10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2022; 18(7):2775-2794. doi:10.7150/ijbs.70691 This issue Cite
Review
New insights into checkpoint inhibitor immunotherapy and its combined therapies in hepatocellular carcinoma: from mechanisms to clinical trials
1. Department of Pathology, Xiangya Hospital, Central South University, Changsha, Hunan, China; and Department of Pathology, School of Basic Medical Science, Xiangya School of Medicine, Central South University, Changsha, Hunan, China.
2. Center for Molecular Medicine, Xiangya Hospital, Central South University, Changsha, Hunan, China.
3. Department of Radiology, Xiangya Hospital, Central South University, Changsha, Hunan, China.
4. National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Central South University, Changsha, Hunan, China.
Abstract
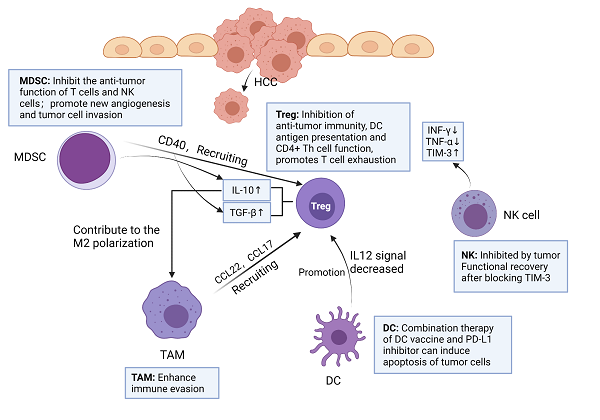
Hepatocellular carcinoma (HCC) is one of the most lethal tumors in China and worldwide, although first-line therapies for HCC, such as atezolizumab and bevacizumab, have been effective with good results, the researches on new therapies have attracted much attention. With the deepening research on tumor immunology, the role and operation mechanism of immune cells in the tumor microenvironment (TME) of HCC have been explained, such as programmed cell death protein 1 (PD-1) binding to ligand could cause T cell exhaustion and reduce IFN-γ T cell secretion, cytotoxic T lymphocyte 4 (CTLA-4) and CD28 mediate immunosuppression by competing for B7 protein and disrupting CD28 signal transduction pathway, which also lays the foundation for the development and application of more new immune checkpoint inhibitors (ICIs). The biological behavior of various immune checkpoints has been proved in HCC, such as PD-1, programmed cell death ligand 1 (PD-L1), CTLA-4 and so on, leading to a series of clinical trials. Currently, FDA approved nivolumab, pembrolizumab and nivolumab plus ipilimumab for the treatment of HCC. However, the treatment of ICI has the disadvantages of low response rate and many side effects, so the combination of ICIs and various other therapies (such as VEGF or VEGFR inhibition, neoadjuvant and adjuvant therapy, locoregional therapies) has been derived. Further studies on immune checkpoint mechanisms may reveal new therapeutic targets and new combination therapies in the future.
Keywords: Hepatocellular carcinoma (HCC), Tumor microenvironment (TME), Programmed cell death protein 1 (PD-1), Cytotoxic T lymphocyte 4 (CTLA-4), Immune checkpoint inhibitors (ICIs)

 Global reach, higher impact
Global reach, higher impact