10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2022; 18(6):2439-2451. doi:10.7150/ijbs.67873 This issue Cite
Research Paper
A SIX1 degradation inducer blocks excessive proliferation of prostate cancer
1. Affiliated Cancer Hospital & institute of Guangzhou Medical University, Guangzhou, Guangdong, 510095, China.
2. Guangzhou Municipal and Guangdong Provincial Key Laboratory of Protein Modification and Degradation, School of Basic Medical Sciences, Guangzhou Medical University, Guangzhou, Guangdong, 511436, China.
3. Department of Pathology, First People's Hospital of Foshan, 528000 Foshan, Guangdong, China.
*These authors contributed equally to this work.
Abstract
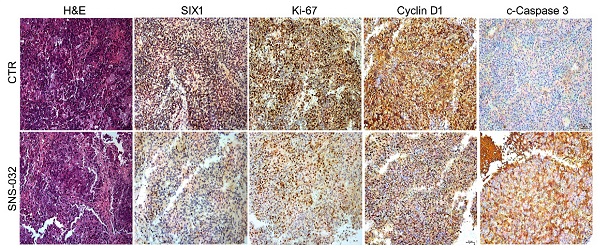
Prostate cancer (PC) remains a great medical challenge due to its high incidence and the development of castration resistance in patients treated with androgen deprivation therapy. Deubiquitinases, the enzymes that specifically hydrolyze ubiquitin chains on their substrates, were recently proposed as a serious of critical therapeutic targets for cancer treatment. Our previous study has been reported that the ubiquitin specific peptidase 1 (USP1) functionally acts as a deubiquitinase of sine oculis homeobox homolog 1 (SIX1) and contributes to the proliferation and castration resistance of PC. The stabilization of SIX1 by USP1 partially depends on the status of glucose-regulated protein 75 (GRP75). In this study, we aimed to identify a SIX1 degradation inducer via inhibiting the USP1-SIX1 axis. we screened a range of kinase inhibitors and showed that SNS-032 is the best candidate to trigger the ubiquitinated degradation of SIX1. SNS-032 not only restrains activity of the USP1-SIX1 axis and cell cycle progression, but also results in apoptosis of PC cells. Moreover, the combination of SNS-032 and enzalutamide synergistically induces apoptosis and downregulates expression of USP1, SIX1, and AR/AR-V7 in AR-V7 highly expressed 22Rv1 cells. Overall, our findings may develop a novel and effective strategy to overcome castration resistance in PC for the identification of a SIX1 degradation inducer via targeting the USP1-SIX1 axis.
Keywords: Prostate cancer, USP1, SIX1, SNS-032, Degradation

 Global reach, higher impact
Global reach, higher impact