2012;68:301-8
16. Xiao F, Liu X, Guo SW. Platelets and Regulatory T Cells May Induce a Type 2 Immunity That Is Conducive to the Progression and Fibrogenesis of Endometriosis. Front Immunol. 2020;11:610963
17. Hanada T, Tsuji S, Nakayama M, Wakinoue S, Kasahara K, Kimura F. et al. Suppressive regulatory T cells and latent transforming growth factor-β-expressing macrophages are altered in the peritoneal fluid of patients with endometriosis. Reprod Biol Endocrinol. 2018;16:9
18. Li MQ, Wang Y, Chang KK, Meng YH, Liu LB, Mei J. et al. CD4+Foxp3+ regulatory T cell differentiation mediated by endometrial stromal cell-derived TECK promotes the growth and invasion of endometriotic lesions. Cell Death Dis. 2014;5:e1436
19. Zhu YZ, Wu W, Zhu Q, Liu X. Discovery of Leonuri and therapeutical applications: From bench to bedside. Pharmacol Ther. 2018;188:26-35
20. Li YY, Lin YK, Liu XH, Wang L, Yu M, Li DJ. et al. Leonurine: From Gynecologic Medicine to Pleiotropic Agent. Chin J Integr Med. 2020;26:152-60
21. Li Y, Lin Y, Huang X, Xu C, Liu X, Wang L. et al. SCM-198 protects endometrial stromal cells from oxidative damage through Bax/Bcl-2 and ERK signaling pathways. Acta Biochim Biophys Sin (Shanghai). 2019;51:580-7
22. Lin Y, Li Y, Li X, Liu X, Wang X, Yu M. et al. SCM-198 ameliorates endometrial inflammation via suppressing the LPS-JNK-cJUN/cFOS-TLR4-NF-κB pathway. Acta Biochim Biophys Sin (Shanghai). 2021;53:1207-15
23. Nie J, Liu X. Leonurine Attenuates Hyperalgesia in Mice with Induced Adenomyosis. Med Sci Monit. 2017;23:1701-6
24. Fantini MC, Dominitzki S, Rizzo A, Neurath MF, Becker C. In vitro generation of CD4+ CD25+ regulatory cells from murine naive T cells. Nat Protoc. 2007;2:1789-94
25. Pluchino N, Mamillapalli R, Wenger JM, Ramyead L, Drakopoulos P, Tille JC. et al. Estrogen receptor-α immunoreactivity predicts symptom severity and pain recurrence in deep endometriosis. Fertil Steril. 2020;113:1224-31.e1
26. Guo X, Ding S, Li T, Wang J, Yu Q, Zhu L. et al. Macrophage-derived netrin-1 is critical for neuroangiogenesis in endometriosis. Int J Biol Macromol. 2020;148:226-37
27. Miller JE, Ahn SH, Marks RM, Monsanto SP, Fazleabas AT, Koti M. et al. IL-17A Modulates Peritoneal Macrophage Recruitment and M2 Polarization in Endometriosis. Front Immunol. 2020;11:108
28. Wang H, Zhang H, Wang Y, Brown ZJ, Xia Y, Huang Z. et al. Regulatory T cell and neutrophil extracellular trap interaction contributes to carcinogenesis in non-alcoholic steatohepatitis. J Hepatol. 2021
29. Polanczyk MJ, Walker E, Haley D, Guerrouahen BS, Akporiaye ET. Blockade of TGF-β signaling to enhance the antitumor response is accompanied by dysregulation of the functional activity of CD4(+)CD25(+)Foxp3(+) and CD4(+)CD25(-)Foxp3(+) T cells. J Transl Med. 2019;17:219
30. Olkowska-Truchanowicz J, Sztokfisz-Ignasiak A, Zwierzchowska A, Janiuk I, Dąbrowski F, Korczak-Kowalska G. et al. Endometriotic Peritoneal Fluid Stimulates Recruitment of CD4(+)CD25(high)FOXP3(+) Treg Cells. J Clin Med. 2021 10
31. Khan KN, Yamamoto K, Fujishita A, Muto H, Koshiba A, Kuroboshi H. et al. Differential Levels of Regulatory T Cells and T-Helper-17 Cells in Women With Early and Advanced Endometriosis. J Clin Endocrinol Metab. 2019;104:4715-29
32. Benvenga S, Elia G, Ragusa F, Paparo SR, Sturniolo MM, Ferrari SM. et al. Endocrine disruptors and thyroid autoimmunity. Best Pract Res Clin Endocrinol Metab. 2020;34:101377
33. Vasanthakumar A, Chisanga D, Blume J, Gloury R, Britt K, Henstridge DC. et al. Sex-specific adipose tissue imprinting of regulatory T cells. Nature. 2020;579:581-5
34. Hong JY, Lim J, Carvalho F, Cho JY, Vaidyanathan B, Yu S. et al. Long-Term Programming of CD8 T Cell Immunity by Perinatal Exposure to Glucocorticoids. Cell. 2020;180:847-61.e15
35. Liao ZH, Huang T, Xiao JW, Gu RC, Ouyang J, Wu G. et al. Estrogen signaling effects on muscle-specific immune responses through controlling the recruitment and function of macrophages and T cells. Skelet Muscle. 2019;9:20
36. Goodman WA, Bedoyan SM, Havran HL, Richardson B, Cameron MJ, Pizarro TT. Impaired estrogen signaling underlies regulatory T cell loss-of-function in the chronically inflamed intestine. Proc Natl Acad Sci U S A. 2020;117:17166-76
37. Luo CY, Wang L, Sun C, Li DJ. Estrogen enhances the functions of CD4(+)CD25(+)Foxp3(+) regulatory T cells that suppress osteoclast differentiation and bone resorption in vitro. Cell Mol Immunol. 2011;8:50-8
38. Suguro R, Chen S, Yang D, Yang Z, Miao L, Wu W. et al. Anti-hypercholesterolemic Effects and a Good Safety Profile of SCM-198 in Animals: From ApoE Knockout Mice to Rhesus Monkeys. Front Pharmacol. 2018;9:1468
39. Luo S, Xu S, Liu J, Ma F, Zhu YZ. Design and synthesis of novel SCM-198 analogs as cardioprotective agents: Structure-activity relationship studies and biological evaluations. Eur J Med Chem. 2020;200:112469
40. Liu Q, Ma P, Liu L, Ma G, Ma J, Liu X. et al. Evaluation of PLGA containing anti-CTLA4 inhibited endometriosis progression by regulating CD4+CD25+Treg cells in peritoneal fluid of mouse endometriosis model. Eur J Pharm Sci. 2017;96:542-50
41. Liao TL, Lee YC, Tzeng CR, Wang YP, Chang HY, Lin YF. et al. Mitochondrial translocation of estrogen receptor β affords resistance to oxidative insult-induced apoptosis and contributes to the pathogenesis of endometriosis. Free Radic Biol Med. 2019;134:359-73
42. Zhang L, Xiong W, Li N, Liu H, He H, Du Y. et al. Estrogen stabilizes hypoxia-inducible factor 1α through G protein-coupled estrogen receptor 1 in eutopic endometrium of endometriosis. Fertil Steril. 2017;107:439-47
43. Zhang B, Zhou WJ, Gu CJ, Wu K, Yang HL, Mei J. et al. The ginsenoside PPD exerts anti-endometriosis effects by suppressing estrogen receptor-mediated inhibition of endometrial stromal cell autophagy and NK cell cytotoxicity. Cell Death Dis. 2018;9:574
Author contact
![]() Corresponding authors: Mei-rong Du, Laboratory for Reproductive Immunology, Hospital of Obstetrics and Gynecology, Fudan University Shanghai Medical College, ZhaoZhou Road 413, 200032, Shanghai, China, E-mail: mrduedu.cn; Min Yu, Obstetrics & Gynecology Hospital of Fudan University, Shanghai JIAI Genetics & IVF Institute, 588#Fangxie road, Shanghai, 200032, P.R. China, E-mail: yumincom; Yi-zhun Zhu, State Key Laboratory of Quality Research in Chinese Medicine and School of Pharmacy, Macau University of Science and Technology, Macau SAR, China, E-mail: yzzhuedu.mo.
Corresponding authors: Mei-rong Du, Laboratory for Reproductive Immunology, Hospital of Obstetrics and Gynecology, Fudan University Shanghai Medical College, ZhaoZhou Road 413, 200032, Shanghai, China, E-mail: mrduedu.cn; Min Yu, Obstetrics & Gynecology Hospital of Fudan University, Shanghai JIAI Genetics & IVF Institute, 588#Fangxie road, Shanghai, 200032, P.R. China, E-mail: yumincom; Yi-zhun Zhu, State Key Laboratory of Quality Research in Chinese Medicine and School of Pharmacy, Macau University of Science and Technology, Macau SAR, China, E-mail: yzzhuedu.mo.

 Global reach, higher impact
Global reach, higher impact