10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2022; 18(8):3313-3323. doi:10.7150/ijbs.72526 This issue Cite
Research Paper
HBO1 induces histone acetylation and is important for non-small cell lung cancer cell growth
1. Respiratory Department I, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
2. Key Laboratory of Neuroregeneration of Jiangsu and Ministry of Education, Nantong University, Nantong, China
3. Department of Radiotherapy and Oncology, Affiliated Kunshan Hospital of Jiangsu University, Kunshan, China
4. Changshu Hospital Affiliated to Nanjing University of Chinese Medicine, Changshu, China
5. Department of Cardiothoracic Surgery, the Second Affiliated Hospital of Soochow University, Suzhou, China
6. Department of Thoracic Surgery, Institute of Thoracic Surgery, The First Affiliated Hospital of Soochow University, Suzhou, China
# Co-first author
Received 2022-3-1; Accepted 2022-4-18; Published 2022-5-9
Abstract
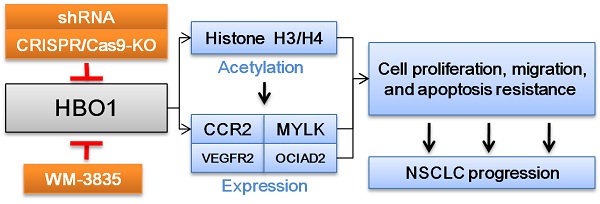
We examined the expression and the potential biological function of HBO1 in non-small cell lung cancer (NSCLC). TCGA and Oncomine databases showed that HBO1 transcripts were elevated in NSCLC. Furthermore, in local NSCLC tumor tissues HBO1 expression was higher than that in matched adjacent lung tissues. In primary and immortalized NSCLC cells, HBO1 shRNA robustly inhibited cell viability, proliferation and migration. Moreover, HBO1 knockout by CRISPR/Cas9 induced significant anti-tumor activity in NSCLC cells. Conversely, ectopic HBO1 overexpression in primary NSCLC cells increased proliferation and migration. H3-H4 histone acetylation and expression of several potential oncogenic genes (CCR2, MYLK, VEGFR2 and OCIAD2) were significantly decreased in NSCLC cells with HBO1 silencing or knockout. They were however increased after HBO1 overexpression. Intratumoral injection of HBO1 shRNA-expressing adeno-associated virus hindered the growth of A549 cell xenografts and primary NSCLC cell xenografts in nude mice. H3-H4 histone acetylation as well as expression of HBO1 and HBO1-dependent genes were decreased in HBO1-silenced NSCLC xenograft tissues. An HBO1 inhibitor WM-3835 potently inhibited NSCLC cell growth. Together, HBO1 overexpression promotes NSCLC cell growth.
Introduction
It is important to explore novel molecular targets essential for uncontrolled non-small cell lung cancer (NSCLC) cell growth [1-5]. Dysregulation in epigenetics is closely associated with pathogenesis and progression of NSCLC [6, 7]. Histone acetylation is a key epigenetic mechanism of gene expression [8-10]. Histone deacetylase (HDAC) and histone acetyltransferase (HAT) and are two primary families of enzymes responsible for histone acetylation [10, 11]. Sun et al., showed that nuclear glycogen metabolism could provide substrates for histone acetylation to increase NSCLC cell proliferation [12]. Mi et al., reported that YEATS domain-containing 2 (YEATS2) acted as a reader of histone H3 acetylation, required for transcriptional activation of multiple key genes for NSCLC tumorigenesis [13].
One HAT family protein, HBO1, also known as KAT7 or MYST2, catalyzes H4 and H3 histone acetylation at multiple lysine residues [14]. HBO1 is required for acetyl-CoA binding and histone acetylation [15]. Through association with native subunits and cofactors, HBO1 regulates multiple key physiological functions and behaviors. It is important for gene transcription and expression, cell self-renewal, immune-response regulation and development [15-20]. HBO1 association with BRPF scaffold proteins is vital for histone H3K14 acetylation (H3K14ac) [15]. Moreover, HBO1 association with JADE (JADE1/2/3) can catalyze histone H4 acetylation [16, 17, 21]. HBO1-mediated H3K14ac is required for replication origin activation. HBO1-catalized histone H4 acetylation is shown to promote DNA replication licensing [16-20].
Zhong et al., demonstrated that HBO1 is overexpressed in hepatocellular carcinoma (HCC), and it is required for cancer growth. Conversely, HBO1 silencing by shRNA or CRISPR/Cas9-induced HBO1 knockout (KO) potently inhibited HCC growth [22]. Gao et al., have shown that HBO1 is a novel and important oncogenic gene of osteosarcoma. HBO1 silencing or inhibition (by a first-in-class inhibitor WM-3835) robustly inhibited osteosarcoma cell growth [23]. The results of this study will show that HBO1 overexpression is important for NSCLC cell growth.
Materials and methods
Chemicals and reagents. WM-3835 was provided by Dr. Cao's group [24]. Cell counting kit -8 (CCK-8), EdU (5-Ethynyl-2'-deoxyuridine), DAPI and TUNEL dyes were all from Invitrogen Thermo-Fisher (Beijing, China). Antibodies were described previously [22, 24].
Cell culture. Provided by Dr. Shi's group [25], the primary human NSCLC cells, pNSCLC-1/-2/-3, were derived from three different written-informed consent patients [25]. Primary NSCLC cells and established cell lines (A549 and H460) were maintained under the described medium [25]. The primary epithelial cells-derived from human lung were also from Dr. Shi [25].
Human tissues. Thirteen (13) NSCLC patients (all male, 42 to 78-year old, stage-III-IV), administrated at authors' institutions, were enrolled in this study. The written-informed consent was obtained from each patient. Fresh tumor tissues and matched surrounding normal lung tissues were obtained at the time of surgery and were stored in liquid nitrogen. The fresh tumor specimens of six NSCLC patients with poor over survival (less than 50 months, 19-43 months) and another six tumors of NSCLC patients (age, sex and disease-stage matched) with better overall survival (over 50 months) were provided by the First Affiliated Hospital of Soochow University (Suzhou, China). The protocols of using human tissues and cells were approved by Zhengzhou University's Ethics Committee and were in according to Declaration of Helsinki priciples.
HBO1 silencing or overexpression. The GV369 lentiviral constructs encoding six different shRNAs targeting human HBO1 (sh-HBO1-Sq1 to sh-HBO1-Sq6) were provided by Genechem (Shanghai, China). The lentiviral construct encoding the full-length HBO1 cDNA was provided by Dr. Cao [24]. The construct, and the lentivirus helper plasmids, were transfected to HEK-293T cells. The achieved lentiviral particles were enriched and transduced to cultured NSCLC cells (maintained under the polybrene containing complete medium). After 48h, puromycin was added to selected stable cells for another 48h.
HBO1 knockout (KO). A LentiCas9-puro construct (Genechem) was transduced to pNSCLC-1 cells and cultured for 72h. Stable cells were formed after selection using puromycin. Cells were then further transfected with Lenti-HBO1-sgRNA-puro construct (from Dr. Cao [24]). A set of five different sgRNAs were tested. The transfected cells were further distributed to 96-well plates and were subjected to HBO1 KO screening. Among the five tested sgRNAs, HBO1 sgRNA-2 (“Sg2”) and HBO1 sgRNA-5 (“Sg5”) resulted in complete HBO1 KO. The single stable HBO1 KO pNSCLC-1 cells were established.
Other methods, including Western blotting, qRT-PCR, CCK-8, colony formation, EdU staining, Transwell assays, TUNEL staining and other apoptosis-related assays were described in the previous studies [25, 26]. Same set of protein lysates were always run in parallel “sister” gels to test different proteins. Each blotting data was repeated for five times and similar results were obtained in each time. The mRNA primers utilized in this study were provided by Dr. Cao [24]. The uncropped blotting images of the study were listed in Figure S1.
Xenograft study. A549 cells or pNSCLC-1 primary cells (at six million cells of each mouse) were inoculated to the flanks of the nude mice via subcutaneous (s.c.) injection. A549 xenografts or pNSCLC-1 xenografts were established within three weeks. Mice were thereafter assigned randomly into two different groups and were intratumorally injected with the applied adeno-associated virus (AAV). Tumor volume was calculated as described [25]. All animal studies were approved by the IACUC and Institute Animal Ethics Review Board of Zhengzhou University.
Statistical analyses. The in vitro experiments of the study were repeated five times, and each time similar results were obtained. Numeric data were always with normal distribution and were presented as mean ± S. D. (standard deviation). Statistical differences of multiple groups were analyzed by ANOVA and Dunnett's test (SPSS 23.0, Chicago, CA). The difference between two specific groups was evaluated by Student's t test. P < 0.05 stands for the statistically significant difference.
Results
HBO1 overexpression in NSCLC
TCGA database was first utilized to examine HBO1 expression in NSCLC tissues. As demonstrated, HBO1 transcripts in NSCLC tissues (“Tumor”, n = 515) were higher than those in normal lung tissues (“Normal”, n = 59) (Figure 1A). Oncomine 4.5 database results further showed that HBO1 mRNA levels in lung adenocarcinoma tissues (n = 40) were significantly higher than those in the normal lung tissues (n = 5, Figure 1B). Furthermore, HBO1 mRNA elevation was detected in large cell lung carcinoma (P < 0.05 versus normal lung tissues, Figure 1C). Therefore, bioinformatics assay results show that HBO1 is elevated in NSCLC tissues.
HBO1 expression in local human NSCLC tissues was tested next. Thirteen (n = 13) pairs of patient-derived NSCLC tumor tissues (“T”) and matched adjacent normal lung tissues (“N”) were analyzed. HBO1 mRNA expression in NSCLC tumor tissues was significantly higher than that in normal lung tissues (Figure 1D). Figure 1E, showed that HBO1 protein expression was elevated in NSCLC tumor tissues in the four representative patients (“Patient-1” to “Patient-4”). Quantitative analyses integrating all 13 sets of human tissues showed that HBO1 protein upregulation in NSCLC tumor tissues was significant (P < 0.05 versus “N” tissues, Figure 1F).
HBO1 overexpression in NSCLC. TCGA cohorts show HBO1 transcripts in 515 cases of lung adenocarcinoma tissues (“Primary Tumor”) and 59 cases of normal lung tissues (“Normal”) (A). Oncomine 4.5 database shows the relative HBO1 mRNA expression in described lung cancer tissues and normal lung tissues (“Normal”) (B and C). Expression of HBO1 mRNA and listed proteins in the described NSCLC tumor tissues and normal lung tissues (“N”) as well as in the listed NSCLC cells and normal lung epithelial cells (“pEpi”) were tested by qRT-PCR (D, G and J) and Western blotting (E, F, H, I and K) assays. *P < 0.05 versus “Normal”/“N” tissues or “pEpi” cells. *P < 0.05 versus “better-survival NSCLC” tissues (G and I).
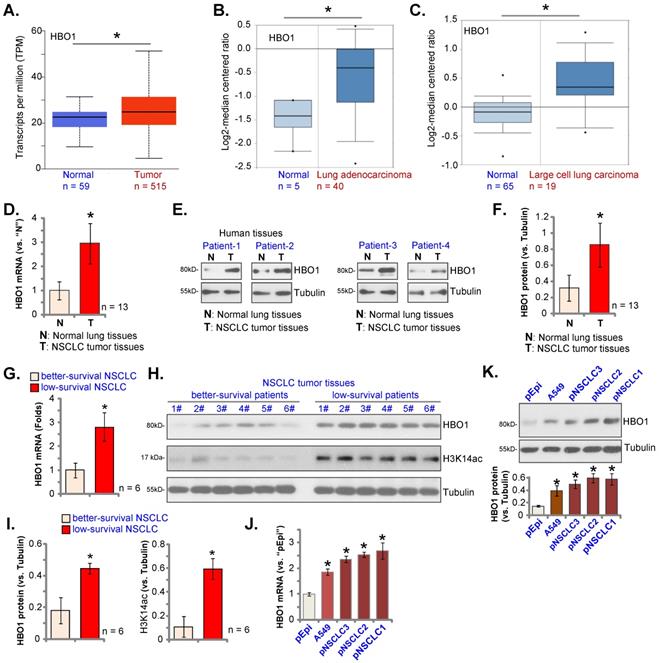
To examine whether HBO1 overexpression in NSCLC is associated with patient survival. The tumor specimens of six NSCLC patients with poor over survival (less than 50 months, 19-43 months) and tumors of other six NSCLC patients (age, sex and disease-stage matched) with better overall survival (over 50 months) were obtained. Expression of HBO1 was tested. mRNA and protein expression of HBO1 in tumor tissues of low-survival NSCLC patients was significantly higher than that in tumors of better-survival NSCLC patients (Figure 1G-I). H3K14ac in tumor tissues of low-survival NSCLC patients was also significantly higher than that in better-survival NSCLC patients (Figure 1H and I).
The expression of HBO1 in NSCLC cells was studied. HBO1 mRNA and protein (Figure 1J and K) levels were relatively low in primary lung epithelial cells (“pEpi”). While HBO1 overexpression was detected in primary NSCLC cells that were derived from three different patients, “pNSCLC-1/2/3”, as well as in the immortalized A549 cell line (Figure 1J and K). These results confirmed HBO1 overexpression in NSCLC.
HBO1 silencing causes anti-NSCLC cell activity
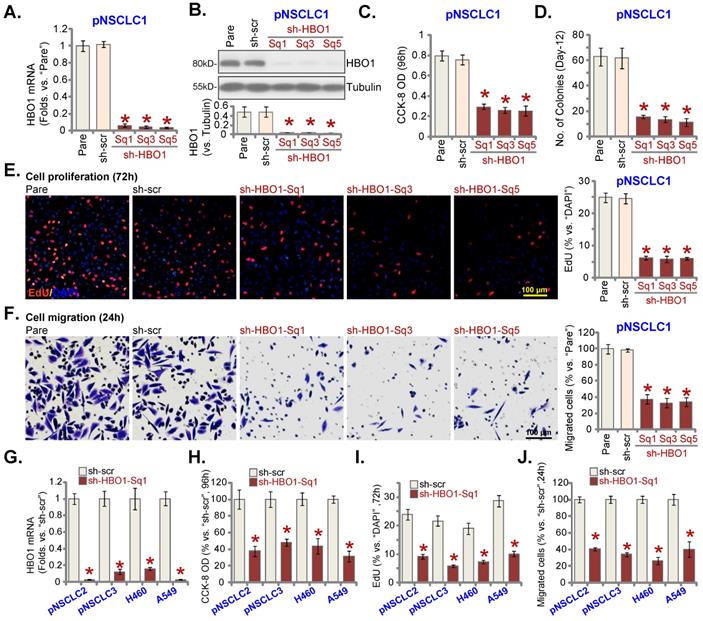
In order to silence HBO1, lentiviral particles encoding six different shRNAs against HBO1 were individually added to pNSCLC-1 cells. Stable cells were formed after puromycin selection. Testing HBO1 mRNA expression in the stable cells, by qRT-PCR, showed that three out of the six tested HBO1 shRNAs (sh-HBO1-Sq1/3/5) led to over 90% silencing of HBO1 mRNA (P < 0.05 versus cells with scramble control shRNA/sh-src, Figure 2A). HBO1 protein levels were robustly downregulated in sh-HBO1-expressing pNSCLC-1 cells (Figure 2B). CCK-8 viability was significantly decreased after HBO1 silencing (Figure 2C). Moreover, pNSCLC-1 cell colony formation was hindered after HBO1 shRNA (Figure 2D).
In HBO1 shRNA-expressing stable pNSCLC-1 cells, the EdU-positive nuclei ratio was significantly decreased (Figure 2E), indicating that HBO1 silencing inhibited pNSCLC-1 cell proliferation. HBO1 shRNA potently suppressed pNSCLC-1 cell migration (Figure 2F). In contrast, the control shRNA (sh-src) failed to significantly alter HBO1 expression (Figure 2A and B) and functions of pNSCLC-1 cells (Figure 2C-F).
To the primary (pNSCLC-2 and pNSCLC-3) and immortalized lines (A549 and H460), sh-HBO1-Sq1 lentiviral particles were added. Stable NSCLC cells were formed after selection. Figure 2G confirmed that sh-HBO1-Sq1 led to robust HBO1 mRNA downregulation in the NSCLC cells and it significantly decreased viability (Figure 2H). Moreover, the ratio of the EdU-positive nuclei was decreased in NSCLC cells bearing the HBO1 shRNA (Figure 2I). Figure 2J further showed that sh-HBO1-Sq1 decreased in vitro migration of the primary and immortalized NSCLC cells.
HBO1 silencing provokes NSCLC cell apoptosis
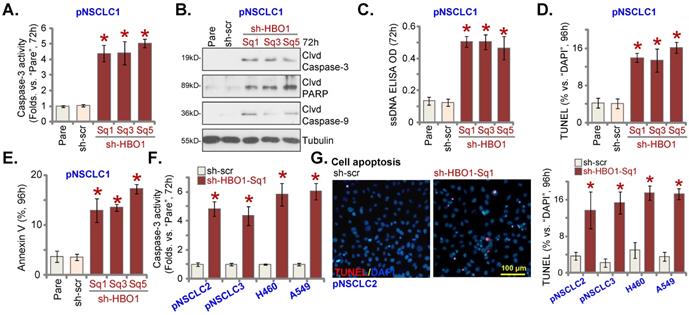
An early RNA-seq analyses in HBO1-depleted cells have identified over 250 differentially regulated genes, including many anti-apoptosis genes [27]. CRISPR/Cas9-induced HBO1 KO induced apoptosis activation in cancer cells [28]. In AML cells, depletion of HBO1 caused apoptosis activation [29]. The relative caspase-3 activity, Figure 3A, was significantly increased in pNSCLC-1 cells stably expressing different HBO1 shRNAs (sh-HBO1-Sq1/3/5). In addition, increased cleavages of caspase-3, caspase-9 and PARP were detected in HBO1-silenced pNSCLC-1 cells (Figure 3B). The single stranded DNA (ssDNA) contents were increased in pNSCLC-1 cells with HBO1 shRNAs (Figure 3C), indicating increased DNA breakage.
In HBO1 shRNA-expressing stable pNSCLC-1 cells, TUNEL-positive nuclei percentage (% versus DAPI) was significantly increased (Figure 3D). Figure 3E demonstrated that HBO1 silencing by targeted shRNAs led to increased Annexin V positive staining, further supporting apoptosis activation. The scramble control shRNA, sh-scr, failed to induce apoptosis activation in pNSCLC-1 cells (Figure 3A-E).
In pNSCLC-2 and pNSCLC-3 primary cells as well as in A549 and H460 immortalized cells, sh-HBO1-Sq1-induced HBO1 silencing increased caspase-3 activity (Figure 3F). TUNEL-positive nuclei increasing supported apoptosis activation in HBO1-silenced NSCLC cells (Figure 3G). Taken together, HBO1 shRNA provoked apoptosis activation in human NSCLC cells.
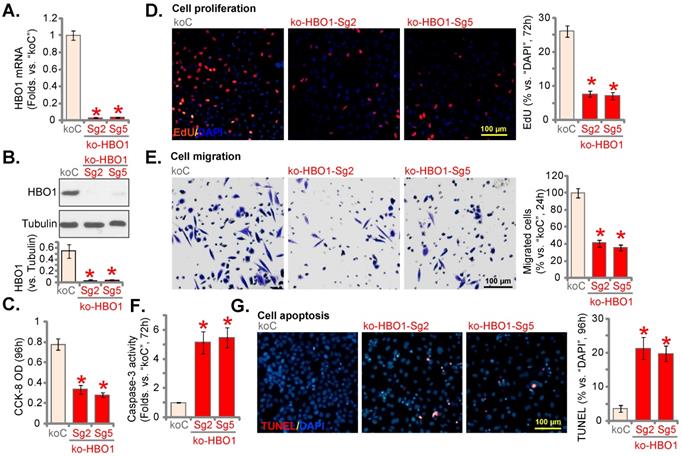
HBO1 knockout by CRISPR/Cas9 produces significant anti-NSCLC cell activity
To completely knockout (KO) HBO1, a set of five different lenti-Cas9-HBO1-KO constructs, encoding different sgRNAs targeting HBO1, were individually transduced to pNSCLC-1 cells. Single stable cells were formed. Among the five tested sgRNAs, HBO1 sgRNA-2 (“Sg2”) and HBO1 sgRNA-5 (“Sg5”) resulted in complete HBO1 mRNA depletion (Figure 4A). HBO1 protein levels were almost depleted (Figure 4B). HBO1 KO decreased CCK-8 viability (Figure 4C) and robustly inhibited cell proliferation (EdU-positive nuclei percentage decreasing, Figure 4D) in pNSCLC-1 cells. HBO1 KO potently inhibited pNSCLC-1 cell in vitro cell migration (Figure 4E). HBO1 KO induced caspase-3 activation (Figure 4F) and apoptosis (Figure 4G) in pNSCLC-1 cells.
HBO1 silencing causes anti-NSCLC cell activity. The described primary or immortalized NSCLC cells, stably expressing the applied HBO1 shRNA or scramble control shRNAs (“sh-scr”), were established; Expression of HBO1 mRNA (A and G) and protein (B) was shown. Cells were further maintained in culturing medium for described hours, cell viability (CCK-8 OD, C and H), colony formation (D), proliferation (by examining the EdU-positive nuclei ratio, E and I) and in vitro cell migration (“Transwell” assays, F and J) were examined. “Pare” stands for parental control cells. *P < 0.05 versus “sh-scr” group. Scale bar = 100 μm (E and F).
HBO1 silencing provokes NSCLC cell apoptosis. The described primary or immortalized NSCLC cells, stably expressing the applied HBO1 shRNA or scramble control shRNAs (“sh-scr”), were established. Cells were further maintained in culturing medium for described hours, the relative caspase-3 activity was tested (A and F); Expression of listed proteins was tested by Western blotting assays (B); Single strand DNA (ssDNA) contents were tested by ELISA assays (C). Cell apoptosis was tested by measuring nuclear TUNEL ratio (D and G) and Annexin V percentage (E). “Pare” stands for parental control cells. *P < 0.05 versus “sh-scr” group. Scale bar = 100 μm (G).
HBO1 knockout by CRISPR/Cas9 produces significant anti-NSCLC cell activity. pNSCLC-1 primary cells with the applied lenti-Cas9-HBO1-KO construct (containing ko-HBO1-Sg2 and ko-HBO1-Sg5) or empty vector (“koC”) were established. Expression of HBO1 mRNA (A) and listed proteins (B) tested. Cells were further maintained in culturing medium for described hours, CCK-8 OD (C), cell proliferation (by testing nuclear EdU staining assays, D) and in vitro cell migration (E) were tested. The relative caspase-3 activity (F) and cell apoptosis (by recording TUNEL-positive nuclei ratio, G) were examined as well. *P < 0.05 versus “koC” cells. Scale bar = 100 μm (D, E and G).
Ectopic HBO1 overexpression further promotes NSCLC cell proliferation and migration. pNSCLC-1 primary cells stably expressing the lentiviral HBO1-expressing construct (“OE-HBO1-SL1” and “OE-HBO1-SL2”, two selections) or the empty vector (“Vec”), were established, with expression of HBO1 mRNA (A) and protein (B) tested. Cells were further maintained in culturing medium for described hours, cell proliferation (EdU assays, C) and in vitro cell migration (D) were tested. *P < 0.05 versus “Vec” cells. Scale bar = 100 μm (C and D).
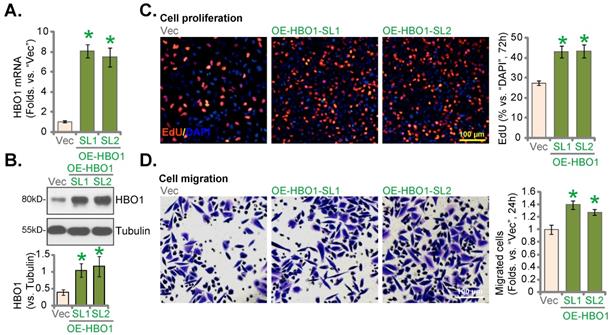
Ectopic HBO1 overexpression further promotes NSCLC cell proliferation and migration
Next a lentiviral HBO1-expressing construct (from Dr. Cao [24]) was transduced to pNSCLC-1 cells. Two stable cell selections, OE-HBO1-SL1 and OE-HBO1-SL2, were formed after selection. As shown HBO1 mRNA increased over seven fold in OE-HBO1 pNSCLC-1 cells (Figure 5A). HBO1 protein overexpression was detected as well (Figure 5B). HBO1 overexpression increased cell proliferation (evidenced by the EdU-positive nuclei percentage increasing, Figure 5C) and in vitro migration (Figure 5D) in pNSCLC-1 cells.
HBO1 is important for H3-H4 acetylation and expression of several oncogenic genes in NSCLC cells. The stable pNSCLC-1 cells with the described HBO1 shRNA (sh-HBO1-Sq1 or sh-HBO1-Sq3), the described lenti-Cas9-HBO1-KO construct (ko-HBO1-Sg2) or the scramble control shRNA plus empty vector (“sh-scr+koC”) were established. Alternatively, pNSCLC-1 cells with the HBO1-expressing lentiviral construct (OE-HBO1-SL1 and OE-HBO1-SL2, two cell selection) or the empty vector (“Vec”) were established as well; Expression of the listed proteins and mRNAs in the described cells were tested by Western blotting (A and B) and qRT-PCR (C and D) assays, respectively. *P < 0.05 versus “sh-scr+koC”/“Vec” cells.
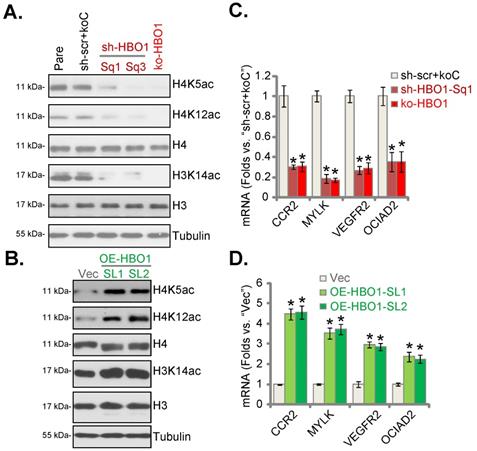
HBO1 is important for H3-H4 acetylation and expression of several oncogenic genes in NSCLC cells
HBO1 catalyzes H4 acetylation (H4K5ac and H4K12ac) and H3K14ac in human cancer [22, 24, 28]. As shown HBO1 silencing or CRISPR/Cas9-mediated HBO1 KO largely inhibited H4K5ac, H4K12ac and H3K14ac in pNSCLC-1 cells (Figure 6A). Conversely, their levels were increased in HBO1-overexpressed pNSCLC-1 cells (Figure 6B). Total H3 and H4 expression was however not significantly altered (Figure 6A and B).
RNA-Seq studies have tested differentially-regulated mRNAs in control cells versus HBO1-depleted cells [27, 28]. Many of these HBO1-dependent genes could exert oncogenic/cancer-promoting functions or being upregulated in NSCLC, including C‑C chemokine receptor type 2 (CCR2) [30, 31], myosin light chain kinase (MYLK) [32], VEGFR2 [33, 34], ovarian cancer immunoreactive antigen domain containing 2 (OCIAD2) [35]. Here, mRNA levels of CCR2, MYLK, VEGFR2 and OCIAD2 were dramatically reduced in HBO1-silenced and HBO1-KO pNSCLC-1 cells (Figure 6C). In contrast, their expression was robustly elevated in OE-HBO1-SL1 and OE-HBO1-SL2 cells (Figure 6D). These results suggest that HBO1 is vital for H3-H4 acetylation and expression of multiple oncogenic genes in NSCLC cells.
HBO1 silencing inhibits NSCLC xenograft growth in mice
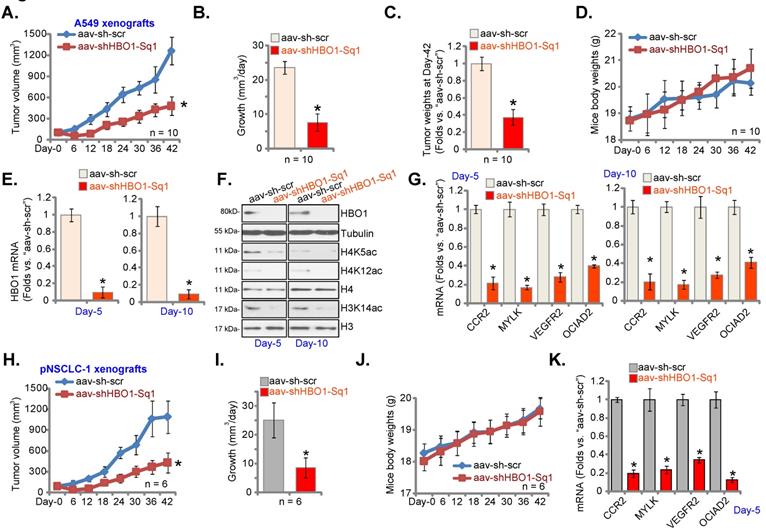
A549 cells (at six million cells of each mouse) were inoculated to the flanks of the nude mice. Within 21 days A549 xenografts were established (tumor volume at close to100 mm3). The xenograft-bearing mice received intratumoral injection of AAV-packed HBO1 shRNA (“aav-shHBO1-Sq1”) or AAV scramble control shRNA (“aav-sh-scr”), with 10 mice in each group. Virus injection was carried out daily for six consecutive days (“Day-0” to “Day-5”).
Figure 7A, demonstrated that injection of HBO1 shRNA AAV largely inhibited A549 xenograft growth. The estimated daily tumor growth was calculated as described [25]. Figure 7B confirmed that HBO1 shRNA potently suppressed A549 xenograft growth. At Day-42, A549 tumors were isolated. Tumors in aav-shHBO1-Sq1 group mice were lighter than those in aav-sh-scr group mice (Figure 7C). Figure 7D confirmed that the animal body weights were not different significantly among the two groups.
At “Day-5” and “Day-10”, one tumor per group was carefully isolated 6h after virus injection, and fresh tumor lysates were achieved. HBO1 mRNA and protein levels (Figure 7E-F) were significantly decreased in aav-shHBO1-Sq1-injected xenograft tissues, where H4K5ac, H4K12ac and H3K14ac levels were largely inhibited (Figure 7F). H3 and H4 protein levels were however unchanged (Figure 7F). In addition, mRNA levels of HBO1-dependent genes, CCR2, MYLK, VEGFR2 and OCIAD2, were robustly reduced in HBO1-shRNA-injected A549 xenografts (Figure 7G). Thus, HBO1 silencing inhibited H3-H4 acetylation and decreased expression of HBO1-dependent genes in A549 xenografts.
Next, pNSCLC-1 primary cells were s.c. injected to the nude mice (again at six million cells of each mouse). Three weeks after cell implantation, pNSCLC-1 xenografts were formed and patient-derived xenograft model was established. Mice were thereafter subject to the intratumoral injection of aav-shHBO1-Sq1 or aav-sh-scr. Virus injection was carried out daily for six consecutive days (“Day-0” to “Day-5”). Recording weekly tumor volumes, Figure 7H, demonstrated that injection of the HBO1 shRNA virus potently inhibited pNSCLC-1 xenograft growth in the experimental animals. The estimated daily tumor growth was inhibited following HBO1 shRNA (Figure 7I). The body weights of the nude mice were again indifferent (Figure 7J). At experimental Day-5, six hours after virus injection, one tumor of each group was isolated. CCR2, MYLK, VEGFR2 and OCIAD2 mRNA levels were reduced in HBO1 shRNA virus-injected pNSCLC-1 xenografts (Figure 7K).
WM-3835 induces significant anti-cancer activity in NSCLC cells
At last, the effect of a first-in-class small molecule inhibitor of HBO1 [24, 28] WM-3835 was studied. Figure 8A demonstrated that WM-3835 decreased pNSCLC1 CCK-8 viability in a time-dependent manner. It required 48-96h for the HBO1 inhibitor to exert a significant effect in decreasing cell viability (Figure 8A). Moreover, WM-3835-induced cell viability reduction was concentration dependent (Figure 8A). The IC-50 was close to 5 μM. H3 and H4 histone acetylation was largely inhibited by WM-3835 (Figure 8B). HBO1 protein expression was unchanged (Figure 8B). CCR2, MYLK, VEGFR2 and OCIAD2 mRNA levels were dramatically decreased in WM-3835-treated pNSCLC1 cells (Figure 8C).
HBO1 silencing inhibits NSCLC xenograft growth in mice. A549 xenograft-bearing nude mice (A-G) or the pNSCLC-1 xenograft-bearing nude mice (H-K) were subjected to intratumoral injection of AAV-packed HBO1 shRNA (“aav-shHBO1-Sq1”) or AAV-packed scramble control shRNA (“aav-sh-scr”). Virus injection was performed daily for six days. Tumor volumes (A and H) and mice body weights (D and J) were recorded every six days (total of 42 days). The estimated daily tumor growth was calculated (B and I). At Day-42, A549 tumors were isolated and individually weighted (C). The described tumors were isolated, and tumor lysates were obtained. Expression of the listed mRNAs (E, G and K) and proteins (F) was tested. *P < 0.05 versus “aav-sh-scr” group.
WM-3835 induces significant anti-cancer activity in NSCLC cells. The listed primary or immortalized NSCLC cells were treated with WM-3835 (at 5 μM, expect for A) or the vehicle control (“Veh”, 0.5% DMSO), and maintained in culturing medium for described hours; Cell viability was tested by CCK-8 assays (A and G), and expression of listed proteins and mRNAs were shown (B and C); Cell proliferation (D and H),in vitro cell migration (E) and apoptosis (F, by measuring TUNEL-positive nuclei percentage) were tested by the described assays. “Pare” stands for parental control cells. * P < 0.05 versus “Veh” group. Scale bar = 100 μm (D and E).
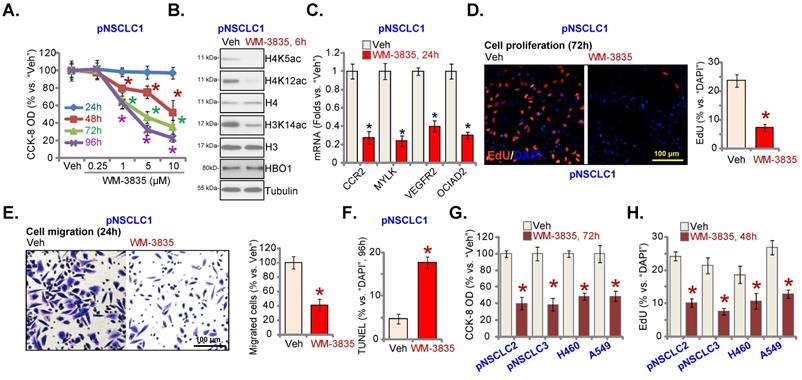
Furthermore, treatment with the HBO1 inhibitor inhibited pNSCLC1 cell proliferation and in vitro cell migration, examined by the nuclear EdU-staining (Figure 8D) and “Transwell” (Figure 8E) assays, respectively. WM-3835 increased the percentage of the TUNEL-positive nuclei in pNSCLC1 cells, suggesting apoptosis activation (Figure 8F). In pNSCLC-2 and pNSCLC-3 primary cells as well as in the immortalized cells (A549 and H460), WM-3835 (5 μM) resulted in significant viability reduction (Figure 8G) and proliferation arrest (EdU-positive cell decreasing, Figure 8H). Thus, the HBO1 inhibitor WM-3835 induced significant anti-cancer activity in different human NSCLC cells.
Discussion
By catalyzing histone lysine acetylation, HBO1 and other HATs are essential in transcriptional regulation of gene expression and various key biological processes [36-38]. Dysregulation of HATs is commonly detected in NSCLC. One HAT hMOF is overexpressed in NSCLC, and it is essential for growth and tumorigenesis of NSCLC [39, 40]. CPTH6, a HAT inhibitor, preferentially inhibited lung cancer stem-like cell growth and viability [41]. Another HAT inhibitor, A485, enhanced the sensitivity of TRAIL in NSCLC cells [42]. C646, a p300 HAT inhibitor, radio-sensitized NSCLC cells by facilitating mitotic catastrophe [43].
HBO1 is vital for H3 and H4 acetylation, gene transcription, DNA replication and repair [15, 44]. Through directly binding to ORC1, HBO1 interacts with pre-replication complex proteins, including MCM2 [19] and chromatin licensing and DNA replication Factor 1 [45], thereby promoting DNA replication. HBO1 acetylated ORC2, MCM2, CDC6, and geminin, and it was essential for replication licensing [46]. By acting as the positive regulator of centromeric Centromere Protein A, HBO1 inhibited inactivation of SUV39H1-mediated centromere [47]. In addition, ATR-dependent HBO1 phosphorylation is import for DNA repair [48].
Recent studies implied a pivotal role of HBO1 in tumorigenesis [15]. MacPherson et al., showed that HBO1 and several known members of the HBO1 protein complex served as the critical regulators for leukaemia stem cells [28]. In acute myeloid leukemia (AML) cells, HBO1 depletion led to a rapid and complete loss of H3K14ac and H4K12ac, and caused proliferation inhibition, apoptosis and cell differentiation [29]. HBO1 was required for Wnt/beta-catenin signaling activation and proliferation of bladder cancer cells [49]. HBO1 expression was elevated in HCC and it was important for cancer cell growth [22]. Gao et al., have proposed HBO1 as a novel oncogenic gene for osteosarcoma. HBO1 overexpression in osteosarcoma was required for cell growth in vitro and in vivo [24].
Our results support that HBO1 could be a key therapeutic target of NSCLC. TCGA and Oncomine bioinformatics analyses have shown that HBO1 is overexpressed in human NSCLC tissues. Furthermore, in local NSCLC tumor tissues HBO1 is significantly overexpressed. HBO1 overexpression was detected in different established and immortalized human NSCLC cells. In different NSCLC cells, HBO1 shRNA or KO inhibited cell viability, proliferation and migration, and provoked apoptosis activation. Conversely, HBO1 overexpression further increased NSCLC cell proliferation and migration. Importantly, AAV-packed HBO1 shRNA intratumoral injection largely hindered NSCLC xenograft tumor growth in nude mice. WM-3835, a first-in-class small molecule HBO1 inhibitor, potently inhibited NSCLC cell growth.
A large number of HBO1-dependent genes, CCR2, MYLK, VEGFR2 and OCIAD2, could exert tumor-promoting activity in NSCLC [15, 22, 28, 44, 50]. VEGFR2 is a well-established oncogene in NSCLC [33, 34]. Moreover, CCR2 expression was shown to be upregulated in NSCLC tissues and cells [31, 51]. CAS445479-97-0, a CCR2 antagonist, potently inhibited viability, motility and invasion of NSCLC cells [31]. Conversely, the CCR2 ligand CCL2 promoted NSCLC cell proliferation, migration and invasion by promoting MMP-9 expression [31]. Tan et al., showed that expression of MYLK, a HBO1-dependent gene, in stages III and IV NSCLC was significantly higher than that in stages I and II NSCLC [32]. Furthermore, MYLK expression in NSCLC with lymphatic metastasis was higher [32]. Moreover, OCIAD2 was associated with progression of early-stage lung adenocarcinoma and overexpression predicted poorer prognosis in patients [35, 52]. Here we showed that CCR2, MYLK, VEGFR2 and OCIAD2 expression was significantly decreased in NSCLC cells with HBO1 silencing or KO. They were however increased after HBO1 overexpression. Moreover, the expression of these genes was significantly reduced in HBO1-shRNA AAV-injected NSCLC xenograft tissues. Therefore, HBO1-driven NSCLC cell growth could be due to its role in promoting H3-H4 acetylation and expression of important NSCLC-promoting genes.
Conclusion
Overexpressed HBO1 induces histone acetylation and is important for NSCLC cell growth.
Supplementary Material
Supplementary figure.
Acknowledgements
This work is supported by the Research Project of Jiangsu Province Health Committee (Z2019054) and National Science Foundation of China (81602704).
Data Availability Statement
All data are available upon request.
Ethics Statement
This study was approved by Ethics Committee of The First Affiliated Hospital of Zhengzhou University.
Author Contributions
TC, YS, CX and SL proposed and designed the research. TC, HH, YZ, XC, HZ, RY, PL, LQ, YS, CX and SL performed the experiments, analyzed the data and organized Figures. TC, YS, CX and SL collected the clinical samples. J.Yao, TC, YS, CX and SL supervised the research. TC, YS, CX and SL are responsible for funding acquisition, project administration and revision. TC, HH, YZ, XC, HZ, LQ, YS, CX and SL participated in writing the manuscript and revising it, and all authors discussed the experiments and approved final manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Vestergaard HH, Christensen MR, Lassen UN. A systematic review of targeted agents for non-small cell lung cancer. Acta Oncol. 2018;57:176-86
2. Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature. 2018;553:446-54
3. Gridelli C, Rossi A, Carbone DP, Guarize J, Karachaliou N, Mok T. et al. Non-small-cell lung cancer. Nat Rev Dis Primers. 2015;1:15009
4. Xu P, Jiang L, Yang Y, Wu M, Liu B, Shi Y. et al. PAQR4 promotes chemoresistance in non-small cell lung cancer through inhibiting Nrf2 protein degradation. Theranostics. 2020;10:3767-78
5. Li F, Li X, Li Z, Ji W, Lu S, Xia W. betaKlotho is identified as a target for theranostics in non-small cell lung cancer. Theranostics. 2019;9:7474-89
6. Schiffmann I, Greve G, Jung M, Lubbert M. Epigenetic therapy approaches in non-small cell lung cancer: Update and perspectives. Epigenetics. 2016;11:858-70
7. Forde PM, Brahmer JR, Kelly RJ. New strategies in lung cancer: epigenetic therapy for non-small cell lung cancer. Clin Cancer Res. 2014;20:2244-8
8. Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293:1074-80
9. Schuldner M, Dorsam B, Shatnyeva O, Reiners KS, Kubarenko A, Hansen HP. et al. Exosome-dependent immune surveillance at the metastatic niche requires BAG6 and CBP/p300-dependent acetylation of p53. Theranostics. 2019;9:6047-62
10. Yang G, Feng J, Liu Y, Zhao M, Yuan Y, Yuan H. et al. HAT1 signaling confers to assembly and epigenetic regulation of HBV cccDNA minichromosome. Theranostics. 2019;9:7345-58
11. Marmorstein R, Zhou MM. Writers and readers of histone acetylation: structure, mechanism, and inhibition. Cold Spring Harb Perspect Biol. 2014;6:a018762
12. Sun RC, Dukhande VV, Zhou Z, Young LEA, Emanuelle S, Brainson CF. et al. Nuclear Glycogenolysis Modulates Histone Acetylation in Human Non-Small Cell Lung Cancers. Cell Metab. 2019;30:903-16 e7
13. Mi W, Guan H, Lyu J, Zhao D, Xi Y, Jiang S. et al. YEATS2 links histone acetylation to tumorigenesis of non-small cell lung cancer. Nat Commun. 2017;8:1088
14. Iizuka M, Takahashi Y, Mizzen CA, Cook RG, Fujita M, Allis CD. et al. Histone acetyltransferase Hbo1: catalytic activity, cellular abundance, and links to primary cancers. Gene. 2009;436:108-14
15. Lan R, Wang Q. Deciphering structure, function and mechanism of lysine acetyltransferase HBO1 in protein acetylation, transcription regulation, DNA replication and its oncogenic properties in cancer. Cell Mol Life Sci. 2020;77:637-49
16. Feng Y, Vlassis A, Roques C, Lalonde ME, Gonzalez-Aguilera C, Lambert JP. et al. BRPF3-HBO1 regulates replication origin activation and histone H3K14 acetylation. EMBO J. 2016;35:176-92
17. Lalonde ME, Avvakumov N, Glass KC, Joncas FH, Saksouk N, Holliday M. et al. Exchange of associated factors directs a switch in HBO1 acetyltransferase histone tail specificity. Genes Dev. 2013;27:2009-24
18. Miotto B, Struhl K. JNK1 phosphorylation of Cdt1 inhibits recruitment of HBO1 histone acetylase and blocks replication licensing in response to stress. Mol Cell. 2011;44:62-71
19. Burke TW, Cook JG, Asano M, Nevins JR. Replication factors MCM2 and ORC1 interact with the histone acetyltransferase HBO1. J Biol Chem. 2001;276:15397-408
20. Iizuka M, Stillman B. Histone acetyltransferase HBO1 interacts with the ORC1 subunit of the human initiator protein. J Biol Chem. 1999;274:23027-34
21. Saksouk N, Avvakumov N, Champagne KS, Hung T, Doyon Y, Cayrou C. et al. HBO1 HAT complexes target chromatin throughout gene coding regions via multiple PHD finger interactions with histone H3 tail. Mol Cell. 2009;33:257-65
22. Zhong W, Liu H, Deng L, Chen G, Liu Y. HBO1 overexpression is important for hepatocellular carcinoma cell growth. Cell Death Dis. 2021;12:549
23. Gao YY, Ling ZY, Zhu YR, Shi C, Wang Y, Zhang XY. et al. The histone acetyltransferase HBO1 functions as a novel oncogenic gene in osteosarcoma. Theranostics. 2021;11:4599-615
24. Gao YY, Ling ZY, Zhu YR, Shi C, Wang Y, Zhang XY. et al. The histone acetyltransferase HBO1 functions as a novel oncogenic gene in osteosarcoma. Theranostics. 2021;11:4599-615
25. Zhou T, Sang Y-H, Cai S, Xu C, Shi M-h. The requirement of mitochondrial RNA polymerase for non-small cell lung cancer cell growth. Cell Death & Disease. 2021;12:751
26. Xia YC, Zha JH, Sang YH, Yin H, Xu GQ, Zhen J. et al. AMPK activation by ASP4132 inhibits non-small cell lung cancer cell growth. Cell Death Dis. 2021;12:365
27. Yan MS, Turgeon PJ, Man HJ, Dubinsky MK, Ho JJD, El-Rass S. et al. Histone acetyltransferase 7 (KAT7)-dependent intragenic histone acetylation regulates endothelial cell gene regulation. J Biol Chem. 2018;293:4381-402
28. MacPherson L, Anokye J, Yeung MM, Lam EYN, Chan YC, Weng CF. et al. HBO1 is required for the maintenance of leukaemia stem cells. Nature. 2020;577:266-70
29. Au YZ, Gu M, De Braekeleer E, Gozdecka M, Aspris D, Tarumoto Y. et al. KAT7 is a genetic vulnerability of acute myeloid leukemias driven by MLL rearrangements. Leukemia. 2021;35:1012-22
30. Abd-Rabou AA, Ahmed HH. Bevacizumab and CCR2 Inhibitor Nanoparticles Induce Cytotoxicity-Mediated Apoptosis in Doxorubicin-Treated Hepatic and Non-Small Lung Cancer Cells. Asian Pac J Cancer Prev. 2019;20:2225-38
31. An J, Xue Y, Long M, Zhang G, Zhang J, Su H. Targeting CCR2 with its antagonist suppresses viability, motility and invasion by downregulating MMP-9 expression in non-small cell lung cancer cells. Oncotarget. 2017;8:39230-40
32. Tan X, Chen M. MYLK and MYL9 expression in non-small cell lung cancer identified by bioinformatics analysis of public expression data. Tumour Biol. 2014;35:12189-200
33. Devery AM, Wadekar R, Bokobza SM, Weber AM, Jiang Y, Ryan AJ. Vascular endothelial growth factor directly stimulates tumour cell proliferation in non-small cell lung cancer. Int J Oncol. 2015;47:849-56
34. Yang F, Tang X, Riquelme E, Behrens C, Nilsson MB, Giri U. et al. Increased VEGFR-2 gene copy is associated with chemoresistance and shorter survival in patients with non-small-cell lung carcinoma who receive adjuvant chemotherapy. Cancer Res. 2011;71:5512-21
35. Sakashita M, Sakashita S, Murata Y, Shiba-Ishii A, Kim Y, Matsuoka R. et al. High expression of ovarian cancer immunoreactive antigen domain containing 2 (OCIAD2) is associated with poor prognosis in lung adenocarcinoma. Pathol Int. 2018;68:596-604
36. Sapountzi V, Cote J. MYST-family histone acetyltransferases: beyond chromatin. Cell Mol Life Sci. 2011;68:1147-56
37. Lee KK, Workman JL. Histone acetyltransferase complexes: one size doesn't fit all. Nat Rev Mol Cell Biol. 2007;8:284-95
38. Marmorstein R, Roth SY. Histone acetyltransferases: function, structure, and catalysis. Curr Opin Genet Dev. 2001;11:155-61
39. Chen Z, Ye X, Tang N, Shen S, Li Z, Niu X. et al. The histone acetylranseferase hMOF acetylates Nrf2 and regulates anti-drug responses in human non-small cell lung cancer. Br J Pharmacol. 2014;171:3196-211
40. Zhao L, Wang DL, Liu Y, Chen S, Sun FL. Histone acetyltransferase hMOF promotes S phase entry and tumorigenesis in lung cancer. Cell Signal. 2013;25:1689-98
41. Di Martile M, Desideri M, De Luca T, Gabellini C, Buglioni S, Eramo A. et al. Histone acetyltransferase inhibitor CPTH6 preferentially targets lung cancer stem-like cells. Oncotarget. 2016;7:11332-48
42. Zhang B, Chen D, Liu B, Dekker FJ, Quax WJ. A novel histone acetyltransferase inhibitor A485 improves sensitivity of non-small-cell lung carcinoma cells to TRAIL. Biochem Pharmacol. 2020;175:113914
43. Oike T, Komachi M, Ogiwara H, Amornwichet N, Saitoh Y, Torikai K. et al. C646, a selective small molecule inhibitor of histone acetyltransferase p300, radiosensitizes lung cancer cells by enhancing mitotic catastrophe. Radiother Oncol. 2014;111:222-7
44. Quintela M, Sieglaff DH, Gazze AS, Zhang A, Gonzalez D, Francis L. et al. HBO1 directs histone H4 specific acetylation, potentiating mechano-transduction pathways and membrane elasticity in ovarian cancer cells. Nanomedicine. 2019;17:254-65
45. Miotto B, Struhl K. HBO1 histone acetylase is a coactivator of the replication licensing factor Cdt1. Genes Dev. 2008;22:2633-8
46. Iizuka M, Matsui T, Takisawa H, Smith MM. Regulation of replication licensing by acetyltransferase Hbo1. Mol Cell Biol. 2006;26:1098-108
47. Ohzeki J, Shono N, Otake K, Martins NM, Kugou K, Kimura H. et al. KAT7/HBO1/MYST2 Regulates CENP-A Chromatin Assembly by Antagonizing Suv39h1-Mediated Centromere Inactivation. Dev Cell. 2016;37:413-27
48. Niida H, Matsunuma R, Horiguchi R, Uchida C, Nakazawa Y, Motegi A. et al. Phosphorylated HBO1 at UV irradiated sites is essential for nucleotide excision repair. Nat Commun. 2017;8:16102
49. Chen Z, Zhou L, Wang L, Kazobinka G, Zhang X, Han X. et al. HBO1 promotes cell proliferation in bladder cancer via activation of Wnt/beta-catenin signaling. Mol Carcinog. 2018;57:12-21
50. Wang Y, Chen S, Tian W, Zhang Q, Jiang C, Qian L. et al. High-Expression HBO1 Predicts Poor Prognosis in Gastric Cancer. Am J Clin Pathol. 2019;152:517-26
51. Zhang XW, Qin X, Qin CY, Yin YL, Chen Y, Zhu HL. Expression of monocyte chemoattractant protein-1 and CC chemokine receptor 2 in non-small cell lung cancer and its significance. Cancer Immunol Immunother. 2013;62:563-70
52. Itoguchi N, Nakagawa T, Murata Y, Li D, Shiba-Ishii A, Minami Y. et al. Immunocytochemical staining for stratifin and OCIAD2 in bronchial washing specimens increases sensitivity for diagnosis of lung cancer. Cytopathology. 2015;26:354-61
Author contact
![]() Corresponding authors: Dr. Chun Xu. Department of Thoracic Surgery, Institute of Thoracic Surgery, The First Affiliated Hospital of Soochow University, Suzhou, China. Email: xuchunedu.cn; Dr. Yong-Hua Sang. Department of Cardiothoracic Surgery, The Second Affiliated Hospital of Soochow University, Suzhou, China. Email: sangyh1983com; Dr. Shao-xia Liu, No. 1, Jianshe East Road, Respiratory Department I, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan, China. Email: zhaohuancc1505com.
Corresponding authors: Dr. Chun Xu. Department of Thoracic Surgery, Institute of Thoracic Surgery, The First Affiliated Hospital of Soochow University, Suzhou, China. Email: xuchunedu.cn; Dr. Yong-Hua Sang. Department of Cardiothoracic Surgery, The Second Affiliated Hospital of Soochow University, Suzhou, China. Email: sangyh1983com; Dr. Shao-xia Liu, No. 1, Jianshe East Road, Respiratory Department I, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan, China. Email: zhaohuancc1505com.

 Global reach, higher impact
Global reach, higher impact