10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2022; 18(10):4135-4150. doi:10.7150/ijbs.71520 This issue Cite
Research Paper
O-GlcNAcylation of ZEB1 facilitated mesenchymal pancreatic cancer cell ferroptosis
1. Laboratory of Medical Imaging, Affiliated Hospital of Jiangsu University, Zhenjiang, China, 212001.
2. Department of Medical Imaging, Affiliated Hospital of Jiangsu University, Zhenjiang, China, 212001.
3. School of Medicine, Jiangsu University, Zhenjiang, China, 212013.
4. Department of Medical Imaging, Affiliated Hospital of Nanjing Medicine University, 211166.
5. Department of Laboratory Medicine, Affiliated Hospital of Jiangsu University, Zhenjiang, China, 212001.
#These authors contributed equally to this work.
Abstract
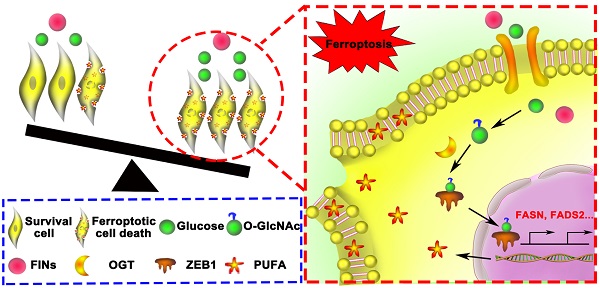
Background: Mesenchymal cancer cells, resistant to the traditional regulated cell death, are exquisitely vulnerable to ferroptosis. However, the underlying mechanism has been rarely studied. While glycolipid metabolism rewiring is a critical determination of both cancer cell mesenchymal phenotype and cell death resistance, we are interested in the underlying cross talk between glycolipid metabolism and mesenchymal cancer cell ferroptosis sensitivity.
Methods: CCK-8, western blot and clone forming assay were used to access the effect of glucose on mesenchymal cancer cell ferroptosis susceptibility and O-GlcNAcylation level. GEPIA database, shRNA knockdown and various pharmacological inhibitors were used to analyze the relationship between O-GlcNAcylation and mesenchymal cancer cell ferroptosis in vitro and in vivo. A series of experiments were conducted to investigate the underlying mechanisms of glucose induced ZEB1 O-GlcNAcylation on mesenchymal cancer cell ferroptosis susceptibility.
Results: Mesenchymal pancreatic cancer cells O-GlcNAcylation level and ferroptosis cell death was significantly increased under high glucose condition in vitro and in vivo. O-GlcNAcylation of ZEB1, rather than other transcription factors, was involved in this process. Mechanistically, glucose triggered ZEB1 O-GlcNAcylation at Ser555 site enhanced its stabilization and nuclear translocation, induced lipogenesis associated genes, FASN and FADS2, transcription activity, which ultimately resulted in lipid peroxidation dependent mesenchymal pancreatic cancer cell ferroptosis.
Conclusions: These results identify a novel role of glycolipid metabolism and O-GlcNAcylation in mesenchymal cancer cells ferroptosis susceptibility, which broaden the molecular mechanism of ferroptosis and suggested a potential clinical therapeutic strategy for refractory tumors.
Keywords: Mesenchymal pancreatic cancer cell, ZEB1, O-GlcNAcylation, Ferroptosis, Glycolipid metabolism

 Global reach, higher impact
Global reach, higher impact