Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2022; 18(14):5221-5229. doi:10.7150/ijbs.67869 This issue Cite
Research Paper
MG53 inhibits cellular proliferation and tumor progression in colorectal carcinoma
1. Department of Pharmaceutical Sciences, College of Pharmacy and Health Sciences, St. John's University, Queens, NY 11439, USA.
2. Department of Surgery, The Ohio State University, Columbus, OH 43210, USA.
# Current affiliation: Albert Einstein College of Medicine, 1300 Morris Park Ave, Bronx, NY 10461, USA.
* These authors contributed equally.
Received 2021-10-8; Accepted 2021-11-12; Published 2022-8-15
Abstract
Cancer is the second leading cause of mortality after cardiovascular diseases in the United States. Chemotherapy is widely used to treat cancers. Since the development of drug resistance is a major contributor towards the failure of chemotherapeutic regimens, efforts have been made to develop novel inhibitors that can combat drug resistance and sensitize cancer cells to chemotherapy. Here we investigated the anti-cancer effects of MG53, a TRIM-family protein known for its membrane repair functions. We found that rhMG53 reduced cellular proliferation of both parental and ABCB1 overexpressing colorectal carcinoma cells. Exogenous rhMG53 protein entered SW620 and SW620/AD300 cells without altering the expression of ABCB1 protein. In a mouse SW620/AD300 xenograft model, the combination of rhMG53 and doxorubicin treatment significantly inhibited tumor growth without any apparent weight loss or hematological toxicity in the animals. Our data show that MG53 has anti-proliferative function on colorectal carcinoma, regardless of their nature to drug-resistance. This is important as it supports the broader value for rhMG53 as a potential adjuvant therapeutic to treat cancers even when drug-resistance develops.
Keywords: Cancer, Drug Resistance, ABCB1, rhMG53, Doxorubicin
Introduction
Cancer has long been known to be a heterogeneous disease that exhibits an uncontrollable growth of cells. [1, 2]. Over the years, several efforts have been made to fight against cancer; the use of chemotherapeutic agents remains the most successful one. Each year the USFDA approves a number of agents for cancer treatment. Studies have shown that the extensive use of these agents leads to multidrug resistance (MDR), a condition in which tumor cells develop resistance to drugs that differ in their chemical composition and mechanism of action [3-7]. MDR has known to be the major contributor towards the failure of chemotherapy and the mortality of cancer patients [8].
Out of the various factors causing MDR, the overexpression of a diverse class of drug efflux transporters, ATP-binding cassette (ABC) transporters, has been known to be the most aggressive one [3, 9, 10]. These transporters are found on the plasma membrane of tumor cells and extrude chemotherapeutic drugs by utilizing energy derived from ATP hydrolysis [11-13]. Amongst the 49 known human ABC transporters, ABCB1 is highly expressed in kidneys, intestine, adrenal glands, blood-brain barrier, and placenta [14]. ABCB1 (commonly referred as P-glycoprotein (P-gp)) is the first member of the B family of ABC transporters [4, 15]. Structural studies have reported that ABCB1 contains two nucleotide and two transmembrane binding domains [16]. In these tissues, ABCB1 exerts protective function against cellular toxicants and xenobiotics. However, in the case of cancer cells, ABCB1 expression leads to a reduced cell concentration of the substrate chemotherapeutic drug, thus causing MDR [17, 18]. Some of the most common substrate chemotherapeutic drugs of ABCB1 include paclitaxel, etoposide, vincristine, colchicine, and doxorubicin [19, 20]. Many studies have been conducted to develop novel inhibitors as modulators of ABC transporter mediated MDR and as potential anti-cancer drugs [21, 22]. These inhibitors sensitize the ABCB1 overexpressing cancer cells to the substrate chemotherapeutic drugs.
We previously identified MG53, a member of the tripartite motif/RING B-box Coiled Coil (TRIM/RBCC) family protein (TRIM72), to be an integral part of the proteins assisting in the repair of the cellular machinery [23, 24]. MG53 mediates membrane repair by its redox-dependent oligomerization; thus, facilitating nucleation of intracellular vesicles to form repair patches at the site of injury [25]. Proteomic analysis has shown that in species such as rat, mouse, human, and monkey, the amino acid sequence of MG53 remains preserved [26]. Studies done in the past have shown that the membrane protective functions of MG53 can be translated into the treatment of clinical conditions such as acute lung injury, myocardial infarction, muscular dystrophy, wound healing and acute kidney injury [25, 27-30].
Chen and colleagues showed that knockout of TRIM72/MG53 through CRISPR-gene silencing led to aggressive lung tumor growth and metastasis in mice, raising the possibility that MG53 might possess tumor suppressor function in lung cancer [31-33]. However, the anti-cancer effects of MG53 in colorectal cancer cells and tumor xenografts have not been studied. Colorectal cancer ranks at number three in terms of cancer-related deaths in the United States [2, 34]. In 2020, approximately 148,000 people will be diagnosed with colorectal cancer with approximately 53,000 mortalities [34, 35]. Thus, it is pertinent to develop novel therapeutic adjuvants for colorectal cancer. In this study, we show that MG53 inhibits proliferation of sensitive SW620 and doxorubicin-resistant and ABCB1 overexpressing SW620/AD300 cells and reduces tumor growth and progression in ABCB1 mouse xenograft models.
Materials and Methods
Chemicals
rhMG53 was purified from a E. coli fermentation system as described previously [25]. Dulbecco's modified Eagle's Medium, fetal bovine serum (FBS), 0.25% trypsin, and penicillin/streptomycin were purchased from Hyclone (Pittsburgh, PA). The ABCB1 antibody was purchased from Thermo Fisher Scientific Inc. (Rockford, IL). The MTT reagent and DMSO were obtained from Sigma Chemical Co. (St. Louis, MO). Alexa FluorTM 647 protein labeling kit was purchased from Invitrogen (Waltham, MA).
Cell lines and cell culture
The drug sensitive parental SW620 cell line and doxorubicin resistant SW620/AD300 cell line were as described previously [36]. The present study also employed HCT-15 (from ATCC), S1, KB-3-1, and KB-C2 cells. These cell lines were cultured in DMEM media containing 10% FBS and 1% P/S at 37°C in a humidified atmosphere of 5% CO2.
MTT assay
The sensitive and drug-resistant cells were trypsinized, seeded into 96 well plates and incubated for 24 h. The next day, cells were treated with 20 μl of rhMG53 at varying concentrations (0-100 μM). To assess the chemosensitizing effects of rhMG53, different concentrations of doxorubicin were added after pre-incubation with rhMG53 or verapamil for 2 h. After 68 h of treatment, MTT dye (4 mg/ml) was added and the plates were further incubated for 4 h. Subsequently, absorbance was determined at 570 nm by Opsys microplate reader (Dynex Technologies, Chantilly, VA) [36-38].
Western blot analysis
Protein concentration was determined using the BCA assay from the treated and control cells. Twenty micrograms of protein was loaded and Western blotting was performed to measure the protein levels of ABCB1 using a previously described method and quantified using ImageJ [37]. The ABCB1 (E1Y7S) rabbit mAb (catalog number 13978S) and β-Actin (13E5) rabbit mAb (catalog number 4970S) were purchased from Cell Signaling Technology (Danvers, MA) and used at 1:1000 dilutions.
rhMG53 up-take and live cell confocal imaging
rhMG53 and bovine serum albumin (BSA) were labeled with Alexa FluorTM 647 protein labeling kit (InvitrogenTM, A20173) according to the manufacturer's instructions. SW620 and SW620/AD300 cells were cultured on glass bottom dishes (MatTek Inc., Ashland, MA). Alex647-rhMG53 or Alex647-BSA was added to the culture medium. Live cell images were taken by Nikon A1R confocal microscope and analyzed using ImageJ software.
Experimental animals and methodology for generating MDR mouse tumor xenograft model
The athymic nude mice, purchased from Taconic Farms (Taconic Biosciences, NY), were housed at the animal facility of St. John's University. All experiments were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC), as described previously [39, 40]. The animal protocol approval number was 1899 and it was approved on 02/14/2017. The tumor xenograft model of SW620 and SW620/AD300 cells was generated as described previously [41]. Animals were divided into four treatment groups: Group (a) vehicle (normal saline, q3d × 4, i.p.); (b) doxorubicin (2 mg/kg, q3d × 4, i.p.); (c) rhMG53 (2mg/kg, q3d × 4, i.v.); and (d) combination of rhMG53 and doxorubicin (2 mg/kg, q3d × 4, i.v., i.p.). The body weight of animals and tumor volume was accessed as described previously [42]. The blood was taken via submandibular puncture on the last treatment day and white blood cells (WBC) and platelet counts were determined in all four groups. At the end, all the animals were euthanized, the tumor tissues were excised, and weighed.
Statistical analysis
All the experiments were conducted in triplicates and ANOVA test followed with Tukey's post-hoc test to determine statistical significance at p < 0.05.
Results
Anti-proliferative effects of rhMG53 on parental and ABCB1 overexpressing cells
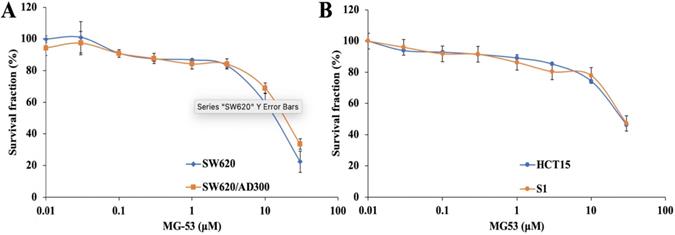
In order to study the chemosensitizing role of rhMG53, its non-cytotoxic concentration was determined by MTT assay. Our results showed that over 85% of both drug sensitive and resistant cell lines were alive at the concentration of 3 μM rhMG53 (Figure 1), indicating that rhMG53 is non-toxic up to 3 μM (Figure 1A). In addition, rhMG53 did not exhibit significant cytotoxicity on two other human colon cancer cell lines, HCT-15 and S1 (Figure 1B). Based on these results, we selected 1 and 3 μM concentrations of rhMG53 to be investigated for their reversal activity in combination with a chemotherapeutic drug. At higher concentrations, rhMG53 reduced survival of both SW620 and SW620/AD300 cells.
Anti-proliferative effect of rhMG53 does not involve changes in ABCB1 function
The drug sensitization effect of rhMG53 on ABCB1 substrate was investigated by performing the MTT assay. As shown in Table 1, SW620/AD300 cells were 315-fold resistant to doxorubicin as compared to the sensitive SW620 cells. Studies from our group have shown that SW620/AD300 also confers resistance to other ABCB1 substrate drugs such as paclitaxel, vincristine, and colchicine but not cisplatin [43-45]. rhMG53 (up to 3 μM) did not significantly decrease the IC50 values of the ABCB1 substrate drug doxorubicin in the SW620/AD300 cells. These results suggested that rhMG53 did not sensitize the drug resistant SW620/AD300 cells to doxorubicin. Verapamil, a widely known ABCB1 inhibitor, was used as a positive control inhibitor of ABCB1, which did reduce the IC50 of doxorubicin from 56.78 ± 2.35 to 1.22 ± 0.21 (p < 0.05).
Similar findings were also observed using KB-3-1 and ABCB1-overexpressing KB-C2 cells derived from HeLa, a cell line widely used for the study of MDR [46, 47]. As shown in Supplemental Figure S1 and Table S1, rhMG53 can inhibit the growth of both KB-3-1 and KB-C2 cells without alteration of the IC50 of KB-C2 cells to doxorubicin treatment.
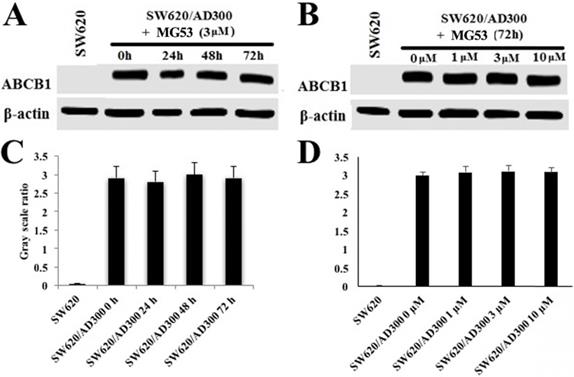
To assess the effect of rhMG53 on the protein expression of ABCB1, Western blot analysis was performed. As shown in Figures 2A and 2C, treatment with rhMG53 (3 µM) did not significantly alter the expression of ABCB1 protein in SW620/AD300 cells following different times of incubation. Similarly, no changes in protein expression of ABCB1 were observed when SW620/AD300 cells were incubated with rhMG53 at indicated concentrations (1, 3, and 10 µM) for 72 h (Figure 2B and 2D). These results suggest that rhMG53 does not change the expression of ABCB1 when treated in a concentration and time-dependent manner.
The effect of rhMG53 on reversal of ABCB1-mediated MDR.
| Treatment | IC50 ± SDa (μM) (FRb) | |
|---|---|---|
| SW620 | SW620/AD300 | |
| Doxorubicin | 0.18 ± 0.04 (1) | 56.78 ± 2.35 (315.45) |
| +1 μM rhMG53 | 0.18 ± 0.06 (1) | 55.14 ± 2.16 (306.34) |
| +3 μM rhMG53 | 0.17 ± 0.09 (0.9) | 51.09 ± 3.01 (283.84) |
| +3 μM Verapamil | 0.24 ± 0.09 (1.34) | 1.22 ± 0.21 (6.78) c |
a IC50 is presented as mean ± SD value of three independent experiments (n=3) each performed in triplicates
b FR: Resistance fold is the IC50 value of substrate drug with or without inhibitor over the IC50 of substrate drug in parental cells without inhibitor
c P < 0.05 compared to the control group in the absence of reversal agent
Anti-proliferative effects of rhMG53 on parental and ABCB1-overexpressing cells. Cytotoxicity assay was conducted to evaluate the anti-proliferative effects of rhMG53 on parental SW620 and ABCB1-overexpressing SW620/AD300 cells (A) and HCT15 and S1 cells (B). The data are the representation of mean ± SD for three independent experiments performed in triplicates.
Effect of rhMG53 on the expression of ABCB1. SW620/AD300 cells were treated with rhMG53 at different time points (A) or different concentrations (B). Similar amounts of cell lysates were used, and Western blot analysis was conducted. The band intensity of ABCB1/β-actin was obtained with Image J and labeled as grayscale ratios (C, D). The differences presented are not statistically significant (p > 0.05).
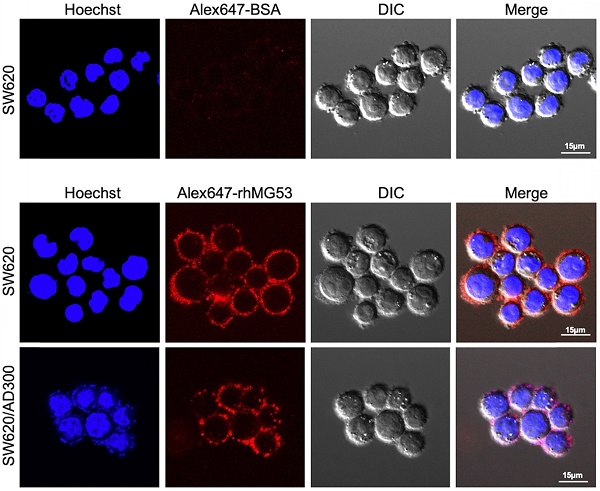
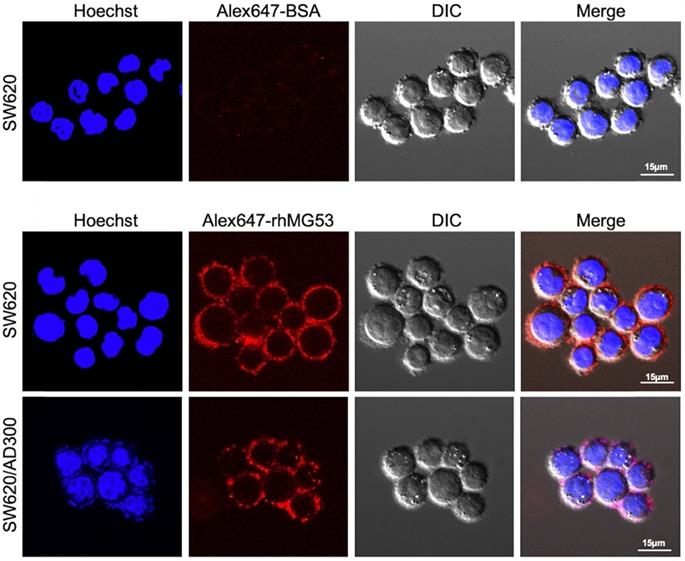
Uptake of rhMG53 in SW620 and SW620/AD300 cells
To assess whether rhMG53 entered the SW620 and SW620/AD300 cells, the cells were incubated with Alexa FluorTM 647 labeled rhMG53, according to the manufacturer's instructions. Our results showed that Alexa647-rhMG53 (red) localizes on the cell membrane and enters the cytosol after 2-hour incubation in both SW620 (middle) and SW620/AD300 (bottom) cells (Figure 3). As control, Alexa647-BSA shows no labeling of these cancer cells (top).
Effect of rhMG53 on the volume and weight of tumors in ABCB1-overexpressing tumor xenograft model
Based on our promising in-vitro findings, we extended our study to evaluate the effect of rhMG53 in mouse tumor xenograft models of colorectal adenocarcinoma. The sensitive and drug resistant cell lines were inoculated into the flank region of mice to generate the parental and ABCB1 overexpressing tumor xenograft model, respectively. The animals were randomly divided into four groups (n=7) and treated as mentioned in 'Material and Methods'. As shown in Figures 4 and 5, the control saline group exhibited a significant increase in volume of tumors derived from transplanted SW620 (Figure 4A and 4C) and SW620/AD300 (Figure 5A and 5C) cells. Upon treatment with rhMG53 (2 mg/kg, q3d × 4, i.v.) or doxorubicin (2 mg/kg, q3d × 4, i.p.), tumor volume reduced significantly in the SW620 tumors. However, since the SW620/AD300 tumors are resistant to doxorubicin, treatment with doxorubicin alone (2 mg/kg, q3d × 4, i.p.) did not reduce the tumor growth or volume in the SW620/AD300 tumors. Interestingly, treatment with rhMG53 (2 mg/kg, q3d × 4, i.v.) significantly reduced the tumor growth and volume in SW620/AD300 tumors. Moreover, the combination of rhMG53 and doxorubicin further reduced the tumor growth and volume in both sensitive and drug resistant tumors. On day 18 of the treatment, we measured the tumor weights from every mouse. As shown in Figure 4B and 5B, a significant reduction in the tumor weight was observed in the rhMG53 and doxorubicin groups as compared to the vehicle control group.
Assessment of potential toxicity of rhMG53
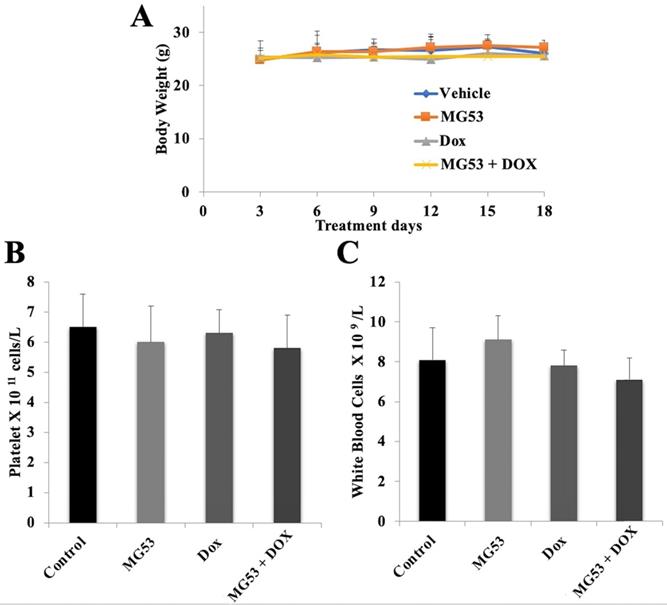
Systemic toxicity is one of the major concerns for any anti-cancer treatment. In order to evaluate the potential toxicity of rhMG53 alone or in combination with doxorubicin, we recorded the body weights of each animal every third day. Our results showed that the body weights of the animals did not change in any of the treatment groups. (Figure 6A). Furthermore, to assess any hematological toxicity, we did the blood cell count on day 18 of the study. As shown in Figures 6B and 6C, no significant change in the platelet and white blood cell count was observed in any of the treatment groups. Our findings show that rhMG53 when combined with doxorubicin exerts an anti-cancer effect on both parental and ABCB1 overexpressing tumors without any apparent weight loss or hematological toxicity.
Discussion
Our findings, for the first time, show the effects of rhMG53 on colorectal carcinoma. We used cell proliferation assay to assess the effect of rhMG53 on both parental SW620 and doxorubicin-resistant SW620/AD300 cells. We found that rhMG53 did not impact the sensitivity of SW620/AD300 cells to doxorubicin treatment, yet it can inhibit the growth of the colorectal cancer cells. As shown in Table 1, doxorubicin alone exhibited higher IC50 in SW620/AD300 cells as compared to SW620 cells. Our results show that no significant changes in IC50 of SW620/AD300 cells to doxorubicin were observed with incubation of rhMG53, suggesting that rhMG53 did not directly impact ABCB1-mediated MDR. Using biochemical assay, we further found that rhMG53 treatment did not alter the protein expression of ABCB1 in SW620/AD300 cells.
rhMG53 up-take by colorectal adenocarcinoma cells. Alexa647-rhMG53 or Alexa647-BSA was added to the culture medium of SW620 and SW620/AD300 cells. Confocal live cell images show that Alexa647-rhMG53 (red) localizes on the cell membrane and enters the cytosol after 2-hour incubation in both SW620 (middle) and SW620/AD300 (bottom) cells. As control, Alexa647-BSA shows no labeling of these cancer cells (top). The cell nuclei were stained with Hoechst 33342 (Blue).
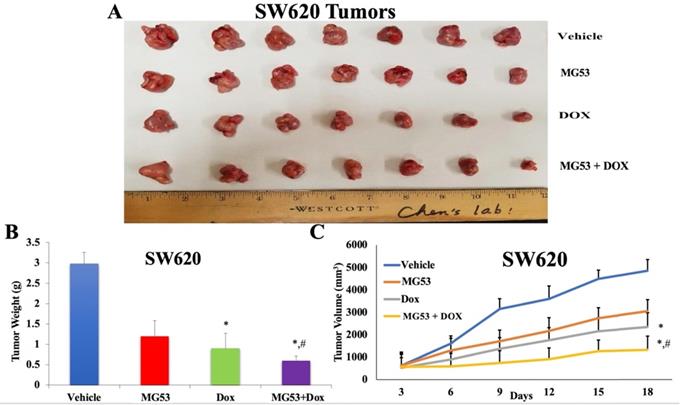
Effect of rhMG53 on the volume and weight of tumors in SW620 tumor xenograft model. (A) Images of SW620 tumors that were taken at the end of the treatment period. (B) The mean weight (n=7) of the SW620 tumors from the mice treated with vehicle, rhMG53, doxorubicin, and the combination of rhMG53 and doxorubicin, at the end of the 12-day treatment period. (C) The changes in tumor volume over time. Each point online graph represents the mean tumor volume (mm3) on each particular day after implantation. Error bars represent SD. *: p < 0.05 versus vehicle group; #: p < 0.05 versus the MG53 alone group.
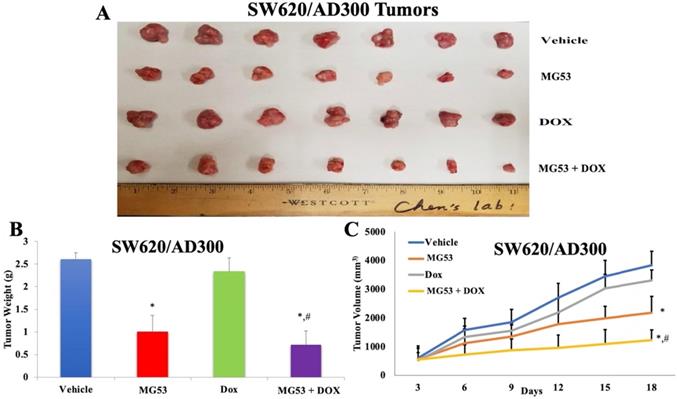
Effect of rhMG53 on the volume and weight of tumors in SW620/AD300 tumor xenograft model. (A) Images of SW620/AD300 tumors that were taken at the end of the treatment period. (B)The mean weight (n=7) of the SW620/AD300 tumors from the mice treated with vehicle, rhMG53, doxorubicin, and the combination of rhMG53 and doxorubicin, at the end of the 18-day treatment period. (C) The changes in tumor volume over time. Each point online graph represents the mean tumor volume (mm3) on each particular day after implantation. Error bars represent SD. *: p < 0.05 versus vehicle group; #: p < 0.05 versus the MG53 alone group.
Our present study demonstrated that rhMG53 has anti-proliferative effect on colorectal cancer cells regardless of their resistance to chemotherapeutic drugs. In our early study, we found that MG53 suppresses tumor progression and stress granule formation by modulating G3BP2 activity in non-small cell lung cancer [48]. In the present study, we provide live cell imaging data to show that the exogenous rhMG53 protein can be taken up by the SW60 and SW60/AD300 cells (Figure 3), further supporting the intracellular action of rhMG53. In separate studies, we demonstrated that the exogenously applied rhMG53 can efficiently target the tumor cells [48]. More studies are required to establish the connection between the mechanistic action of the intracellular MG53 on tumor cell growth.
Assessment of potential toxicity of rhMG53. The body weight of animals bearing the sensitive and drug-resistant tumors is plotted over the 18-day treatment period (A). (B) Platelet and (C) white blood cell count were measured on day 18 of the treatment. Bar graphs represent the mean of the blood cell count, and error bars represent SD.
The promising findings from our in-vitro studies inspired us to investigate the effect of rhMG53 in combination with doxorubicin in the parental and ABC overexpressing cancer cells using mouse tumor xenograft models [11, 49]. While the xenograft formed by the implantation of the SW620/AD300 cells are resistant to doxorubicin treatment, the combination of rhMG53 and doxorubicin could inhibit their tumor growth and progression. rhMG53 in combination with doxorubicin did not produce significant hematological toxicity or weight loss to the animals during the study period, suggesting the safe nature of rhMG53 in the mouse model of colorectal carcinoma. However, the safety profile of the combination of rhMG53 and doxorubicin is yet to be established in large animal models and humans in future studies.
Conclusions
In conclusion, our results show that rhMG53 inhibits proliferation of both drug-sensitive SW620 and doxorubicin resistant ABCB1 overexpressing SW620/AD300 cells, without altering the expression of ABCB1 protein. As evidenced by our in-vivo results, the combination of rhMG53 and doxorubicin attenuates the growth and volume of parental and ABCB1 overexpressing tumors. The combination of rhMG53 and doxorubicin presents a potential new regimen to treat cancer patients that are both susceptible and resistant to chemotherapy due to ABCB1.
Abbreviations
MDR: multidrug resistance; ABCB1: ATP-binding cassette subfamily B member 1; USFDA: US Food and Drug Administration; ABC: ATP-binding cassette; Pgp: P-glycoprotein 1; FBS: fetal bovine serum; DMSO: dimethyl sulfoxie; CO2: carbon dioxide; BSA: bovine serum albumin; WBC: white blood cells.
Supplementary Material
Supplementary figure and table.
Acknowledgements
The authors are thankful to Drs. Susan E. Bates and Robert Robey (NIH, MD) for providing the SW620, SW620/AD300, and S1 cells. The authors are also grateful to Dr. Shin-Ichi Akiyama (Kagoshima University, Japan) for providing the KB-3-1 and KB-C2 cells.
Availability of data and materials
The data and materials supporting the conclusions of this study are included within the article and its additional files.
Funding
This work was supported by the St. John's University Research Seed Grant (No. 579-1110-7002) for Zhe-Sheng Chen and was supported by the National Institute of Health (RO1-AR070752) to Jianjie Ma. We thank the partial support from Department of Pharmaceutical Sciences, College of Pharmacy and Health Sciences, St. John's University.
Author contributions
Pranav Gupta, Haichang Li, Jianjie Ma, and Zhe-Sheng Chen designed the study. Pranav Gupta, Haichang Li, Guan-Nan Zhang, Anna Maria Barbuti, Yuqi Yang1, Pei-hui Lin, and Chuanxi Cai performed the experiments. Pranav Gupta, Tao Tan, Jianjie Ma, and Zhe-Sheng Chen analyzed the data. Tao Tan, Jianjie Ma, and Zhe-Sheng Chen provided the resources. Pranav Gupta, Tao Tan, Jianjie Ma, and Zhe-Sheng Chen wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Competing Interests
Tao Tan and Jianjie Ma hold equity interest in TRIM-edicine, Inc., a university spin-off biotechnology company that develops MG53 for regenerative medicine application. Intellectual properties related to MG53 were licensed by Rutgers University and Ohio State Innovation Foundation.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7-30
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7-30
3. Anreddy N, Gupta P, Kathawala RJ, Patel A, Wurpel JN, Chen ZS. Tyrosine kinase inhibitors as reversal agents for ABC transporter mediated drug resistance. Molecules. 2014;19:13848-77
4. Kathawala RJ, Gupta P, Ashby CR, Chen ZS. The modulation of ABC transporter-mediated multidrug resistance in cancer: a review of the past decade. Drug Resist Updat. 2015;18:1-17
5. Zhang YK, Wang YJ, Gupta P, Chen ZS. Multidrug Resistance Proteins (MRPs) and Cancer Therapy. AAPS J. 2015;17:802-12
6. Zeng L, Gupta P, Chen Y, Wang E, Ji L, Chao H. et al. The development of anticancer ruthenium(ii) complexes: from single molecule compounds to nanomaterials. Chem Soc Rev. 2017;46:5771-804
7. Ashar YV, Zhou J, Gupta P, Teng QX, Lei ZN, Reznik SE. et al. BMS-599626, a Highly Selective Pan-HER Kinase Inhibitor, Antagonizes ABCG2-Mediated Drug Resistance. Cancers (Basel). 2020 12
8. Housman G, Byler S, Heerboth S, Lapinska K, Longacre M, Snyder N. et al. Drug resistance in cancer: an overview. Cancers (Basel). 2014;6:1769-92
9. Gottesman MM, Ambudkar SV. Overview: ABC transporters and human disease. J Bioenerg Biomembr. 2001;33:453-8
10. Beretta GL, Cassinelli G, Pennati M, Zuco V, Gatti L. Overcoming ABC transporter-mediated multidrug resistance: The dual role of tyrosine kinase inhibitors as multitargeting agents. Eur J Med Chem. 2017;142:271-89
11. Anreddy N, Patel A, Zhang YK, Wang YJ, Shukla S, Kathawala RJ. et al. A-803467, a tetrodotoxin-resistant sodium channel blocker, modulates ABCG2-mediated MDR in vitro and in vivo. Oncotarget. 2015;6:39276-91
12. Zhang XY, Zhang YK, Wang YJ, Gupta P, Zeng L, Xu M. et al. Osimertinib (AZD9291), a Mutant-Selective EGFR Inhibitor, Reverses ABCB1-Mediated Drug Resistance in Cancer Cells. Molecules. 2016 21
13. Gao HL, Gupta P, Cui Q, Ashar YV, Wu ZX, Zeng L. et al. Sapitinib Reverses Anticancer Drug Resistance in Colon Cancer Cells Overexpressing the ABCB1 Transporter. Front Oncol. 2020;10:574861
14. Dean M, Hamon Y, Chimini G. The human ATP-binding cassette (ABC) transporter superfamily. J Lipid Res. 2001;42:1007-17
15. Wen Y, Zhao RQ, Zhang YK, Gupta P, Fu LX, Tang AZ. et al. Effect of Y6, an epigallocatechin gallate derivative, on reversing doxorubicin drug resistance in human hepatocellular carcinoma cells. Oncotarget. 2017;8:29760-70
16. Alam A, Kowal J, Broude E, Roninson I, Locher KP. Structural insight into substrate and inhibitor discrimination by human P-glycoprotein. Science. 2019;363:753-6
17. Gottesman MM. Mechanisms of cancer drug resistance. Annu Rev Med. 2002;53:615-27
18. Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG. Cancer drug resistance: an evolving paradigm. Nat Rev Cancer. 2013;13:714-26
19. Li W, Zhang H, Assaraf YG, Zhao K, Xu X, Xie J. et al. Overcoming ABC transporter-mediated multidrug resistance: Molecular mechanisms and novel therapeutic drug strategies. Drug Resist Updat. 2016;27:14-29
20. Yang GJ, Wang W, Lei PM, Leung CH, Ma DL. A 7-methoxybicoumarin derivative selectively inhibits BRD4 BD2 for anti-melanoma therapy. Int J Biol Macromol. 2020;164:3204-20
21. Beeran AA, Udupa N, Maliyakkal N. The Dichloromethane Fraction of Vernonia cinerea Impart Pro-Apoptotic, Genotoxic, Cell Cycle Arrest, and Drug Efflux Inhibitory Effects on Human Adenocarcinoma Cells. Recent Pat Anticancer Drug Discov. 2020;15:239-56
22. Yang GJ, Wang W, Mok SWF, Wu C, Law BYK, Miao XM. et al. Selective Inhibition of Lysine-Specific Demethylase 5A (KDM5A) Using a Rhodium(III) Complex for Triple-Negative Breast Cancer Therapy. Angew Chem Int Ed Engl. 2018;57:13091-5
23. Weisleder N, Takeshima H, Ma J. Immuno-proteomic approach to excitation-contraction coupling in skeletal and cardiac muscle: molecular insights revealed by the mitsugumins. Cell Calcium. 2008;43:1-8
24. Cai C, Masumiya H, Weisleder N, Matsuda N, Nishi M, Hwang M. et al. MG53 nucleates assembly of cell membrane repair machinery. Nat Cell Biol. 2009;11:56-64
25. Weisleder N, Takizawa N, Lin P, Wang X, Cao C, Zhang Y. et al. Recombinant MG53 protein modulates therapeutic cell membrane repair in treatment of muscular dystrophy. Science translational medicine. 2012;4:139ra85
26. Li Z, Wang L, Yue H, Whitson BA, Haggard E, Xu X. et al. MG53, A Tissue Repair Protein with Broad Applications in Regenerative Medicine. Cells. 2021 10
27. Jia Y, Chen K, Lin P, Lieber G, Nishi M, Yan R. et al. Treatment of acute lung injury by targeting MG53-mediated cell membrane repair. Nature communications. 2014;5:4387
28. Duann P, Li H, Lin P, Tan T, Wang Z, Chen K. et al. MG53-mediated cell membrane repair protects against acute kidney injury. Science translational medicine. 2015;7:279ra36
29. Li H, Duann P, Lin PH, Zhao L, Fan Z, Tan T. et al. Modulation of wound healing and scar formation by MG53 protein-mediated cell membrane repair. J Biol Chem. 2015;290:24592-603
30. Liu J, Zhu H, Zheng Y, Xu Z, Li L, Tan T. et al. Cardioprotection of recombinant human MG53 protein in a porcine model of ischemia and reperfusion injury. J Mol Cell Cardiol. 2015;80:10-9
31. Chen S, Sanjana NE, Zheng K, Shalem O, Lee K, Shi X. et al. Genome-wide CRISPR screen in a mouse model of tumor growth and metastasis. Cell. 2015;160:1246-60
32. Chow RD, Wang GC, Ye LP, Codina A, Kim HR, Shen L. et al. In vivo profiling of metastatic double knockouts through CRISPR-Cpf1 screens. Nat Methods. 2019;16:405 -+
33. Fernández-Aceñero MJ, Cruz M, Sastre-Varela J, Casal JI, Nieto MAC, Del Puerto-Nevado L. et al. TRIM72 Immunohistochemical Expression Can Predict Relapse in Colorectal Carcinoma. Pathol Oncol Res. 2020;26:861-5
34. Siegel RL, Miller KD, Goding Sauer A, Fedewa SA, Butterly LF, Anderson JC. et al. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70:145-64
35. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71:7-33
36. Gupta P, Zhang YK, Zhang XY, Wang YJ, Lu KW, Hall T. et al. Voruciclib, a Potent CDK4/6 Inhibitor, Antagonizes ABCB1 and ABCG2-Mediated Multi-Drug Resistance in Cancer Cells. Cell Physiol Biochem. 2018;45:1515-28
37. Gupta P, Kathawala RJ, Wei L, Wang F, Wang X, Druker BJ. et al. PBA2, a novel inhibitor of imatinib-resistant BCR-ABL T315I mutation in chronic myeloid leukemia. Cancer Lett. 2016;383:220-9
38. Gupta P, Kumar RV, Kwon CH, Chen ZS. Synthesis and anticancer evaluation of sulfur containing 9-anilinoacridines. Recent Pat Anticancer Drug Discov. 2021
39. De Vera AA, Gupta P, Lei Z, Liao D, Narayanan S, Teng Q. et al. Immuno-oncology agent IPI-549 is a modulator of P-glycoprotein (P-gp, MDR1, ABCB1)-mediated multidrug resistance (MDR) in cancer: In vitro and in vivo. Cancer Lett. 2018;442:91-103
40. Wang YJ, Zhang YK, Zhang GN, Al Rihani SB, Wei MN, Gupta P. et al. Regorafenib overcomes chemotherapeutic multidrug resistance mediated by ABCB1 transporter in colorectal cancer: In vitro and in vivo study. Cancer Lett. 2017;396:145-54
41. Zhang GN, Zhang YK, Wang YJ, Gupta P, Ashby CR, Alqahtani S. et al. Epidermal growth factor receptor (EGFR) inhibitor PD153035 reverses ABCG2-mediated multidrug resistance in non-small cell lung cancer: In vitro and in vivo. Cancer Lett. 2018;424:19-29
42. Tiwari AK, Sodani K, Wang SR, Kuang YH, Ashby CR, Chen X. et al. Nilotinib (AMN107, Tasigna) reverses multidrug resistance by inhibiting the activity of the ABCB1/Pgp and ABCG2/BCRP/MXR transporters. Biochem Pharmacol. 2009;78:153-61
43. Ji N, Yang Y, Cai CY, Wang JQ, Lei ZN, Wu ZX. et al. Midostaurin Reverses ABCB1-Mediated Multidrug Resistance, an in vitro Study. Front Oncol. 2019;9:514
44. Liao D, Zhang W, Gupta P, Lei ZN, Wang JQ, Cai CY. et al. Tetrandrine Interaction with ABCB1 Reverses Multidrug Resistance in Cancer Cells Through Competition with Anti-Cancer Drugs Followed by Downregulation of ABCB1 Expression. Molecules. 2019 24
45. Yang Y, Ji N, Cai CY, Wang JQ, Lei ZN, Teng QX. et al. Modulating the function of ABCB1: in vitro and in vivo characterization of sitravatinib, a tyrosine kinase inhibitor. Cancer Commun (Lond). 2020;40:285-300
46. Tachiwada T, Chen ZS, Che XF, Matsumoto M, Haraguchi M, Gotanda T. et al. Isolation and characterization of arsenite-resistant human epidermoid carcinoma KB cells. Oncol Rep. 2007;18:721-7
47. Mukai M, Kanzaki A, Chen ZS, Miyashita H, Sumizawa T, Furukawa T. et al. Enhanced nucleotide excision repair in cisplatin resistant human KB carcinoma cells. Oncol Rep. 2002;9:839-44
48. Li H, Lin PH, Gupta P, Li X, Zhao SL, Zhou X. et al. MG53 suppresses tumor progression and stress granule formation by modulating G3BP2 activity in non-small cell lung cancer. Mol Cancer. 2021;20:118
49. Wang YJ, Huang Y, Anreddy N, Zhang GN, Zhang YK, Xie M. et al. Tea nanoparticle, a safe and biocompatible nanocarrier, greatly potentiates the anticancer activity of doxorubicin. Oncotarget. 2016;7:5877-91
Author contact
![]() Corresponding authors: Zhe-Sheng Chen, MD, PhD, Professor, Department of Pharmaceutical Sciences, Director, Institute for Biotechnology, College of Pharmacy and Health Sciences, St. John's University, Queens, NY, 11439, USA. Email: chenzedu, Phone: 1-718-990-1432, Fax: 1-718-990-1877. Haichang Li, PhD, Department of Surgery, The Ohio State University Wexner Medical Center, Columbus, OH 43210, USA. Email: Haichang.liedu, Phone: 1-614-292-3012.
Corresponding authors: Zhe-Sheng Chen, MD, PhD, Professor, Department of Pharmaceutical Sciences, Director, Institute for Biotechnology, College of Pharmacy and Health Sciences, St. John's University, Queens, NY, 11439, USA. Email: chenzedu, Phone: 1-718-990-1432, Fax: 1-718-990-1877. Haichang Li, PhD, Department of Surgery, The Ohio State University Wexner Medical Center, Columbus, OH 43210, USA. Email: Haichang.liedu, Phone: 1-614-292-3012.

 Global reach, higher impact
Global reach, higher impact