10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2022; 18(14):5369-5390. doi:10.7150/ijbs.73949 This issue Cite
Review
Tumor Microenvironment and its Implications for Antitumor Immunity in Cholangiocarcinoma: Future Perspectives for Novel Therapies
1. Hepatobiliary/Liver Transplantation Center, The First Affiliated Hospital of Nanjing Medical University, Key Laboratory of Living Donor Transplantation, Chinese Academy of Medical Sciences, Nanjing, Jiangsu, China.
2. Stomatological college of Nanjing Medical University, Nanjing, Jiangsu, China.
3. Department of General Surgery, Nanjing First Hospital, Nanjing Medical University, Nanjing, Jiangsu, China.
4. Department of Anesthesiology, The First Affiliated Hospital of Nanjing Medical University, Nanjing, Jiangsu, China.
5. Department of Gastroenterology, The First Affiliated Hospital of Nanjing Medical University,Nanjing, Jiangsu, China.
*Indicates first authors.
Received 2022-4-13; Accepted 2022-8-8; Published 2022-8-21
Abstract
The incidence of cholangiocarcinoma (CCA) has been increasing over the past few years. Although there are surgery, chemotherapy and other conventional treatment methods, the effect is not as expected. At present, immunotherapy has become the research frontier of cancer treatment, and CCA tumor microenvironment (TME) is becoming a hot exploration direction of immunobiology. TME can affect tumor progression through changes in metabolism, secretion and immunity. Accordingly, understanding the role played by immune cells and stromal cells in TME is important for the study of CCA immunotherapy. This review will discuss the interactions between immune cells (including CD8+ T cells, CD4+ T cells, macrophages, natural killer cells, dendritic cells, myeloid suppressor cells, mast cells, and neutrophils) and stromal cells (including cancer-associated fibroblasts, endothelial cells) in the TME of CCA. In addition, we will also discuss current research results on TME of CCA and recent advances in immunotherapy.
Keywords: cholangiocarcinoma, tumor microenvironment, immune mechanism, targeted therapy, prognostic markers, immunotherapy
Introduction
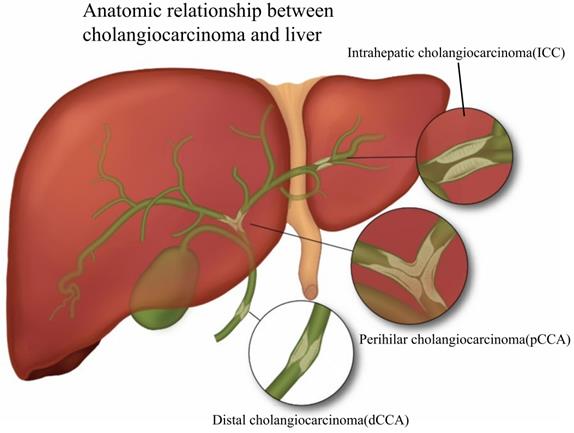
Cholangiocarcinoma (CCA) is an epithelial malignant tumor originating from the biliary tract and is the second most common primary liver malignancy after hepatocellular carcinoma [1]. Based on anatomical site, CCA is divided into intrahepatic cholangiocarcinoma (ICC), perihilar cholangiocarcinoma (pCCA), as well as distal cholangiocarcinoma (dCCA) [2]. Fig. 1 presents the classification of CCA based on its relative location to the liver. ICC is malignant tumors caused by the biliary epithelium, and like hepatocellular carcinoma, ICC is usually classified as primary liver cancer, and in the review, we classified it into CCA. According to the American NCCN guidelines [3], ICC is classified into three types, including mass-forming, periductal-infiltrating, and intraductal-growing. The definition of pCCA has been controversial, and we believe that the location of pCCA is defined as the secondary branch of the hepatic duct from the common bile duct above the cystic duct to the liver, which is histologically a Klatskin tumor. dCCA is uncontroversial in definition, and it is the carcinoma of the distal extrahepatic bile duct [4]. Existing research has suggested that dCCA accounts for approximately 50% of the total number of CCAs, pCCA accounts for nearly 40%, and the rest is ICC [5].
According to the relative anatomical location with the liver, CCA is divided into three types: ICC, pCCA and dCCA. ICC is defined as bile duct cancer located inside the liver, and the histological types are classified as mass-forming, periductal infiltrating, and intraductal growing. pCCA is defined as a secondary branch located in the hepatic duct (from the common bile duct above the cystic duct to the liver) and histologically as a Klatskin tumor. dCCA refers to distal cholangiocarcinoma located extrahepatically, and the histological type is mainly adenocarcinoma.
With the change over time in environmental and immunological factors (e.g., the effects of hepatolithiasis or hepatitis virus infection), the incidence of ICC increases. The reported incidence of ICC in the United States increased from 0.44 cases per 100,000 to 1.18 cases per 100,000, with an annual percentage change (APC) of 2.30%. The above trend has accelerated to 4.36% over the past decade [6]. Early detection and diagnosis of CCA is still a challenge due to its clinical characteristics, such as deep intrahepatic anatomical location, high fibrous hyperplasia, and few tumor cells. Currently, surgery is considered an effective treatment for CCA. For instance, the 5-year survival rate of patients with early CCA after liver transplantation is as high as 65%, but is limited to early nonmetastatic disease [7]. For non-early CCA, recurrence rate after surgical resection exceeds 50% [8]. Besides surgical treatment, the OS (overall survival) of chemotherapy drugs such as GEMCIS is only 11.7 months [9]. Compared with other cancers (e.g., hepatocellular carcinoma), CCA is less effective in chemotherapy and has an unsatisfactory prognosis. Thus, molecular targets and effective therapeutic measures for the treatment of CCA are urgently required.
Tumor microenvironment (TME) has been increasingly reported over the past few years, opening a new approach for immunotherapy targeting CCA. TME is an ever-changing dynamic heterogeneous collection of tumor cells, infiltrating and hosting host cells, secretory factors, and extracellular matrix [10]. It includes not only the structure, function, and metabolism of the tissue where the tumor is located, but also the internal environment of the tumor cell itself (nucleus and cytoplasm), promoting the occurrence and development of the tumor. The TME of CCA is mainly composed of immune-related cells, tumor cells and tumor stromal cells [11]. Thanks to the development of technologies such as single-cell sequencing, research on immune-related cells in CCA TME has promoted the development of CCA immunotherapy. Based on the application of immunosuppressive agents in the clinical treatment of HCC, many immunotherapy clinical trials on CCA are also being carried out successively and have achieved some results. For instance, immunosuppressive agents alone for the treatment of advanced BTC [12] or immunosuppressive agents in combination with chemotherapeutic drugs [13]. A phase II study of lenvatinib plus PD-1 inhibitor as first-line treatment for patients with unresectable BTC showed an encouraging result with mOS (median overall survival) of 17.7 months [14]. In this study, we will review the various components of TME and their mechanisms of action on CCA tumors, and highlight the important role played by TME in immunotherapy for CCA. Fig. 2 contains a schematic representation of the interactions of each component of CCA TME.
CCA TME refers to an intrinsic environment for the interaction between various cells and tumor cells including CD4 + T cells, CD8 + T cells, Tregs, DCs, NK cells, vascular endothelial cells, macrophages, neutrophils, CAFs, mast cells and MDSCs as well as body tissues.
CCA-associated tumor stromal cells in CCA
Cancer is a systemic disease that consists of multiple components of the tumor cells and host stromal cells. It has been proven that stromal cells in the TME play a vital role in cancer development. It is possible to define the molecular events by which reactive stromal cells affect cancer cells and thus to identify biomarkers and therapeutic targets. In general, TME stromal cells comprise endothelial cells in blood and lymph, stem cells, as well as tumor-associated fibroblasts [15]. Vascular endothelial cells and cancer-associated fibroblasts (CAFs) take on a great significance in CCA.
CAFs in CCA
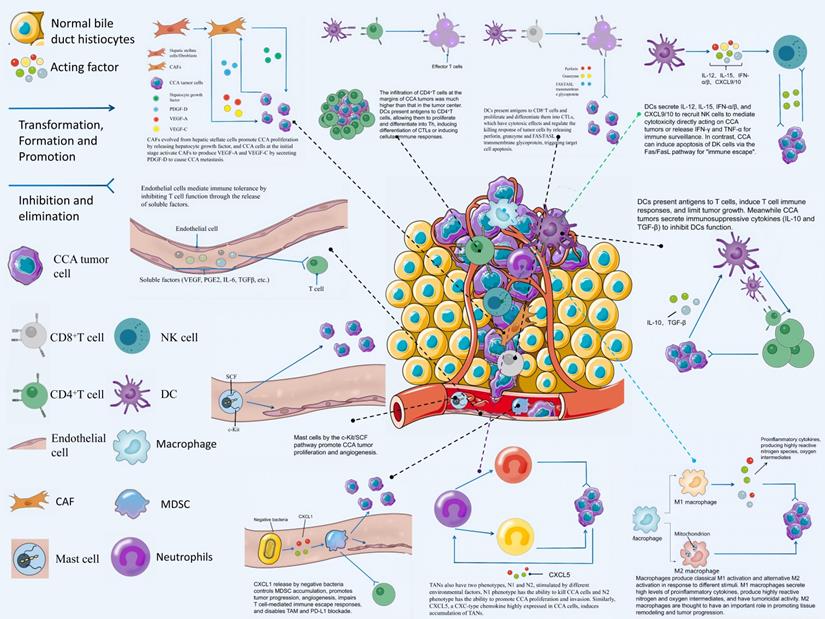
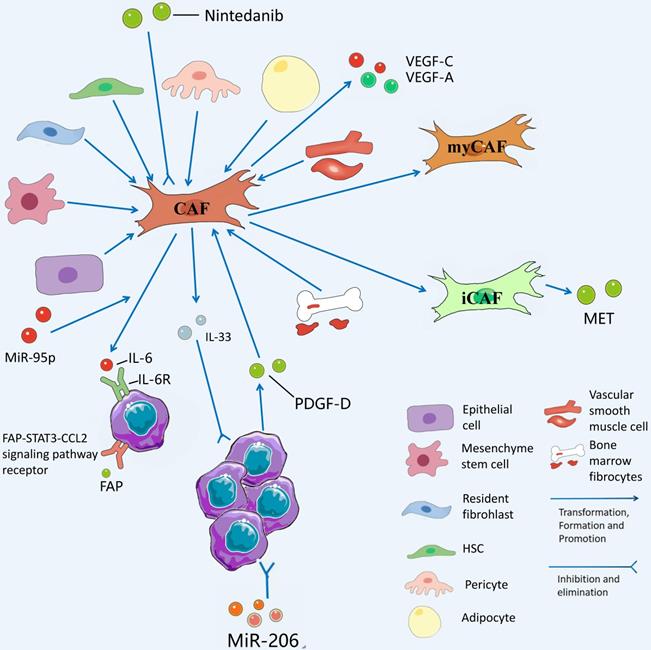
Among the tumor stromal cells involved in CCA TME, CAF mechanisms are the most abundant and essential for driving tumor proliferation, which are investigated in this study. Unlike normal fibroblasts, CAFs are the most abundant source and the major components of tumor stroma and secreted growth factors, inflammatory ligands, and extracellular matrix (ECM) proteins [16]. Thanks to the development of RNA single-cell sequencing technology, we can gain insights into the heterogeneity of CAFs with normal fibroblasts and its mechanism of action. The role played by CAFs in the TME of CCA is not single, the interior of CAFs is a heterogeneous collection of cell populations, and CAFs can either facilitate CCA development or inhibit CCA. CAFs from hepatic stellate cells (HSCs) are capable of mediating the release of hepatocyte growth factor from inflammatory CAFs through the direct interaction of the HSC-CAF-tumor pathway that facilitates the proliferation of ICCs via tumor-expressed MET (mesenchymal to epithelial transition factor) [17]. Zhang et al. have suggested that IL-6 (interleukin-6) secretion by vCAFs (vascular cancer-associated fibroblasts) significantly enhances the malignancy of ICC cells through IL-6/IL-6R axis interaction between CAFs and tumor cells, while exosomal miR-95p derived from ICC cells can induce IL-6 expression in vCAFs [18]. However, high expression of miR-34c, another tumor-derived exosome, can target and inhibit Wnt-1 to activate the Wnt signaling pathway in CCA and inhibit the conversion of fibrocytes into CAFs [19]. It is necessary to conduct in-depth research on the mechanism of action of different exosomes on CAFs in the TME of CCA. At the initial stage of ICC, ICC cells recruit and activate CAF to stimulate fibroblasts to produce VEGF-C and VEGF-A by secreting PDGF-D, resulting in lymphangiectasis and tumor cell injection, which causes tumor metastasis [20]. Another study has suggested that secretion of FAP (fibroblast activation protein) by CAFs activates the FAP-STAT3-CCL2 signaling pathway to facilitate connective tissue proliferation in ICC [21].
CAFs and their secreted products play a role in inhibiting the proliferation of CCA. Some studies have suggested that the prognosis of patients with high IL-33 content in CAFs in CCA patients is significantly better than that of patients with low IL-33 content in CAFs, suggesting that IL-33 is a marker of good prognosis in CCA and strengthening IL-33 in CAFs is a promising therapeutic direction [22]. Moreover, the interaction between CCA and CAFs to facilitate tissue proliferation and tumor metastasis provides a novel entry point for targeting CAFs to treat CCA. For instance, Takahiro et al. suggested that nintedanib is capable of treating refractory ICC by inhibiting the activation of CAFs after the use of nintedanib [23]. It has also been confirmed that MiR-206 inhibits the deterioration of ICC and increases chemosensitivity by inhibiting the interaction with interstitial CAFs [24]. Table 1 lists the promoting and inhibiting effects of CAFs on CCA. Fig. 3 presents the role played by CAFs in CCA TME.
CAFs has diverse sources and is differentiated from epithelial cells, mesenchyme stem cells, resident fibrohlast, HSCs, pericyte, adipocyte, vascular smooth muscle cells, and bone marrow fibrocytes. CAFs from hepatic stellate cells (HSCs) mediate the release of hepatocyte growth factor from inflammatory CAFs through direct interaction of the hsc-cafa-tumor pathway and promote the proliferation of ICC through tumor-expressed MET. VCAFs (vascular carcinoma-associated fibroblasts) secrete IL-6 (interleukin-6) to enhance the malignancy of ICC cells through the interaction of the IL-6/IL-6R axis with tumor cells, while exosomal miR-95p of ICC cells can induce IL-6 expression in vCAFs. High expression of miR-34c in tumor-derived exosomes can target and inhibit Wnt1, allowing it to activate the Wnt signaling pathway in CCA and slow the conversion of fibrocytes to CAFs. Nintedanib can treat refractory CCA by inhibiting the activation of CAFs.
Summary of tumor stromal actions in the TME of CCA
| Cells | The research direction | Result | Reference | Reference number |
|---|---|---|---|---|
| CAF | The immune mechanism | Hepatic stellate cell-derived CAFs are the main tumor-interacting population in ICC. | Affo et al. (2021) | [17] |
| CAF | The immune mechanism | Tumor exosomal miR-9-5p elicited IL-6 expression in vCAFs,thereby leading to epigenetic alterations in ICC. | Zhang et al. (2020) | [18] |
| CAF | Prognostic marker | Cellular senescence, represented by CAV1 levels, may be a marker of CAFs and a prognostic indicator of ICC through FOXP3+ TILs regulation. CAV1 expression in CAFs can be a therapeutic target for ICC. | Lan et al. (2021) | [124] |
| CAF | The immune mechanism | PDGF-D stimulates VEGF-C and VEGF-A production by fibroblasts, resulting in expansion of the lymphatic vasculature and tumor cell intravasation. | Cadamuro et al. (2019) | [20] |
| CAF | Prognostic marker | The ICC patients with immature CAF phenotype had a more aggressive feature and significantly poorer OS than those with mature phenotype. | Zhang et al. (2017) | [125] |
| CAF | The immune mechanism | Downregulation of tumor‐derived exosomal miR-34c induces cancer‐associated fibroblast activation to promote CCA progress. | Qin et al. (2021) | [19] |
| CAF | The immune mechanism | FAP Promotes Immunosuppression by Cancer-Associated Fibroblasts in the TME via STAT3-CCL2 Signaling. | Yang et al. (2016) | [21] |
| CAF | The immune mechanism | CAFs promoted proliferation, mi-gration, and invasion of CCA cells in vitro and boosted tumor growth in vivo. Furthermore, it was demonstrated that stroma enriched with α- SMA- positive CAFs was associated with poor prognosis of patients with ICC. | Sha et al. (2018) | [126] |
| CAF | Immunotherapy | The potential of repeated CAF-targeted PTT for the treatment of desmoplastic CCA after intra-tumoral administration. | Nicolás-Boluda et al. (2020) | [127] |
| CAF | The immune mechanism | IL-6 Secreted by CAFs Inhibits Autophagy and Reduces the Chemosensitivity of CCA Cells. | Thongchot et al. (2021) | [128] |
| CAF | The immune mechanism | ZEB1 plays a key role in CCA progression by regulating tumor cell-CAF crosstalk, leading to tumor dedifferentiation and CAF activation. | Lobe et al. (2021) | [129] |
| CAF | Prognostic marker | FAP overexpression is evident in dCCA. There was a positive association between epithelial FAP expression and better survival. | Byrling et al. (2020) | [130] |
| CAF | The immune mechanism | Treatment with LTB4 that were elevated in CAF-educated MDSCs or blockade of BLT2 that was preferentially expressed in stem-like ICC cells significantly reduced stemness-enhancing effects of CAF-educated MDSCs. | Lin et al. (2022) | [131] |
| CAF | The immune mechanism | To escape EGFR-TKI treatment, CCA tumor cells develop an adaptive mechanism by undergoing an IR/IGF1R-dependent phenotypic switch. | Vaquero et al. (2018) | [132] |
| CAF | immunotherapy | PlGF blockade leads to a reduction in intratumorous hypoxia and metastatic dissemination, enhanced chemotherapy sensitivity and increased survival in mice- bearing aggressive ICC. | Aoki et al. (2021) | [133] |
| CAF | Immunotherapy | Conditioned culture medium from CCA-derived CAFs further stimulated IL-6 secretion in CCA cells and promoted the migration of invasive cholangiocytes, while the nutrient resveratrol strongly counteracted this effect. | Thongchot et al. (2018) | [134] |
| CAF | Prognostic marker | High level of interleukin-33 in cancer cells and cancer-associated fibroblasts correlates with good prognosis and suppressed migration in CCA. | Yangngam et al. (2020) | [22] |
| CAF | The immune mechanism | Fibroblast growth factor receptor inhibition induces loss of matrix MCL1 and necrosis in CCA. | Kabashima et al. (2018) | [135] |
| CAF | The immune mechanism | CCA Cells Secreting PDGF-D Strongly Stimulate Fibroblast Recruitment, an Effect That Is Significantly Reduced by PDGFRb Antagonism and by PDGF-D siRNA. | Cadamuro et al. (2013) | [136] |
| CAF | Immunotherapy | MiR-206 suppresses the deterioration of intrahepatic CCA and promotes sensitivity to chemo-therapy by inhibiting interactions with stromal CAFs. | Yang et al. (2022) | [24] |
| CAF | The immune mechanism | The microRNA-15a-PAI-2 axis in CCA-associated fibroblasts promotes migration of cancer cells. | Utaijaratrasmi et al. (2018) | [137] |
| CAF | Immunotherapy | Nintedanib inhibited the cancer-promoting effect of CAFs via the suppression of CAF activation and secretion of cancer-promoting cytokines. | Yamanaka et al. (2020) | [23] |
| CAF | Immunotherapy | Navitoclax treatment triggered CAF apoptosis, diminishing expression of the desmoplastic extracellular matrix protein tenascin C, suppressing tumor outgrowth, and improving host survival. | Mertens et al. (2013) | [138] |
| Endothelial cell | immunotherapy | Anti-Glypican-1 Antibody-drug Conjugate targets CCA cells and GPC1 in vascular endothelial cells, and directly or indirectly inhibits CCA growth by inhibiting tumor angiogenesis. | Yokota et al. (2021) | [37] |
| Endothelial cell | Prognostic marker | CD105 is a tumor-associated endothelial cell marker. High expression of CD105 was independently associated with poor survival in patients with CCA. | Nair et al. (2020) | [38] |
| Endothelial cell | The immune mechanism | Plasmalemma vesicle-associated protein (PLVAP) is associated with angiogenesis in CCA. DKK1 secreted by CCA cells promotes tumor angiogenesis through the DKK1/CKAP4/PI3K/PLVAP pathway. | Wang et al. (2021) | [28] |
| Endothelial cell | The immune mechanism | ERK5 is highly expressed in human CCA cells, regulates the content of VEGF and angiopoietin 1 in the tumor microenvironment, and induces angiogenesis in tumor-associated endothelial cells. | Gentilini et al. (2021) | [30] |
| Endothelial cell | The immune mechanism | Circ-CCAC1 of CCA-derived EVs was transferred into CCA vascular endothelial monolayer cells, disrupting endothelial barrier integrity and inducing angiogenesis. | Xu et al. (2021) | [31] |
| Endothelial cell | The immune mechanism | Pcca cell-derived HMGB1 upregulates VEGFR2 expression in vascular endothelial cells and induces ectopic angiogenesis by in vitro and in vivo experiments. | Xu et al. (2019) | [29] |
| Endothelial cell | The immune mechanism | The secretion of PDGF-D by CCA cells resulted in increased levels of VEGF-C and VEGF-A secreted by fibroblasts and increased endothelial cell permeability. | Cadamuro et al. (2019) | [20] |
| Endothelial cell | The immune mechanism | TCF21 was decreased in CCA tissues or cell lines compared to normal tissues. TCF21 exerts anti-angiogenic activity through PI3K/Akt and ERK1/2 signaling pathways. | Duan et al. (2019) | [36] |
| Endothelial cell | The immune mechanism | THBS1, THBS2 and PEDF are up-regulated in the ICC microenvironment. The ability of THBS1, THBS2 and PEDF to inhibit endothelial cell angiogenesis was demonstrated by in vivo experiments. | Carpino et al. (2021) | [139] |
| Endothelial cell | The immune mechanism | eNOS is upregulated in CCA tissues and their cell lines, promoting angiogenesis and metastasis in CCA. | Suksawat et al. (2017) | [26] |
Endothelial cells in CCA
Tumor blood vessels primarily comprise endothelial cells, which are critical to cancer progression and provide essential nutrients for tumor growth. Tumor-associated endothelial cells can facilitate tumor progression and metastasis [25]. eNOS (endothelial Nitric Oxide Lyase) expressed in endothelial cells significantly affects the metastasis and angiogenesis of CCA[26]. In addition, endothelial cells mediate immune tolerance through the unbalanced expression of surface molecules and the release of soluble factors (e.g., VEGF, PGE-2, IL-6, TGF-β) that inhibit T cell function [27]. In the tumor microenvironment, CCA cells DKK1 (paracrine dickkopf-related protein 1) to enhance the angiogenic potential of tumor-associated endothelial cells [28]. VEGFR1 and VEGFR2 are critical receptors in the process of angiogenesis. Xu et al. suggested that HMGB-1 (high mobility group box 1) released by perihilar CCA cells is capable of up-regulating the expression of VEGFR2 in vascular endothelial cells [29]. ERK5 silencing down-regulates the expression of angiopoietin-1 and VEGF in the CCA tumor microenvironment to induce angiogenesis, which reduces the ability of HUVEC (human umbilical veins) [30]. Circ-CCAC1 in CCA-derived extracellular vesicles can disrupt endothelial cell barrier integrity and induce angiogenesis in tumor tissues [31]. Besides the formation of blood vessels, endothelial cells also play a role in the formation of lymphatic vessels in tumor tissues. Cadamuro et al. suggested that PDGF-D from ICC recruits lymphatic endothelial cells (LECs) to aggregate by stimulating paracrine signals produced by CAFs to facilitate the formation of tumor-associated lymphatic vessels [20]. Therefore, the current research hotspot is to explore the targets and methods of inhibiting tumor angiogenesis. Ramucirumab is a fully human immunoglobulin G1 monoclonal antibody targeting VEGFR-2 [32], and it has been confirmed as a novel second-line treatment for gastric adenocarcinoma and non-small cell lung adenocarcinoma [33, 34]. In BTC, a clinical study of ramucirumab combined with pembrolizumab involving 26 samples suggested that although there is no significant survival improvement, the OS of PD-L1-positive patients is 11.3 months, which lays the foundation for the next study on how to develop immunotherapy regimens from the Endothelial cell direction [35]. Existing research has suggested that TCF21 is capable of inhibiting tumor-associated angiogenesis via PI3K/Akt and ERK1/2 signaling pathways [36]. Yokota et al. suggested that GPC1 (glypican-1) is significantly related to the clinical prognosis of CCA patients, and it is significantly expressed in CCA cells and vascular endothelial cells in CCA tissue. Thus, targeting GPC1 by ADC (Antibody-drug conjugates) can effectively inhibit the development of CCA. Grow [37]. CD105 is highly expressed in vascular endothelial cells of pCCA, and it is an independent poor prognostic factor, which can serve as a feasible target for clinical treatment [38]. Table 1 lists the role played by endothelial cells in the TME of CCA.
Advances of Immune cells in CCA
The immune environment in the TME comprises different immune cell types, and different immune cells play different roles [39]. To be specific, effector cells perform cell killing function, both in innate and adaptive immune responses. Antitumor cells in the adaptive immune response consist of CD4 + and CD8+ Teff cells, killing cancer cells by different mechanisms. There are also immunosuppressive cell populations in the TME (e.g., CD4+FOXP3+Tregs, myeloid-suppressor cells (MDSCs), macrophages, as well as some B cells). Antigen-presenting cells (e.g., intratumoral dendritic cells (DCs)) play a vital role in maintaining adaptive immune responses in the TME. The role played by immune cells in the tumor microenvironment can be either inhibition of tumor formation (antitumor microenvironment) or promotion of tumorigenesis (immunosuppressive microenvironment), which is dependent on contexts and tumor types.
Adaptive immune response
Adaptive immune response is the whole process of autoactivation, proliferation and differentiation of antigen-specific T/B lymphocytes into effector cells after antigen stimulation in vivo, producing a series of biological effects.
CD8+ T cells in CCA
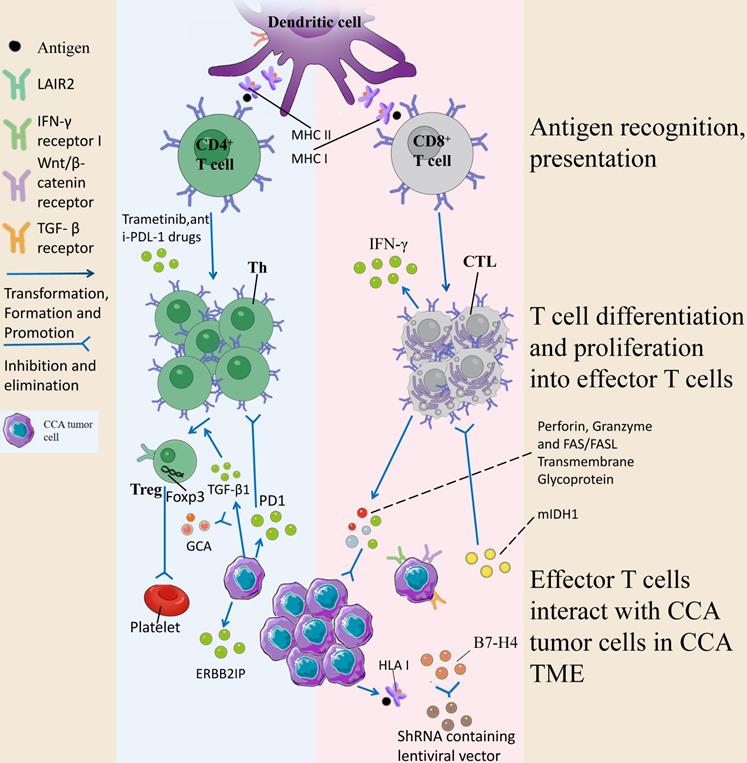
CD8+ T cells are expressed in 30%-35% T cells, recognize antigenic peptides composed of 8-10 amino acid residues, differentiate into cytotoxic T lymphocytes (CTLs) after activation, and have cytotoxic effects. They regulate the killing response of tumor cells by releasing perforin, granzyme and FAS/FASL transmembrane glycoprotein, trigger target cell apoptosis, and specifically kill target cells. In the TME, CTLs play an important role in the development of tumors, and the risk profile based on CD8+ T cell status is significantly related to CCA tumor status. It has been demonstrated that tumor-associated neutrophils are negatively related to CD8+ T cells, while low CD8 + T cells are significantly related to low OS [40]. Wu et al. suggested that isocitrate dehydrogenase 1 mutations (mIDH1) promotes TET2 dependent induction of interferon γ (IFN-γ)-responsive genes in tumor cells by inhibiting the recruitment of stimulated CD8+ T cells and the expression of IFN-γ. CD8+ T cell depletion or tumor cell-specific ablation of IFN-γ receptor 1 thus causes improved tumor resistance. However, immune checkpoint activation limits the efficacy of mIDH1 inhibitors and restores the normal recruitment of CD8+ T cells thereby reducing tumor drug resistance and improving therapeutic efficacy [41]. At the same time, the potential of CD8+ T lymphocytes as a prognostic marker for CCA has also been pointed out, and a significant increase in CD8+ T cell density, in the lymphoepithelial subtype of Epstein-Barr virus-associated CCA, is significantly related to a favorable outcome in ICC [42]. Asahi et al. suggested that the number of CD8+ T cells in the outer edge of the tumor increased and then the number of HLA class I increased, and the role played by HLA class I expression was to present tumor antigen-derived peptides to the immune system, ultimately stimulating CD8+ T cells to show anti-tumor effects and optimize the prognosis of ICC [43].
Numerous studies on immune mechanisms of action and prognostic factors have paved the way for the use of CD8+ T cells for CCA treatment. Existing research has suggested the positive relationships between PD-L1 (programmed cell death ligand 1) expression, CD8+ tumor-infiltrating lymphocytes (TILs) infiltration, T cell inflammatory gene feature expression and estimated CD8 + TIL abundance in ICC tumor cells, while the characteristic genes of Wnt/β-catenin and TGF-β signaling pathways are enriched in tumors with a low proportion of CD69+ CD103+ tissue-resident memory-like CD8+ TIL. The above results reveal that the proliferation and recovery potential of CD69+ CD103+ double-positive, coupled with the expression of checkpoint receptors, can induce CD8 + T cell anti-tumor response and play a certain role in the treatment of ICC [44]. B7-H4, or PD-L1, is a negative regulator of T cell responses, and it is expressed in different human tumors (e.g., breast and gastric cancers) [45, 46]. Existing research has suggested that B7-H4 in CCA may play a negative regulatory role in T cells by inhibiting the recruitment or survival of lymphocytes in the TME, thus reducing the total number of infiltrating lymphocytes in the tumor stroma, especially CD8+ TILs. When the expression of B7-H4 is inhibited by the lentiviral vector encoding shRNA, the decrease of B7-H4 in tumor cells can be directly observed, and stable tumor cell lines with low expression of B7-H4 can be screened, and CD8 + T cell-mediated cytotoxicity is increased, providing a new idea for the treatment of CCA [47]. Table 2 summarizes the current research on CD8+ T cells in CCA and confirms the importance of CD8+ T cells in the immune mechanism, prognostic factors and anti-CCA treatment of CCA.
CD4+ T cells in CCA
CD4+ T cells are a collective term for a class of mature T cells expressing CD4, which are suggested in 60% of T cells and some NKT cells, recognize CD4 antigenic peptides composed of 13- 17 amino acid residues, and after activation, differentiate into Th cells, while some CD4+ effector T cells have cytotoxic and immunosuppressive effects. CD4+ T cell infiltration was present in the interface area around the tumor in the liver surrounding the CCA tumor tissue, while CD8+ T cell infiltration was present inside the tumor. This finding was consistent with HCC T cell infiltration. This is independent of their histogenetic origin, but they are two distinct immune functions, suggesting that even lymphocytes of the same origin may have different functions in these tumor areas [48]. Existing research has suggested that in patients with advanced local CCA, the infiltration of immune cells comprising CD4+ lymphocytes and other immune cells significantly increases compared with metastatic lesions. The above change in TME infiltration is primarily related to microvascular density (MVD) proliferation, and this proliferation is negatively related to CD4+ T cells [49]. Similarly, Kida et al. analyzed peripheral blood samples from 41 patients with CCA and 10 healthy volunteers through flow cytometry. They suggested that t cells and immune checkpoint markers are enriched at the tumor margin compared with the tumor center, whereas a higher frequency of CD4+ T cells is an essential factor for the recurrence of CCA [50]. It is necessary to conduct in-depth research on the relationship between CD4+T cells accumulation at the tumor margin of CCA and the prognosis. T cells and immune checkpoint markers are enriched at the tumor margin in comparison with the tumor center, and Carapeto et al. suggested that high PD-1 or CD4-inducible T cell costimulatory factors in lymphocyte-activated tumor centers contribute to poor survival in patients with ICC [51]. A study on hepatobiliary tumors treated with hypofractionated proton therapy (HPT) radioactivity has suggested that overall survival (OS) is significantly related to CD4+ CD25+ T cell and CD4+ CD127+ T cell fractions in intrahepatic ICC, which reveals that CD4+ T cell and its related cytokines may be vital factors for the prognosis of CCA [52].
Summary of the role of CD8 + T Cells, CD4 + T Cells and Tregs in the TME of CCA
| Cells | The research direction | Result | Reference | Reference number |
|---|---|---|---|---|
| CD8+ T Cell | Prognostic marker | The LEL subtype of EBV aICC, which had a significantly increased density of CD8+T cells, was significantly related to favorable outcome in ICC. | Huang et al. (2021) | [42] |
| CD8+ T Cell | Prognostic marker | Higher number of CD8+T cells in the outer boundary area, means lower number of HLA class I antigen value and predicts better prognosis in ICC patients. | Asahi et al. (2020) | [43] |
| CD8+T Cell | Prognostic marker | The density of CD8+ T-cells in the tumor and stroma correlated with OS and DFS. The number of CD8-positive TILs was significant independent risk factors for a poorer prognosis. | Deng et al. (2021) | [140] |
| CD8+T Cell | Prognostic marker | CD8+ TILs levels were significantly correlated with low LCR (lymphocyte-C-reactive protein ratio), while low LCR was significantly correlated with age, high crp-albumin ratio, and advanced disease, which is an indicator of postoperative prognosis in ICC patients. | Miyazaki et al. (2021) | [141] |
| CD8+T Cell | Prognostic marker | eCCA associated neutrophils were inversely correlated with CD8 + T cells. | Kitano et al. (2018) | [40] |
| CD8+T Cell | Prognostic marker | TILs are associated with prognosis of ICC patients after complete surgery. CD3+ and CD8+ infiltrate is associated with higher survival and lower recurrence risk. | Vigano et al. (2019) | [142] |
| CD8+T Cell | The immune mechanism | The expression of B7-H4 serves a role in shielding tumors from immune surveillance by suppression of tumor-infiltrating CD8+ T lymphocytes in CCA. | Zhao et al. (2016) | [47] |
| CD8+T Cell | The immune mechanism | The mIDH1 supports CCA tumor maintenance through suppressing CD8 + T cell activity, Immune checkpoint activation can inhibit the effect. | Wu et al. (2022) | [41] |
| CD8+T Cell | Immunotherapy | CD69+CD103+TRM-like CD8+TILs represent prominent tumour-specific immune responses and hold promise as a potential therapeutic target in ICC patients. | Kim et al. (2021) | [44] |
| CD4+T Cell | Immunotherapy | TILs from a patient with metastatic CCA contained CD4+ Th1 cells recognizing a mutation in ERBB2IP expressed by the cancer reduces target lesions and prolongs disease stability | Tran et al. (2014) | [53] |
| CD4+T Cell | Immunotherapy | The combination of Trametinib and PD-L1 can lead to enhanced cytotoxicity of hepatic effector memory CD4 + T cells, reduced tumor burden, and improved survival in tumor-bearing mice. | Wabitsch et al. (2021) | [54] |
| CD4+T Cell | The immune mechanism | In patients with advanced local CCA, the infiltration of CD4+ lymphocytes and CD8+ lymphocytes, macrophages, and mast cells was significantly increased compared with metastatic lesions, and this change in TME infiltration was mainly associated with MVD proliferation, and this proliferation was negatively correlated with CD8+ and CD4+ cells. | Tamma et al. (2019) | [49] |
| CD4+T Cell | The immune mechanism | T-cell and immune checkpoint markers are enriched at the tumor margins compared to the tumor center. high PD-1 or lymphocyte-activation gene 3 and low CD3/CD4/inducible T-cell costimulator specifically in the tumor center as associated with poor survival. | Carapeto et al. (2022) | [51] |
| CD4+T Cell | The immune mechanism | The percentage of CD4+CD45RO+ T cells producing IL-22 what significantly higher in liver fluke-infected patients than in healthy controls or even CCA patients without liver fluke infection. In samples was significantly higher in patients with CCA than in patients without it, and the percentages in these two groups were significantly higher than that in controls. | Su et al. (2017) | [143] |
| CD4+T Cell | The immune mechanism | CCAs contained a heterogeneous amount of TILs, composed mainly of CD3+ T cells with a predominance of CD8+ cells in the tumor tissue and of CD4+ cells in the interface region. | Kasper et al. (2009) | [48] |
| CD4+T Cell | Prognostic marker | T cells and immune checkpoint markers were enriched at the tumor margin compared to the tumor center. At the same time, a higher frequency of CD4 + T cells is an important factor in CCA recurrence. | Kida et al. (2021) | [50] |
| CD4+T Cell | Prognostic marker | Longer OS was significantly correlated with greater CD4+CD25+ T cell and CD4+CD127+ T cell fractions at baseline only in ICC patients.This findings suggest a differential relevance of immuno-modulation by HPT in these liver cancers. | Grassberger et al. (2018) | [52] |
| Treg | Prognostic marker | The density of FOXP3+ CD4+ regulatory T cells was higher in eCCA regardless of the tumor site is a key metric associated with clinical outcomes in BTC patients. | Kim et al. (2021) | [58] |
| Treg | Prognostic marker | LAIR2 is expressed by Tregs and some GZMB + CD8+ T cells, is associated with survival, and has increased expression in tumor tissues, which can be used as a prognostic marker for CCA. | Chen et al. (2021) | [60] |
| Treg | The immune mechanism | The mIHC demonstrated that both T follicular helper and regulatory T cells were significantly increased in intra-tumoral TLSs compared to peri-tumoral counterparts. | Ding et al. (2022) | [144] |
| Treg | The immune mechanism | The cells at the Biliary Cancer cancer Center secrete TGF-β1 and induce Tregs, which creates an immune-suppressive environment. | Kinoshita et al. (2020) | [59] |
| B Cell | The immune mechanism | Oxidative stress-mediated reduction in EBF1 expression induces CCA progression. | Armartmuntree et al. (2018) | [64] |
| B Cell | Prognostic marker | PNOC expressed by infiltrating B cells in CCA predicts better survival of patients. | Chen et al. (2021) | [60] |
The above evidence suggests that the human body will develop a mutation-specific T lymphocyte response to CCA derived from epithelial cells. Tran et al. suggested that TILs from patients with metastatic CCA contained CD4+ T helper 1 (Th1) cells using whole exogenomic sequencing and were able to identify cancer-expressed erbb2-interacting protein (ERBB2IP) mutations. They transplanted patients with TILs containing about 25% mutation-specific multifunctional Th1 cells and experienced shrinkage of tumor lesions and higher disease stability. At the time of deterioration, the patient was treated with a pure population of > 95% mutation-responsive Th1 cells and again experienced regression of the tumor tissue. The above results suggest that the response of CD4 + T cells to mutant antigens can be used to mediate the regression of metastatic epithelial cell cancer tissues and may be a potential targeted therapy for CCA [53]. Treatment with trametinib alone leads to the up-regulation of major histocompatibility complex (MHC-I) and PD-L1 on tumor cells in vitro, and it has been suggested that the combination of trametinib with anti-PD-1 drugs can lead to enhanced cytotoxicity of hepatic effector memory CD4 + T cells, reduced tumor burden, and improved survival in tumor-bearing mice [54]. Table 2 lists more studies on CD4 + T lymphocytes. As depicted in this table, CD4+ T lymphocytes take on a great significance in the TME of CCA, and targeting these cells may achieve the purpose of immunotherapy.
Tregs in CCA
Regulatory T cells (Treg) are usually CD4+CD25+FOXP3+T cells, accounting for 5% -10% of the total number of peripheral blood CD4 + T cells, and have a special mechanism of action in the TME of CCA, so we discuss them separately. FOXP3 is a transcription factor, FOXP3 deficiency can lead to severe autoimmune diseases [55], and Tregs are divided into natural regulatory T cells (nTregs) that come from thymic differentiation and peripherally derived inducible regulatory T cells (iTregs) according to the source [56]. Tregs play an immunosuppressive role by down-regulating CD80 and CD86 on dendritic cells mainly by acting on CTLA-4 to inhibit effector cell activation [57]. At present, there are relatively few studies on the role played by Tregs in CCA, but its important role in CCA has been confirmed: Kim et al. showed that the number of FOXP3 + CD4 + regulatory T cells in extrahepatic CCA was more than that in CCA, and the number of FOXP3 + CD4 + regulatory T cells at the tumor margin predicted a better prognosis [58]. Kinoshita et al. suggested that Tregs accumulate in the tumor center of CCA, related to the expression of TGF-β1 in tumor cells. TGF-β1, a cytokine produced by tumor cells, induces Tregs heterogeneity in the TME, shapes an environment conducive to proliferation, anti-apoptosis and angiogenesis for tumor cells, makes tumor cells immune escape and promotes the development of CCA, while GCA (e.g., gemcitabine, and albumin-bound paclitaxel neoadjuvant therapy) can prevent the mechanism [59]. Another study suggested that Treg-expressed leukocyte-associated immunoglobulin-like receptor 2 (LAIR2) can interfere with platelet activation and adhesion by blocking the binding of LAIR1 by competing ligands, inhibit the classical pathway and lectin pathway of the complement system to kill pathogens, and act as a clinical biomarker for evaluation of patients before immunotherapy, thereby predicting patient survival and tumor immune infiltration [60]. The role played by Tregs in CCA is listed in Table 2. The interaction mechanism of T cells with CCA tumor cells in TME is detailed in Fig. 4.
B cells in CCA
TME is a complex structure composed of tumor cells, stromal and endothelial cells, and immune cells with dynamic interactions between the above cells. B cells are the second adaptive immune cell population suggested in the TME. B cells have been considered to have oncogenic properties for the past two decades [61]. In renal cancer and glioblastoma, B cells have been demonstrated to be related to poor prognosis [62]. It has also been shown that B cells play a role in TME by inhibiting tumors, such as B cells and CD4 + T follicular helper cells collaborating to promote anti-tumor CD8 + T cell responses[63]. Both of the above effects have a relatively clear basis and have different factual bases, and different infiltration patterns or TME induce the differentiation of B cells into different directions that can affect their final overall effects. In CCA TME, DNA hypermethylation of the EBF1 (early B cell factor 1) promoter inhibits EBF1 expression in CCA and induces CCA progression. Meanwhile, long-term oxidative stress would inhibit EBF1 expression in cholangiocytes as an adaptive response for cells to survive under continuous stress conditions, which in turn promotes CCA development[64]. However, there are also some studies with different findings: high B cells infiltration levels may be related to better OS and can serve as a biomarker for the evaluation of immune infiltration in CCA[60], this happens to prove that B cells enhance T cell responses by producing antibodies, stimulatory cytokines, and chemokines, act as local antigen-presenting cells, and organize the formation of TLS that maintain immune responses, and TLS has been shown to be related to a positive prognosis in a variety of tumors[51]. Existing studies have illustrated the complex and heterogeneous role played by B cells in TME. Accordingly, further characterization of how B cell responses are initiated and regulated within the TME will help to develop clinical strategies for cancer immunotherapy. Contents regarding B cells are summarized in Table 2.
Innate immune response
Innate immunity is an innate immune defense function formed by the body during germline development and evolution, i.e., non-specific defense function that has been possessed since birth, also known as non-specific immunity. It is a series of defense mechanisms formed by organisms during long-term evolution. Innate immunity is the physiological rejection of the body to a variety of antigenic substances.
DCs in CCA
As professional antigen-presenting cells, dendritic cells (DCs) contribute to the activation of T cells and are the initiators of the induction of T cell immune responses. Activation of the CD40/CD40L immune checkpoint leads to DCs maturation and affects the release of cytokines and chemokines [65]. CD40 molecules are highly expressed on the surface of DCs. Existing research has suggested that CD40 agonist combined with anti-PD1 antibody treatment can significantly increase the number of DC cells in ICC tissue and limit tumor growth [66]. Through flow cytometry analysis, Martín-Sierra et al. suggested that the number of Myeloid Dendritic Cells in the peripheral blood of CCA patients was significantly decreased, and the expression of TNF-α was decreased [67]. In view of the important role played by DCs in inducing T cell immune responses, related studies have used DCs as a starting point to explore ways to enhance tumor immunity. Jiraviriyakul et al. suggested that DC cells sensitized with cell lysates produced by CCA cells after Honokiol treatment enhanced the antitumor immune response of T cells [68]. In the tumor microenvironment, immunosuppressive cytokines (IL-10 and TGF-β) secreted by CCA cells affect DCs function and inhibit the activation of effector T cells [69]. A clinical trial showed that DCs vaccine combined with activated T cell transfer can improve the prognosis of patients with CCA [70]. Summary of the role played by DCs in the TME of CCA is listed in Table 3.
DCs activate naive T cells by presenting antigens by phagocytosis, initiate immune responses, secrete chemotactic cytokines, chemotactic T/B cells, and present antigens to CD8 + T cells/CD4 + T cells by MHC class I/II cells. Upon activation of CD8 + T cells, perforin is released, and granzymes and FAS/FASL transmembrane glycoproteins kill tumors. mlDH1 inhibited the recruitment of CD8 + T cells and the expression of IFN-γ, whereas mlDH1 inhibitor could contact this inhibition. At the same time, HLA class I is expressed and presents tumor antigen-derived peptides to the immune system, which ultimately stimulate CD8 + T cells to show anti-tumor effects. Inhibition of B7-H4 by lentiviral transcription encoding shRNA could enhance CD8 + T cell-mediated cytotoxicity. The response of CD4 + T cells to mutated ERBB2IP antigen can be used to mediate the degeneration of metastatic epithelial cell carcinoma tissues, trametinib can lead to the up-regulation of MHC-I and PD-L1 on tumor cells in vitro, and the combination of trametinib with anti-PD-L1 drugs can enhance the anti-tumor toxicity of hepatic effector memory CD4 + T cells. The increased expression of TGF-β1 in tumor cells induces Tregs heterogeneity in TME, forms an environment conducive to tumor cell proliferation, anti-apoptosis and angiogenesis, and promotes tumor progression, a mechanism that can be inhibited by the combination of GCA. LAIR2 expressed by Tregs blocks the binding of LAIR1 by competing ligands, interferes with platelet activation and adhesion, and inhibits the classical pathway of the complement system and the lectin pathway to kill pathogens.
Summary of the role of DCs and NK cells in the TME of CCA
| Cells | The research direction | Result | Reference | Reference number |
|---|---|---|---|---|
| DC | Immunotherapy | CD40 mediates DC activation in ICC. Anti-CD40/PD-1 combination therapy significantly inhibited tumor growth in murine ICC models. | Diggs et al. (2021) | [66] |
| DC | Immunotherapy | The 5-year PFS and OS of ICC patients who received dendritic cell vaccine plus activated T cell transfer after surgery were longer than those of the control group. It shows that this therapy has the potential to become the standard adjuvant therapy for ICC. | Shimizu et al. (2012) | [70] |
| DC | Immunotherapy | Tumor lysates generated after Honokiol treatment of CCA cells can enhance the antigen presentation of DCs and stimulate the specific killing effects of T cells. | Jiraviriyakul et al. (2019) | [68] |
| DC | The immune mechanism | In vitro activation of CD40/CD40L immune checkpoints can affect the immune regulation function of DCs, providing a new entry point for the immunotherapy of malignant tumors such as CCA. | Sadeghlar et al. (2021) | [145] |
| DC | The immune mechanism | IL-10 and TGF-β secreted by CCA cells inhibit the function of DCs. Inhibition of IL-10 and TGF-β receptors on DCs by specific neutralizing antibodies can enhance the activity of effector T cells. | Thepmalee et al. (2018) | [69] |
| DC | Prognostic marker | The degree of infiltration of BDCA2+ plasmacytoid dendritic cells (PDCs) in peritumoral tissue may serve as a novel prognostic predictor in ICC patients undergoing radical resection. | Hu et al. (2020) | [146] |
| DC | The immune mechanism | Self-differentiated dendritic cells were transduced with short-hairpin RNAs lentiviral to knock down TGF-βRII and IL-10RA mRNAs, and the anti-CCA activity of T cells was enhanced. | Thepmalee et al. (2020) | [147] |
| NK | Immunotherapy | In a randomized placebo-controlled phase I clinical trial, oral administration of Atractylodes lancea (Thunb) DC. (AL) significantly increased the number of NK cells in blood samples from patients with CCA, showing a favorable anticancer effect. | Kulma et al. (2021) | [148] |
| NK | Immunotherapy | Globo H is highly expressed in ICC. The anti-Globo H mAbVK9 can increase the number of NK cells in the tumor microenvironment and limit tumor growth, indicating that Globo H is a valuable therapeutic marker for ICC. | Hung et al. (2022) | [76] |
| NK | Immunotherapy | Cordycepin can increase the expression of DR4 and DR5 in CCA cell line KKU-213A, thereby inducing NK cell cytotoxicity. | Panwong et al. (2021) | [77] |
| NK | The immune mechanism | In vitro studies showed that ICC cells induced apoptosis of T cells and NK cells through the Fas/FasL pathway. | Carnevale et al. (2017) | [75] |
| NK | Prognostic marker | The results of immunohistochemical analysis showed that the high expression of CXCL9 in ICC was significantly correlated with the infiltration of NK cells. CXCL9 can also serve as an effective prognostic marker. | Fukuda et al. (2020) | [74] |
| NK | The immune mechanism | NK cells in CCA express KIR receptors. Multiple alterations of KIR and HLA loci in patients with CCA may affect the immune surveillance function of NK cells. | Cornillet et al. (2019) | [72] |
| NK | Immunotherapy | In vitro and in vivo experiments have shown that NK cells have significant cytotoxicity to CCA cells, providing a research basis for the clinical application of NK cell therapy. | Jung et al. (2018) | [78] |
NK cells in CCA
Natural killer (NK) cells are a type of cytotoxic lymphocytes of the innate immune system. The markers commonly used to detect human NK cells consist of CD16, CD56, CD57, etc. Activated NK cells are capable of mediating direct cytotoxicity to tumor cells through “self-deletion” and ADCC effects, and they can perform immune monitoring functions by releasing cytokines (e.g., IFN-γ and TNF-α) [71]. In patients with CCA, the immune monitoring function of NK cells is often affected, and this abnormality may be related to the alteration of KIR (killer cell immunoglobulin-like receptor) and HLA gene loci in patients with CCA [72]. IL-12, IL-15, IFN-α/β, CXCL9/10 secreted by DCs play a vital role in the activation and recruitment of NK cells [73]. CXCL9 produced by DCs, macrophages, endothelial cells, and others belongs to the ELR motif-negative CXC chemokine family. In the TME, endogenous CXCL9 is capable of regulating the number of tumor-infiltrating NK cells, and it is significantly related to the prognosis of patients with ICC [74]. CCA is involved in regulating immune cells in the tumor microenvironment. Existing research has suggested that CCA cells can induce the apoptosis of CD4+, CD8+ T cells and CD56+ NK cells via the Fas/FasL pathway to produce immune evasion [75]. To study how to maintain or increase the antitumor activity of NK cells, Hung et al. constructed a thioacetamide (TAA)-induced rat ICC model and suggested that the anti-Globo H antibody VK9 can facilitate the activation of NK cells in the tumor microenvironment, increase the Direct cytotoxicity and enhance ADCC function [76]. Panwong et al. have suggested that cordycepin can increase the anti-CCA activity of NK-92, and TRAIL signaling may play a role in the immune regulation process of cordycepin [77]. There has not been any immune cell therapy for CCA clinically, whereas studies in nude mice have confirmed the antitumor effect of NK cells on CCA cells, which provides a reference for the clinical application of NK cells therapy [78]. Table 3 lists the role played by NK cells in the TME of CCA.
TANs in CCA
As professional phagocytes, neutrophils mainly mediate inflammatory responses, and their role in cancer has not been fully investigated. Neutrophils are affected by a variety of factors in the tumor microenvironment and have certain immunomodulatory functions [79]. There are still many controversies about whether TANs play a tumor-promoting or tumor-suppressing role in cancer. Similar to the M1/M2 polarization of macrophages, TANs also have both N1 and N2 phenotypes when stimulated by different environmental factors. Type I IFNs induce TANs to display an anti-tumor N1 phenotype, while TGF-β can modulate TANs to a pro-cancer N2 phenotype [80]. Neutrophils recruited by Methotrexate-loaded tumor-cell-derived microvesicles (MTX-TMP) perfusion display an N1 phenotype with the ability to kill extrahepatic CCA (eCCA) [81]. CXCL5, as a pro-angiogenic CXC-type chemokine, is highly expressed in CCA cells, and induces the recruitment of neutrophils in tumor tissues through PI3K-Akt and ERK1/2-MAPK, promoting ICC progression [82]. Gu et al. suggested that the infiltration of IL+17 cells and neutrophils in ICC tissue was closely related to tumor aggressiveness and increased risk of postoperative mortality in patients [83]. By analyzing the postoperative specimens of 254 patients with CCA by immunohistochemical staining, Mao et al. suggested that the distribution of neutrophils in CCA tissue may indicate poor prognosis and affect the overall survival (OS) of patients [84]. Research on TANs in the CCA microenvironment is limited, and more studies are needed to support their role in tumor immune regulation. Studies on the role played by TANs in the TME of CCA are listed in Table 4. The role played by two immune-related cells with dual effects on tumors in CCA TME is shown in Fig. 5.
Summary of the role of macrophages and neutrophils in the TME of CCA
| Cells | The research direction | Result | Reference | Reference number |
|---|---|---|---|---|
| Macrophages | The immune mechanism | The spatial distribution of TANs and TAMs correlated with each other in samples from patients with intrahepatic CCA. STAT3 is an important molecule in the interaction between TANs and TAMs to promote the progression of ICC. | Zhou et al. (2021) | [98] |
| Macrophages | Immunotherapy | Periostin secreted by intrahepatic CCA stem cells (ICSCs) promotes the recruitment of TAMs in the TME, providing a new target for immunotherapy. | Zeng et al. (2018) | [149] |
| Macrophages | The immune mechanism | The presence of macrophages exacerbates the cytotoxicity, DNA damage and ROS generation of 1,2-Dichloropropane (1,2-DCP) on cholangiocytes, which may be the underlying mechanism of 1,2-DCP-induced carcinogenesis of CCAs. | Ekuban et al. (2021) | [150] |
| Macrophages | The immune mechanism | M2-TAM may promote CCA metastasis through EMT process in the early stage of live Hodgella opioides (Ov)-induced CCA (CCA). High density of M2 TAMs in patient CCA tissue was significantly associated with extrahepatic metastasis. | Thanee et al. (2015) | [92] |
| Macrophages | The immune mechanism | M2 macrophage-derived TGFβ1 promotes CCA progression and chemoresistance through aPKCɩ-mediated NF-κB signaling pathway. CCL5 secreted by CCA cells undergoing aPKCɩ-induced EMT in turn regulates macrophage recruitment and polarization. | Yang et al. (2022) | [151] |
| Macrophages | The immune mechanism | The lncRNA PCAT6 (PCAT6) plays an important role in regulating the function and differentiation of immune cells. In a CCA xenograft mouse model, the PCAT6/miR-326/RohA pathway regulates M2 macrophage polarization. | Tu et al. (2020) | [88] |
| Macrophages | The immune mechanism | Interaction between M2 macrophages and ICC, M2 polarized macrophages induce EMT in CCA cells via IL-10/STAT3. | Yuan et al. (2020) | [152] |
| Macrophages | The immune mechanism | In the ICC mouse model, the recruitment of hepatic macrophage-derived Wnt3a due to inflammation may promote the malignant transformation of hepatocytes into ICC cells. | Saito et al. (2018) | [95] |
| Macrophages | Immunotherapy | The number of CD68+ TAMs infiltrated in tumor tissue was associated with OS in patients undergoing resection. Anti-GM-CSF therapy can relieve the establishment of an immunosuppressive microenvironment through the repolarization of TAMs and MDSCs. | Ruffolo et al. (2022) | [99] |
| Macrophages | The immune mechanism | CCA-sphere conditioned medium constructs an immune niche suitable for the growth of CCA by regulating macrophages. | Raggi et al. (2017) | [153] |
| Macrophages | The immune mechanism | Exosomes are important mediators of the crosstalk between tumor cells and the tumor microenvironment. miR-183-5p upregulates PD-L1 expression in macrophages through the miR-183-5p/PTEN/AKT/PD-L1 pathway, mediating CCA Immunosuppression in the process. | Luo et al. (2022) | [97] |
| Macrophages | The immune mechanism | The TAM-secreted exosome Circ_002056 regulates the proliferation, migration and invasion of CCA cells in the tumor microenvironment through the Circ_002056/miR-432-5p/E2F3 axis. CircRNAs produced by tumor-associated macrophages can serve as a new molecular target for clinical therapy. | Chen et al. (2022) | [96] |
| Macrophages | Prognostic marker | Immunohistochemical techniques were used to assess the abundance of TAMs in tumor samples from patients with intrahepatic CCA. The number of TAMs in the tumor invasive front is a meaningful prognostic marker in routine histopathological evaluation. | Atanasov et al. (2017) | [100] |
| Macrophages | Prognostic marker | In ICC patient tumor samples, the number of macrophages was positively correlated with the number of blood vessels and regulatory T cells, but not with the OS of the patients. | Hasita et al. (2010) | [154] |
| Macrophages | Prognostic marker | Intratumoral M2 macrophages are associated with OS in patients with CCA, suggesting the possibility of targeting macrophages for therapy. | Kunk et al. (2021) | [155] |
| Macrophages | The immune mechanism | When ICC cells were co-cultured with M2-TAMs, the core cytokines (GM-CSF, TNF-α, ICAM-1, IL-6) secreted by M2-TAMs activated the AKT3/PRAS40 signaling pathway to promote EMT in ICC cells. | Sun et al. (2020) | [93] |
| Macrophages | The immune mechanism | 1,2-DCP stimulates macrophages to induce high expression of the pro-inflammatory factor TNF-α, thereby inducing CCA. | Zong et al. (2019) | [156] |
| Macrophages | The immune mechanism | Compared with normal liver tissue, Wnt mRNA was significantly elevated in CCA tissue. LPS induces upregulation of Wnt3 mRNA in macrophages and induces CCA through the Wnt/β-catenin pathway. | Loilome et al. (2014) | [157] |
| Neutrophils | The immune mechanism | Overexpression of CXCL5 in CCA recruits neutrophils to aggregate and promote ICC growth and metastasis. | Zhou et al. (2014) | [82] |
| Neutrophils | Prognostic marker | Analysis of different microanatomical regions of intrahepatic CCA by tissue microarray and immunohistochemistry demonstrated that neutrophils were an independent factor for evaluating the prognosis of patients with intrahepatic CCA. | Gu et al. (2012) | [83] |
| Neutrophils | Immunotherapy | Methotrexate-containing plasma membrane microvesicles induce neutrophil aggregation in eCCA and relieve obstructive eCCA. | Gao et al. (2020) | [81] |
| Neutrophils | Prognostic marker | CD15 neutrophil staining was performed on tissue sections by immunohistochemical staining. The level of neutrophils in CCA tissues was higher than that in adjacent tissues. The expression of CD15 was significantly associated with DFS and OS in patients with CCA. | Mao et al. (2015) | [84] |
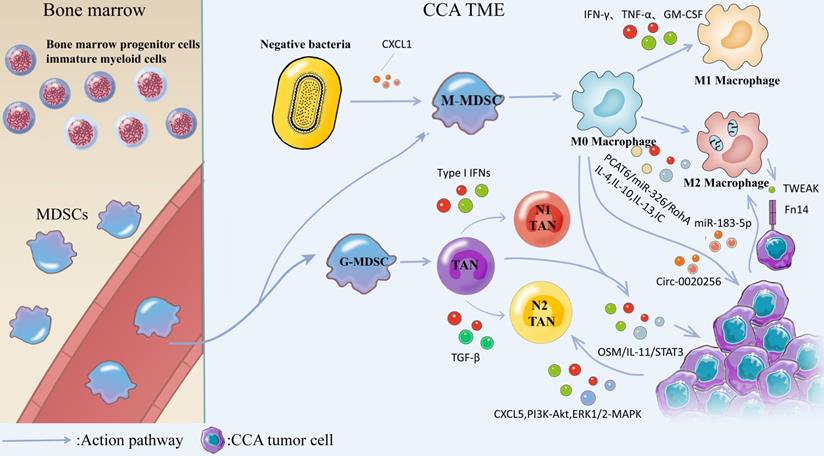
MDSCs are generated from bone marrow progenitor cellsimmature myeloid cells in the bone marrow and subsequently differentiate from blood vessels into M-MDSCs and G-MDSCs. Negative bacteria secrete CXCL1 to promote M-MDSCs transformation and generation, and M-MDSCs are converted into M0 Macrophages. Stimulation with cytokines such as IFN-γ, TNF-α, GM-CSF, or bacterial endotoxin induced the conversion of M0 Macrophages into M1 Macrophages, and IL-4, IL-10, IL-13, and IC induced the conversion of M0 Macrophages into M2 Macrophages. M2 macrophages induce the production of EMT through IL-10/STAT3 and AKT3/PRAS40 pathways. TWEAK produced by macrophages interacts with the Fn14 receptor on the surface of CCA cells and affects CCA progression. Tumor-derived exosome miR-183-5p up-regulates macrophage PD-L1 expression in TME through 315 miR-183-5p/PTEN/AKT/PD-L1 pathway, which promotes the development of immunosuppression in ICC. TAMs can interact with TANs, activate OSM/IL-11/STAT3 signaling pathway, and promote ICC progression. G-MDSCs were converted to N0 TANs and subsequently IFNs induced TANs to exhibit an anti-tumor N1 phenotype, whereas TGF-β could modulate TANs to exhibit a pre-malignant N2 phenotype. CXCL5 is highly expressed in CCA cells and promotes CCA progression by inducing neutrophil recruitment in tumor tissues via PI3K-Akt and ERK1/2-MAPK.
Macrophages in CCA
Macrophages can generate canonical M1 activation and alternative M2 activation under different environmental stimuli. M1 macrophages are mainly induced by the stimulation of cytokines such as IFN-γ, TNF-α, GM-CSF or bacterial endotoxin, while IL-4, IL-10, IL-13, immune complexes (IC) and other factors trigger macrophage transformation to M2 phenotype [85]. M1 macrophages secrete high levels of pro-inflammatory cytokines, produce highly reactive nitrogen and oxygen intermediates, and have strong tumoricidal activity. M2 macrophages are considered to be of great significance in facilitating tissue remodeling and tumor progression, and they have immunomodulatory functions [86]. Macrophages accumulating within tumor tissue are termed tumor-associated macrophages (TAMs). Macrophages in the CCA immune microenvironment are the predominant infiltrating inflammatory cells, often exhibiting a M2 phenotype [87]. PCAT6, as a LncRNA, is highly expressed in CCA patients, and previous studies have suggested that PCAT6/miR-326/RohA is an essential pathway that plays a role in M2 polarization of CCA-related macrophages [88]. Th2 cytokine IL-4 also plays a role in stimulating the conversion of macrophages to the M2 phenotype [89]. TAM-mediated inflammatory responses take on a critical significance in tumor transformation and progression, and they have been widely studied [90]. A bioinformatics analysis based on the gene expression omnibus database suggested that activated dendritic cells, M2 macrophages and regulatory T cells (Tregs) were expressed at higher levels in ICC than normal tissues, while M1 macrophages, monocytes, and helper T cells were expressed at lower levels in ICC than in normal tissues [91]. Thus, M2-TAMs has been the most studied macrophage cell type in the CCA tumor microenvironment. There is a close relationship between M2 TAMs and the development, immunosuppression, and clinical prognosis of ICC. M2 polarized macrophages play a role in promoting the growth and invasion of CCA and this potential mechanism may be the production of EMT (epithelial-mesenchymal transition) induced by M2 macrophages through the IL-10/STAT3 and AKT3/PRAS40 pathways [92, 93]. Tumor necrosis factor-like weak inducer of apoptosis (TWEAK) produced by macrophages interacts with Fn14 receptors on the surface of CCA cells to influence CCA development [94]. Macrophage-derived Wnt3a appears to play a role in the transformation of ICC cells [95]. Several studies have shown that exosomes are important mediators linking TAMs and CCA. Circ_0020256 in TAM-derived exosomes can promote the proliferation, invasion, and migration of CCA [96]. However, tumor-derived exosomal miR-183-5p upregulates the expression of macrophage PDL-1 in TME through the miR-183-5p/PTEN/AKT/PD-L1 pathway, thereby promoting the occurrence of immunosuppression in ICC [97]. Hou et al. suggested that TAMs can interact with tumor-associated neutrophils (TANs) to activate the OSM/IL-11/STAT3 signaling pathway to promote ICC progression [98]. Overall, TAMs mainly plays a role in promoting cancer in CCA. In order to inhibit the tumor-promoting effect of M2 macrophages, Ruffolo et al. built an orthotopic tumor-bearing mouse model of ICC and confirmed that anti-GM-CSF monoclonal antibody treatment can reverse tumor-induced M2 polarization of TAMs, thus stimulating the immune activity of T cells and preventing the establishment of an immunosuppressive microenvironment [99]. TAMs are meaningful markers in evaluating the clinical prognosis of patients with ICC [100]. Table 5 lists the existing studies on the role played by macrophages in the TME of CCA.
MDSCs
Myeloid-derived Suppressor Cells (MDSCs) are a heterogeneous group of bone marrow-derived cells that is a highly diverse cell population. MDSCs are classified into two categories, including mononuclear myeloid cells (M-MDSCs) (e.g., macrophages and dendritic cells (DCs)), as well as monocytes, and granulocytic myeloid cells (G-MDSCs) (e.g., terminally differentiated polymorphonuclear neutrophils, eosinophils, basophils, and mast cells [101]. The major functions of MDSCs consist of immunosuppression, promoting tumor progression, upregulating tumor proliferation pathways, promoting angiogenesis, and supporting tumor spillage by disrupting the ECM [102]. Extensive studies that have suggested a promoting effect of MDSCs on CCA, and the goal is to study inhibition of MDSCs to treat CCA. Previous findings have suggested that blocking TAMs and PD-L1 in CCA can reduce CCA progression, but G-MDSCs disable TAM and PD-L1 blockade by impairing T-cell-mediated immune escape responses. Loeuillard et al. suggested that dual inhibition of TAMs and G-MDSCs enhances immune checkpoint blockade (ICB), which highlights the therapeutic potential of ICB coupled to immunotherapy targeting immunosuppressive myeloid cells in CCA [103]. In vivo, lipopolysaccharide (LPS) induces CXCR2+ and polymorphonuclear myeloid-derived suppressor cells (PMN-MDSC) accumulation through the production of TLR4-dependent CXCL1, controls CCA tumor cells to form an immunosuppressive microenvironment, and facilitates CCA tumor growth [104]. Table 5 lists the studies on the role played by MDSC in the TME of CCA.
Mast cells in CCA
Mast cells were initially discovered by Paul Ehrlich in 1878 [104]. They originate from bone marrow hematopoietic progenitor cells migrating to and maturing in the surrounding tissue when not fully differentiated [105]. Mast cells function is primarily divided into two aspects: from a physiological perspective, mast cells are involved in tissue remodeling and neovascularization [106], while from a pathological perspective, it is not only an important factor in IgE-mediated allergic diseases, but also involved in non-IgE-mediated allergic reactions [107, 108]. A clinical specimen analysis suggested that immune cell infiltration consisting of CD8+, CD4+ lymphocytes, macrophages, and mast cells was significantly increased in locally advanced CCA patients compared with metastatic lesions, and this change in TME infiltration was related to a significant increase in MVD due to mast cell involvement in angiogenesis in local CCA lesions [49]. However, the use of sodium chromolybdate can inhibit CCA proliferation, angiogenesis, and the presence of mast cell markers by blocking the mast cell c-Kit/SCF pathway [109]. SCF (the stem cell factor) is a c-Kit ligand, and it is also known as steel factor. The c-Kit/SCF pathway is a vital pathway to facilitate tumor proliferation and angiogenesis [110]. This study suggests that targeting c-Kit/SCF interaction by sodium chromomolybdate may be a potential therapeutic option to inhibit tumorigenesis and progression. Table 5 lists the role played by mast cells in the TME of CCA.
Current Status of Immunotherapy for CCA
The treatment methods for CCA primarily comprise surgical treatment, radiation therapy, and systemic therapy in accordance with the guidelines for liver cancer issued by the US NCCN in 2022. To be specific, surgical treatment consists of subtotal hepatectomy, wedge resection and resection of the liver for ICC [8], pancreaticoduodenectomy for dCCA [111], as well as extensive hepatectomy for pCCA [112]. However, the prognosis of surgical treatment does not achieve the ideal goal due to the complexity of the anatomy of hepatobiliary tumors and the multiple extrahepatic lymph node metastases, while functional reconstruction after resection surgery is a major problem. Radiation therapy is generally considered a supplement to surgical treatment. For instance, postoperative EBRT using conventional 3D-CRT or intensity-modulated radiotherapy is an option for resection of extrahepatic CCA and gallbladder cancer [113]. However, as a radiosensitive organ, the liver and its surrounding tissues toxicity and other side effects caused by radiation therapy cannot be ignored [114]. The recommended regimen given by the NCCN for the treatment of resectable CCA system, which has attracted much attention, is 5-fluorouracil, capecitabine or gemcitabine in combination with a chemotherapeutic drug, such as oxaliplatin, of which capecitabine is the preferred regimen for adjuvant therapy. Existing research has shown signs of improved survival in the experimental group compared with no capecitabine, whereas these signs did not achieve statistical significance [115]. In unresectable CCA, the preferred regimen is gemcitabine combined with cisplatin [9], and although the mPFS can reach 8.0 months, the mOS is only 11.7 months, which is far from the expectations of clinicians. Another factor affecting the therapeutic effect of chemotherapeutic drugs is microsatellite instability-high (MSI-H) caused by the presence of mismatch repair (MMR) gene [116]. However, if CCA belongs to MSI-H/dMMR type, it often has many gene mutations, but is sensitive to PD-1/PD-L1 inhibitor treatment. At the same time, with the increasing use of immunotherapies such as immune checkpoint inhibitors Immune checkpoint inhibitors (ICIs) in other cancers, such as PD-L1 monoclonal antibody combined with VEGFR monoclonal antibody regimen has become the first-line systemic treatment regimen for advanced HCC [117], methods for hepatobiliary tumors have also entered our horizon.
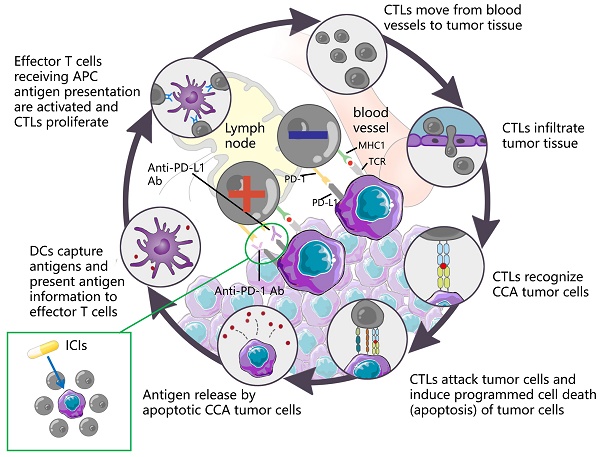
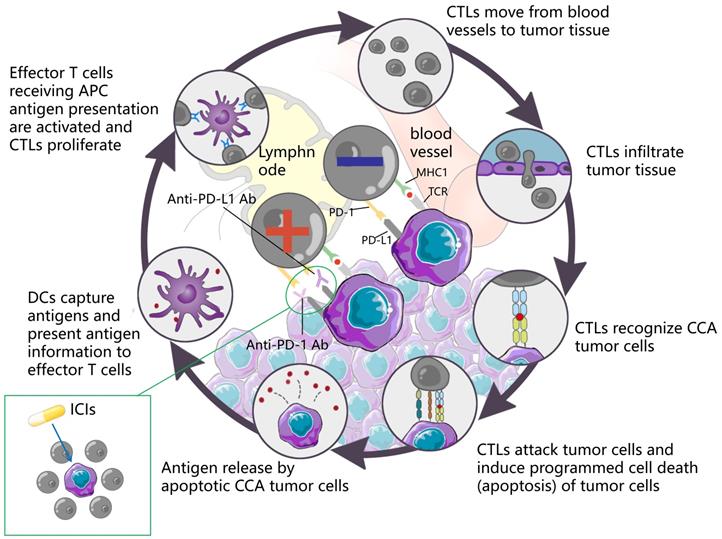
ICI is capable of restoring T cell-mediated tumor cell killing and depleting regulatory Tregs by blocking immune checkpoint molecules (e.g., cytotoxic T lymphocyte antigen 4 (CTLA4), PD-L1, as well as PD-1. This mechanism of action has aroused wide attention of investigators. The understanding of the antitumor activity of PD-1/PD-L1 antibodies has been deepened over the past few years, and significant results have been achieved in the clinical treatment of HCC with similar biliary anatomy. In other words, camrelizumab combined with apatinib achieved ORR in 33.3% of cases of resectable HCC [118] in a recent phase II clinical trial. Accordingly, the application of immunotherapy in the treatment of CCA has attracted much attention. Durvalumab is an IgG1 κ monoclonal antibody, which acts as a PD-L1 blocker, can bind to PD-L1 on tumor cells, and block its interaction with PD-1 of T cells and antigen-presenting cells, thereby relieving PD-1/PD-L1-mediated immunouppression, promoting T cell proliferation, and strengthening the immune killing effect on tumors. A controlled trial involving 685 cases showed that Durvalumab combined with GEMCIS significantly prolonged OS compared with GEMCIS [119], and similar regimens were Camrelizumab combined with GEMOX and Pmbrolizumab combined with CAPOX [120, 121]. The above studies showed that the combination of PD-L1 blocker and chemotherapeutic drugs had good efficacy in patients with advanced CCA. As an emerging treatment before the advent of immunotherapy, the combination of targeted therapy and immune drugs is also one of the hot directions for clinicians. In a study of lenvatinib in combination with PD-1 antagonists (including pembrolizumab, tislelizumab, sintilimab, camrelizumab, and toripalima) [14], the mOS and ORR reached 17.7 months and 42.1%, a result that is alarming and exciting. This may be related to the fact that targeted drugs act directly on the target thereby enhancing the therapeutic effect. In contrast, the therapeutic effect of ICIs alone, although good, is not as satisfactory as that of ICIs combined with chemical drugs or targeted drugs, such as nivolumab or pembrolizumab alone [122, 123], although the efficacy of ICIs alone is significant compared with traditional methods. However, not all the study results were positive. The mOS of ramucirumab combined with pembrolizumab was only 6.44 months, which did not show a significant improvement in survival rate, whereas the mOS of PD-L1 positive patients was 11.33 months [35], suggesting that PD-L1 positive can serve as a tumor marker to provide a reference for the designation of future immunotherapy methods. Table 6 lists more studies on immunotherapy in CCA. The mechanism of action of the ICIs is presented in Fig. 6.
Immunotherapy plays a certain role in the clinical treatment of CCA, whereas its efficacy and safety should be analyzed in more studies. Furthermore, the development of effective combination therapies with ICIs and chemotherapeutic drugs or targeted drugs may be the right direction to prolong the survival of CCA patients.
Conclusion and perspectives
Novel treatments are urgently required due to the increasing incidence of CCA, limited efficacy of surgical treatment and chemotherapy, and poor prognosis. Targeted immunotherapy, a cutting-edge therapy in cancer treatment, is arousing rising attention. Although much remains to be explored, many studies have shown that immunotherapy has some advantages over conventional therapies, such as fewer side effects. In this review, some studies have proposed immunotherapeutic methods that are increasingly used in clinical practice (e.g., inhibiting MDSCs and enhancing immune checkpoint blocking). The involvement and regulation of cytokines and chemists, and the interaction between receptors and related ligands are of great significance in the function, maturation and differentiation of immune cells. The above factors, together with stromal cells and tumor cells, constitute TME. With the development of several technologies (e.g., single-cell RNA sequencing), the understanding of the complex mechanisms of TME should be deepened. Immunotherapy is not the end of cancer treatment. Instead, TME-based immunotherapy is the beginning of a new chapter in cancer treatment.
Summary of MDSCs and Mast cells in the TME of CCA
| Cells | The research direction | Result | Reference | Reference number |
|---|---|---|---|---|
| MDSC | The immune mechanism | G-MDSCs accelerate CCA progression by impairing T cell-mediated immune escape responses and disabling TAM and PD-L1 blockade. | Loeuillard et al. (2020) | [102] |
| MDSC | The immune mechanism | Conditioned media (CM) from CAF-educated MDSCs drastically promoted tumorsphere formation efficiency and stemness marker gene expression in ICC cells. CAF-CM stimulation increased expression and activity of 5-LO in MDSCs, while 5-LO inhibitor impaired the stemness-enhancing capacity of MDSCs in vitro and in vivo. Furthermore, IL-6 and IL-33 primarily expressed by CAFs mediated hyperactivated 5-LO metabolism in MDSCs. | Lin et al. (2022) | [131] |
| MDSC | The immune mechanism | In vivo, bacteria/(LPS) induce CXCR2 + PMN-MDSC accumulation through TLR4-dependent CXCL1 production, control CCA tumor cells to form an immunosuppressive microenvironment, and promote CCA tumor growth. | Zhang et al. (2020) | [18] |
| Mast cell | The immune mechanism | In locally advanced CCA patients, there is a significant increase of immune cell infiltrate constituted by CD8+ and CD4+ lymphocytes, macrophages and mast cells as compared to the metastatic ones. | Tamma et al. (2019) | [49] |
| Mast cell | The immune mechanism | Inhibition of mast cell-derived histamine decreases human CCA growth and differentiation via c-Kit/SCF-dependent signaling. | Johnson et al. (2016) | [109] |
| Mast cell | The immune mechanism | Mast cells increase during carcino- genesis in HCC and ICC, and they may play a role in fibrosis or tumor immunology in HCC and ICC. | Terada et al. (2000) | [158] |
DCs capture antigens, present antigen information to effector T cells, and effector T cells receiving APC antigen presentation are activated and CTLs proliferate. CTLs move from blood vessels to tumor tissue, recognize tumor cells and infiltrate tumor tissue, attack tumor cells, induce programmed cell death (apoptosis) of tumor cells, and subsequently apoptotic tumor cells release antigens to form a dynamic cycle. PD-1 is an important immunosuppressive molecule. It prevents the immune system from killing cancer cells by regulating the immune system's response to human cells downward, as well as regulating the immune system and promoting self-tolerance by suppressing T-cell inflammatory activity. PD-L1 is a ligand expressed on the surface of tumor cells. PD-L1 is up-regulated in a variety of tumor cells. It binds to PD-1 on CTLs and inhibits CTLs proliferation and activation, so that CTLs are in an inactivated state and finally induce immune escape. ICIs can block the binding of PD-1 and PD-L1, up-regulate the growth and proliferation of CTLs, enhance the recognition of CTLs to tumor cells, activate their attack and killing function, and achieve anti-tumor effect by mobilizing the body 's own immune function.
Summary of Immunotherapy in CCA
| Study | Immunotherapy regimen | Type of mechanism of action | Sample size | Median follow-up duration, months | Disease status | mPFS, months | mOS, months | ORR, % | Significantly improved survival compared to historical controls | Reference number |
|---|---|---|---|---|---|---|---|---|---|---|
| Arkenau et al. (2018) NCT02443324 | Ramucirumab plus Pembrolizumab | IgG1 VEGFR-2Antagonists and IgG4 PD-1 Antagonists | 26 | 15.7 | Advanced BTC | 1.64 | 6.44 | 3.8 | No | [35] |
| Chen et al. (2020) NCT03486678 | Camrelizumab plus GemOx | IgG4-κ; PD- 1 Antagonists and Chemotherapy | 36 | 11.8 | Advanced BTC | 6.1 | 11.8 | 80.0 (patients with PD- L1 TPS ≥1%) 53.8 (patients with PD- L1 TPS <1%) | Yes | [120] |
| Chen et al. (2021) NCT03092895 | Camrelizumab plus FolFox4 | IgG4-κ; PD- 1; Antagonists and Chemotherapy | 29 | 8.7 | Advanced BTC | 5.5 | 12.9 | 10.3 | Yes | [159] |
| Camrelizumab plus GemOx | 63 | 3.8 | 13.6 | 19.0 | ||||||
| Zhang et al. (2021) ChiCTR2100044476 | Lenvatinib plus PD-1 inhibitors (pembrolizumab/tislelizumab/sintilimab/camrelizumab/toripalimab) | Tyrosine kinase inhibitor and PD-1 Antagonists | 38 | 13.7 | Unresectable BTC | 8.0 | 17.7 | 42.1 | Yes | [14] |
| Oh et al. (2022) NCT03875235 | Durvalumab plus GemCis | IgG1-κ; PD- 1 Antagonists and Chemotherapy | 341 | 13.7 | Advanced BTC | 7.2 | 12.8 | 26.7 | Yes | [160] |
| Placebo plus GemCis | Chemotherapy | 344 | 12.6 | 5.7 | 11.5 | 18.7 | ||||
| Monge et al. (2022) NCT03111732 | Pmbrolizumab plus CapOx | IgG4-κ; PD- 1 Antagonists and Chemotherapy | 11 | 34.8 | Advanced BTC | 4.1 | 9.9 | NA | Yes | [121] |
| Kim et al. (2020) NCT02829918 | Nivolumab | IgG4; PD- 1 Antagonists | 54 | 12.4 | Advanced BTC | 3.7 | 14.2 | 22.0 | Yes | [122] |
| Piha-Paul et al. (2020); NCT02628067, NCT02054806 | Pembrolizumab | IgG4; PD- 1 Antagonists | 104 | 7.5 | Advanced BTC | 2.0 | 7.4 | 5.8 | Yes | [12] |
| 24 | 5.7 | 1.8 | 5.7 | 13 | ||||||
| Lee et al. (2020) NA | Pembrolizumab | IgG4; PD- 1 Antagonists | 51 | 3.8 | Metastatic BTC | 2.1 | 6.9 | 17 | Yes | [123] |
| Goyal et al. (2020) NCT02375880 | DKN-01 plus GemCis | A humanized monoclonal antibody targeting DKK1 and chemotherapy | 51 | NA | Advanced BTC | 8.7 | 12.4 | 21.3 | Yes | [161] |
| Feng et al. (2020) NCT03311789 | Nivolumab plus GemCis | IgG4-κ; PD- 1 Antagonists and Chemotherapy | 32 | 12.8 | Unresectable or Metastatic BTC | 6.1 | 8.5 | 33.3 | Yes | [13] |
| Xie et al. (2019) NCT01853618 | Tremelimumab plus Microwave ablation | CTLA-4 and Targeted therapy | 20 | NA | Refractory BTC | 3.4 | 6.0 | NA | Yes | [162] |
| Boilève et al. (2021) NCT03704480 | Durvalumab plus Tremelimumab plus paclitaxel | IgG1-κ; PD- 1 Antagonists plus CTLA-4 Antagonists plus Chemotherapy | 20 | NA | Advanced BTC | NA | NA | NA | NO | [163] |
Abbreviations
CCA: cholangiocarcinoma; TME: tumor microenvironment; APC: annual percentage change; BTC: biliary tract cancer; PD-1: programmed cell death protein 1; PD-L1: programmed cell death-ligand 1; OS: overall survival; mOS: median overall survival; CAFs: cancer-associated fibroblasts; ECM: extracellular matrix; HSCs: hepatic stellate cells; MET: mesenchymal to epithelial transition factor; IL-6: interleukin-6; vCAF: vascular cancer-associated fibroblast; IL-6R: interleukin 6 receptor; miR: micro RNA; Wnt: wingless/integrated; VEGF: vascular endothlial growth factor; PDGF: platelet-derived growth factor; FAP: fibroblast activation protein; STAT: signal transducer and activator of transcription; CCL: c-c motif chemokine ligand 2; eNOS: endothelial nitric oxide lyase; PGE: prostaglandin E; TGF: transforming growth factor; DKK-1: dickkopf-related protein 1; VEGFR: vascular endothelial growth factor receptor; HMGB: high mobility group box; ERK: extracellular signal-regulated kinase; HUVEC: human umbilical veins; Circ-CCAC1: CCA-associated circRNA-1; LEC: lymphatic endothelial cells; TCF: transcription factor; GPC: phosphatidylinositol proteoglycan; ADC: antibody-drug conjugates; FOXP3: forkhead box P3; MDSCs: myeloid-suppressor cells; DCs: dendritic cells; CTLs: cytotoxic T lymphocytes; FAS/FASL: fasrymndrit/fasrymndrit ligand; TANs: tumor-associated neutrophils; mIDH1: isocitrate dehydrogenase 1 mutation; TET2: tet methylcytosine dioxygenase 2; IFN: interferon; HLA: human leukocyte antigen; TILs: tumor-infiltrating lymphocytes; shRNA: short hairpin RNA; MVD: microvascular density; HPT: hypofractionated proton therapy; Th1: T helper 1; ERBB2IP: erbb2-interacting protein; MHC: major histocompatibility complex; Tregs: Regulatory T cells; CTLA: cytolytic T lymphocyte-associated antigen; LAIR: leukocyte-associated immunoglobulin-like receptor; EBF1: early B cell factor 1; TLS: tumor lysis syndrome; TNF: tumor necrosis factor; NK: natural killer; ADCC: antibody-dependent cell-mediated cytotoxicity; KIR: killer cell immunoglobulin-like receptor; CXCL: C-X-C motif chemokine ligand; TAA: thioacetamide; TRAIL: tumor necrosis factor-related apoptosis-inducing ligand; GM-CSF: granulocyte-macrophage colony-stimulating factor; IC: immune complexes; TAMs: tumor-associated macrophages; PACT: Prostate cancer associated transcript; ICB: immune checkpoint blockade; LPS: lipopolysaccharide; SCF: stem cell factor; mPFS: median progression free survival; MSI-H: microsatellite instability-high; MMR: mismatch repair; ICI: Immune checkpoint inhibitor; HCC: hepatocellular carcinoma; GEMCIS: Gemcitabine+Cisplatin; CAPOX: Capecitabine+Oxaliplatin; GEMOX: Gemcitabine+Oxaliplatin; FolFox4: Leucovorin+5-fluorouracil+oxaliplatin; EMT: epithelial-mesenchymal transition.
Acknowledgements
Funding
We are grateful for the grants from the National Natural Science Foundation of China (No. 82070676).
Author contribution
There are 4 first authors in this manuscript and they have equally contributed to this project. Dr. HSC, TH, MRD and XYK were responsible for gathering information of the related research and designing the review. Dr. MRD was responsible for drawing the pictures. Furthermore, we have two corresponding authors in this manuscript. Dr. WWT has contributed to information interpretation, editing and critical revision of the manuscript. Dr.YXX has contributed to study design and critical revision of the manuscript. Dr. WWT and YXX were also responsible for handling the revisions and re-submission of revised manuscripts. Other authors were responsible for gathering information. All authors read and approved the final manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Pellino A, Loupakis F, Cadamuro M, Dadduzio V, Fassan M, Guido M. et al. Precision medicine in cholangiocarcinoma. Transl Gastroenterol Hepatol. 2018;3:40
2. Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet. 2014;383:2168-79
3. Benson AB, D'Angelica MI, Abbott DE, Anaya DA, Anders R, Are C. et al. Hepatobiliary Cancers, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19:541-65
4. Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P. et al. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-8
5. DeOliveira ML, Cunningham SC, Cameron JL, Kamangar F, Winter JM, Lillemoe KD. et al. Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Ann Surg. 2007;245:755-62
6. Saha SK, Zhu AX, Fuchs CS, Brooks GA. Forty-Year Trends in Cholangiocarcinoma Incidence in the U.S.: Intrahepatic Disease on the Rise. Oncologist. 2016;21:594-9
7. Sapisochin G, Facciuto M, Rubbia-Brandt L, Marti J, Mehta N, Yao FY. et al. Liver transplantation for "very early" intrahepatic cholangiocarcinoma: International retrospective study supporting a prospective assessment. Hepatology. 2016;64:1178-88
8. Endo I, Gonen M, Yopp AC, Dalal KM, Zhou Q, Klimstra D. et al. Intrahepatic cholangiocarcinoma: rising frequency, improved survival, and determinants of outcome after resection. Ann Surg. 2008;248:84-96
9. Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A. et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273-81
10. Anderson NM, Simon MC. The tumor microenvironment. Curr Biol. 2020;30:R921-r5
11. Fabris L, Sato K, Alpini G, Strazzabosco M. The Tumor Microenvironment in Cholangiocarcinoma Progression. Hepatology. 2021;73(Suppl 1):75-85
12. Piha-Paul SA, Oh DY, Ueno M, Malka D, Chung HC, Nagrial A. et al. Efficacy and safety of pembrolizumab for the treatment of advanced biliary cancer: Results from the KEYNOTE-158 and KEYNOTE-028 studies. Int J Cancer. 2020;147:2190-8
13. Feng K, Liu Y, Zhao Y, Yang Q, Dong L, Liu J. et al. Efficacy and biomarker analysis of nivolumab plus gemcitabine and cisplatin in patients with unresectable or metastatic biliary tract cancers: results from a phase II study. J Immunother Cancer. 2020 8
14. Zhang Q, Liu X, Wei S, Zhang L, Tian Y, Gao Z. et al. Lenvatinib Plus PD-1 Inhibitors as First-Line Treatment in Patients With Unresectable Biliary Tract Cancer: A Single-Arm, Open-Label, Phase II Study. Front Oncol. 2021;11:751391
15. Raffaghello L, Dazzi F. Classification and biology of tumour associated stromal cells. Immunol Lett. 2015;168:175-82
16. Biffi G, Tuveson DA. Diversity and Biology of Cancer-Associated Fibroblasts. Physiol Rev. 2021;101:147-76
17. Affo S, Nair A, Brundu F, Ravichandra A, Bhattacharjee S, Matsuda M. et al. Promotion of cholangiocarcinoma growth by diverse cancer-associated fibroblast subpopulations. Cancer Cell. 2021;39:866-82.e11
18. Zhang M, Yang H, Wan L, Wang Z, Wang H, Ge C. et al. Single-cell transcriptomic architecture and intercellular crosstalk of human intrahepatic cholangiocarcinoma. J Hepatol. 2020;73:1118-30
19. Qin X, Lu M, Li G, Zhou Y, Liu Z. Downregulation of tumor-derived exosomal miR-34c induces cancer-associated fibroblast activation to promote cholangiocarcinoma progress. Cancer Cell Int. 2021;21:373
20. Cadamuro M, Brivio S, Mertens J, Vismara M, Moncsek A, Milani C. et al. Platelet-derived growth factor-D enables liver myofibroblasts to promote tumor lymphangiogenesis in cholangiocarcinoma. J Hepatol. 2019;70:700-9
21. Yang X, Lin Y, Shi Y, Li B, Liu W, Yin W. et al. FAP Promotes Immunosuppression by Cancer-Associated Fibroblasts in the Tumor Microenvironment via STAT3-CCL2 Signaling. Cancer Res. 2016;76:4124-35
22. Yangngam S, Thongchot S, Pongpaibul A, Vaeteewoottacharn K, Pinlaor S, Thuwajit P. et al. High level of interleukin-33 in cancer cells and cancer-associated fibroblasts correlates with good prognosis and suppressed migration in cholangiocarcinoma. J Cancer. 2020;11:6571-81
23. Yamanaka T, Harimoto N, Yokobori T, Muranushi R, Hoshino K, Hagiwara K. et al. Nintedanib inhibits intrahepatic cholangiocarcinoma aggressiveness via suppression of cytokines extracted from activated cancer-associated fibroblasts. Br J Cancer. 2020;122:986-94
24. Yang R, Wang D, Han S, Gu Y, Li Z, Deng L. et al. MiR-206 suppresses the deterioration of intrahepatic cholangiocarcinoma and promotes sensitivity to chemotherapy by inhibiting interactions with stromal CAFs. Int J Biol Sci. 2022;18:43-64
25. Hida K, Maishi N, Annan DA, Hida Y. Contribution of Tumor Endothelial Cells in Cancer Progression. Int J Mol Sci. 2018 19
26. Suksawat M, Techasen A, Namwat N, Yongvanit P, Khuntikeo N, Titapun A. et al. Upregulation of endothelial nitric oxide synthase (eNOS) and its upstream regulators in Opisthorchis viverrini associated cholangiocarcinoma and its clinical significance. Parasitol Int. 2017;66:486-93
27. De Sanctis F, Ugel S, Facciponte J, Facciabene A. The dark side of tumor-associated endothelial cells. Semin Immunol. 2018;35:35-47
28. Wang Y, Yu H, Xie X, Deng T, Ye L, Wu L. et al. Plasmalemma vesicle-associated protein promotes angiogenesis in cholangiocarcinoma via the DKK1/CKAP4/PI3K signaling pathway. Oncogene. 2021;40:4324-37
29. Xu YF, Liu ZL, Pan C, Yang XQ, Ning SL, Liu HD. et al. HMGB1 correlates with angiogenesis and poor prognosis of perihilar cholangiocarcinoma via elevating VEGFR2 of vessel endothelium. Oncogene. 2019;38:868-80
30. Gentilini A, Lori G, Caligiuri A, Raggi C, Di Maira G, Pastore M. et al. Extracellular Signal-Regulated Kinase 5 Regulates the Malignant Phenotype of Cholangiocarcinoma Cells. Hepatology. 2021;74:2007-20
31. Xu Y, Leng K, Yao Y, Kang P, Liao G, Han Y. et al. A Circular RNA, Cholangiocarcinoma-Associated Circular RNA 1, Contributes to Cholangiocarcinoma Progression, Induces Angiogenesis, and Disrupts Vascular Endothelial Barriers. Hepatology. 2021;73:1419-35
32. Spratlin JL, Cohen RB, Eadens M, Gore L, Camidge DR, Diab S. et al. Phase I pharmacologic and biologic study of ramucirumab (IMC-1121B), a fully human immunoglobulin G1 monoclonal antibody targeting the vascular endothelial growth factor receptor-2. J Clin Oncol. 2010;28:780-7
33. Wilke H, Muro K, Van Cutsem E, Oh SC, Bodoky G, Shimada Y. et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15:1224-35
34. Garon EB, Ciuleanu TE, Arrieta O, Prabhash K, Syrigos KN, Goksel T. et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet. 2014;384:665-73
35. Arkenau HT, Martin-Liberal J, Calvo E, Penel N, Krebs MG, Herbst RS. et al. Ramucirumab Plus Pembrolizumab in Patients with Previously Treated Advanced or Metastatic Biliary Tract Cancer: Nonrandomized, Open-Label, Phase I Trial (JVDF). Oncologist. 2018;23:1407-e136
36. Duan HX, Li BW, Zhuang X, Wang LT, Cao Q, Tan LH. et al. TCF21 inhibits tumor-associated angiogenesis and suppresses the growth of cholangiocarcinoma by targeting PI3K/Akt and ERK signaling. Am J Physiol Gastrointest Liver Physiol. 2019;316:G763-g73
37. Yokota K, Serada S, Tsujii S, Toya K, Takahashi T, Matsunaga T. et al. Anti-Glypican-1 Antibody-drug Conjugate as Potential Therapy Against Tumor Cells and Tumor Vasculature for Glypican-1-Positive Cholangiocarcinoma. Mol Cancer Ther. 2021;20:1713-22
38. Nair A, Ingram N, Verghese ET, Wijetunga I, Markham AF, Wyatt J. et al. CD105 is a prognostic marker and valid endothelial target for microbubble platforms in cholangiocarcinoma. Cell Oncol (Dordr). 2020;43:835-45
39. Leone RD, Powell JD. Metabolism of immune cells in cancer. Nat Rev Cancer. 2020;20:516-31
40. Kitano Y, Okabe H, Yamashita YI, Nakagawa S, Saito Y, Umezaki N. et al. Tumour-infiltrating inflammatory and immune cells in patients with extrahepatic cholangiocarcinoma. Br J Cancer. 2018;118:171-80
41. Wu MJ, Shi L, Dubrot J, Merritt J, Vijay V, Wei TY. et al. Mutant IDH Inhibits IFNγ-TET2 Signaling to Promote Immunoevasion and Tumor Maintenance in Cholangiocarcinoma. Cancer Discov. 2022;12:812-35
42. Huang YH, Zhang CZ, Huang QS, Yeong J, Wang F, Yang X. et al. Clinicopathologic features, tumor immune microenvironment and genomic landscape of Epstein-Barr virus-associated intrahepatic cholangiocarcinoma. J Hepatol. 2021;74:838-49
43. Asahi Y, Hatanaka KC, Hatanaka Y, Kamiyama T, Orimo T, Shimada S. et al. Prognostic impact of CD8+ T cell distribution and its association with the HLA class I expression in intrahepatic cholangiocarcinoma. Surg Today. 2020;50:931-40
44. Kim HD, Jeong S, Park S, Lee YJ, Ju YS, Kim D. et al. Implication of CD69(+) CD103(+) tissue-resident-like CD8(+) T cells as a potential immunotherapeutic target for cholangiocarcinoma. Liver Int. 2021;41:764-76
45. Chen LJ, Sun J, Wu HY, Zhou SM, Tan Y, Tan M. et al. B7-H4 expression associates with cancer progression and predicts patient's survival in human esophageal squamous cell carcinoma. Cancer Immunol Immunother. 2011;60:1047-55
46. Sun Y, Wang Y, Zhao J, Gu M, Giscombe R, Lefvert AK. et al. B7-H3 and B7-H4 expression in non-small-cell lung cancer. Lung Cancer. 2006;53:143-51
47. Zhao X, Guo F, Li Z, Jiang P, Deng X, Tian F. et al. Aberrant expression of B7-H4 correlates with poor prognosis and suppresses tumor-infiltration of CD8+ T lymphocytes in human cholangiocarcinoma. Oncol Rep. 2016;36:419-27
48. Kasper HU, Drebber U, Stippel DL, Dienes HP, Gillessen A. Liver tumor infiltrating lymphocytes: comparison of hepatocellular and cholangiolar carcinoma. World J Gastroenterol. 2009;15:5053-7
49. Tamma R, Annese T, Ruggieri S, Brunetti O, Longo V, Cascardi E. et al. Inflammatory cells infiltrate and angiogenesis in locally advanced and metastatic cholangiocarcinoma. Eur J Clin Invest. 2019;49:e13087
50. Kida A, Mizukoshi E, Kido H, Toyama T, Terashima T, Arai K. et al. The characteristics of the immune cell profiles in peripheral blood in cholangiocarcinoma patients. Hepatol Int. 2021;15:695-706
51. Carapeto F, Bozorgui B, Shroff RT, Chagani S, Solis Soto L, Foo WC. et al. The immunogenomic landscape of resected intrahepatic cholangiocarcinoma. Hepatology. 2022;75:297-308
52. Grassberger C, Hong TS, Hato T, Yeap BY, Wo JY, Tracy M. et al. Differential Association Between Circulating Lymphocyte Populations With Outcome After Radiation Therapy in Subtypes of Liver Cancer. Int J Radiat Oncol Biol Phys. 2018;101:1222-5
53. Tran E, Turcotte S, Gros A, Robbins PF, Lu YC, Dudley ME. et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science. 2014;344:641-5
54. Wabitsch S, Tandon M, Ruf B, Zhang Q, McCallen JD, McVey JC. et al. Anti-PD-1 in Combination With Trametinib Suppresses Tumor Growth and Improves Survival of Intrahepatic Cholangiocarcinoma in Mice. Cell Mol Gastroenterol Hepatol. 2021;12:1166-78
55. Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330-6
56. Göschl L, Scheinecker C, Bonelli M. Treg cells in autoimmunity: from identification to Treg-based therapies. Semin Immunopathol. 2019;41:301-14
57. Oderup C, Cederbom L, Makowska A, Cilio CM, Ivars F. Cytotoxic T lymphocyte antigen-4-dependent down-modulation of costimulatory molecules on dendritic cells in CD4+ CD25+ regulatory T-cell-mediated suppression. Immunology. 2006;118:240-9
58. Kim HD, Kim JH, Ryu YM, Kim D, Lee S, Shin J. et al. Spatial Distribution and Prognostic Implications of Tumor-Infiltrating FoxP3- CD4+ T Cells in Biliary Tract Cancer. Cancer Res Treat. 2021;53:162-71
59. Kinoshita M, Kobayashi S, Gotoh K, Kubo M, Hayashi K, Iwagami Y. et al. Heterogeneity of Treg/Th17 According to Cancer Progression and Modification in Biliary Tract Cancers via Self-Producing Cytokines. Dig Dis Sci. 2020;65:2937-48
60. Chen Z, Yu M, Yan J, Guo L, Zhang B, Liu S. et al. PNOC Expressed by B Cells in Cholangiocarcinoma Was Survival Related and LAIR2 Could Be a T Cell Exhaustion Biomarker in Tumor Microenvironment: Characterization of Immune Microenvironment Combining Single-Cell and Bulk Sequencing Technology. Front Immunol. 2021;12:647209
61. Zhang B, Vogelzang A, Miyajima M, Sugiura Y, Wu Y, Chamoto K. et al. B cell-derived GABA elicits IL-10(+) macrophages to limit anti-tumour immunity. Nature. 2021;599:471-6
62. Garaud S, Zayakin P, Buisseret L, Rulle U, Silina K, de Wind A. et al. Antigen Specificity and Clinical Significance of IgG and IgA Autoantibodies Produced in situ by Tumor-Infiltrating B Cells in Breast Cancer. Front Immunol. 2018;9:2660
63. Cui C, Wang J, Fagerberg E, Chen PM, Connolly KA, Damo M. et al. Neoantigen-driven B cell and CD4 T follicular helper cell collaboration promotes anti-tumor CD8 T cell responses. Cell. 2021;184:6101-18.e13
64. Armartmuntree N, Murata M, Techasen A, Yongvanit P, Loilome W, Namwat N. et al. Prolonged oxidative stress down-regulates Early B cell factor 1 with inhibition of its tumor suppressive function against cholangiocarcinoma genesis. Redox Biol. 2018;14:637-44
65. Eriksson E, Moreno R, Milenova I, Liljenfeldt L, Dieterich LC, Christiansson L. et al. Activation of myeloid and endothelial cells by CD40L gene therapy supports T-cell expansion and migration into the tumor microenvironment. Gene Ther. 2017;24:92-103
66. Diggs LP, Ruf B, Ma C, Heinrich B, Cui L, Zhang Q. et al. CD40-mediated immune cell activation enhances response to anti-PD-1 in murine intrahepatic cholangiocarcinoma. J Hepatol. 2021;74:1145-54
67. Martín-Sierra C, Martins R, Laranjeira P, Abrantes AM, Oliveira RC, Tralhão JG. et al. Functional Impairment of Circulating FcεRI(+) Monocytes and Myeloid Dendritic Cells in Hepatocellular Carcinoma and Cholangiocarcinoma Patients. Cytometry B Clin Cytom. 2019;96:490-5
68. Jiraviriyakul A, Songjang W, Kaewthet P, Tanawatkitichai P, Bayan P, Pongcharoen S. Honokiol-enhanced cytotoxic T lymphocyte activity against cholangiocarcinoma cells mediated by dendritic cells pulsed with damage-associated molecular patterns. World J Gastroenterol. 2019;25:3941-55
69. Thepmalee C, Panya A, Junking M, Chieochansin T, Yenchitsomanus PT. Inhibition of IL-10 and TGF-β receptors on dendritic cells enhances activation of effector T-cells to kill cholangiocarcinoma cells. Hum Vaccin Immunother. 2018;14:1423-31
70. Shimizu K, Kotera Y, Aruga A, Takeshita N, Takasaki K, Yamamoto M. Clinical utilization of postoperative dendritic cell vaccine plus activated T-cell transfer in patients with intrahepatic cholangiocarcinoma. J Hepatobiliary Pancreat Sci. 2012;19:171-8
71. Myers JA, Miller JS. Exploring the NK cell platform for cancer immunotherapy. Nat Rev Clin Oncol. 2021;18:85-100
72. Cornillet M, Jansson H, Schaffer M, Hertwig L, Berglin L, Zimmer CL. et al. Imbalance of Genes Encoding Natural Killer Immunoglobulin-Like Receptors and Human Leukocyte Antigen in Patients With Biliary Cancer. Gastroenterology. 2019;157:1067-80.e9
73. Cazzetta V, Franzese S, Carenza C, Della Bella S, Mikulak J, Mavilio D. Natural Killer-Dendritic Cell Interactions in Liver Cancer: Implications for Immunotherapy. Cancers (Basel). 2021 13
74. Fukuda Y, Asaoka T, Eguchi H, Yokota Y, Kubo M, Kinoshita M. et al. Endogenous CXCL9 affects prognosis by regulating tumor-infiltrating natural killer cells in intrahepatic cholangiocarcinoma. Cancer Sci. 2020;111:323-33
75. Carnevale G, Carpino G, Cardinale V, Pisciotta A, Riccio M, Bertoni L. et al. Activation of Fas/FasL pathway and the role of c-FLIP in primary culture of human cholangiocarcinoma cells. Sci Rep. 2017;7:14419
76. Hung TH, Hung JT, Wu CE, Huang Y, Lee CW, Yeh CT. et al. Globo H Is a Promising Theranostic Marker for Intrahepatic Cholangiocarcinoma. Hepatol Commun. 2022;6:194-208
77. Panwong S, Wathikthinnakon M, Kaewkod T, Sawasdee N, Tragoolpua Y, Yenchitsomanus PT. et al. Cordycepin Sensitizes Cholangiocarcinoma Cells to Be Killed by Natural Killer-92 (NK-92) Cells. Molecules. 2021 26
78. Jung IH, Kim DH, Yoo DK, Baek SY, Jeong SH, Jung DE. et al. In vivo Study of Natural Killer (NK) Cell Cytotoxicity Against Cholangiocarcinoma in a Nude Mouse Model. In vivo. 2018;32:771-81
79. Shaul ME, Fridlender ZG. Neutrophils as active regulators of the immune system in the tumor microenvironment. J Leukoc Biol. 2017;102:343-9
80. Giese MA, Hind LE, Huttenlocher A. Neutrophil plasticity in the tumor microenvironment. Blood. 2019;133:2159-67
81. Gao Y, Zhang H, Zhou N, Xu P, Wang J, Gao Y. et al. Methotrexate-loaded tumour-cell-derived microvesicles can relieve biliary obstruction in patients with extrahepatic cholangiocarcinoma. Nat Biomed Eng. 2020;4:743-53
82. Zhou SL, Dai Z, Zhou ZJ, Chen Q, Wang Z, Xiao YS. et al. CXCL5 contributes to tumor metastasis and recurrence of intrahepatic cholangiocarcinoma by recruiting infiltrative intratumoral neutrophils. Carcinogenesis. 2014;35:597-605
83. Gu FM, Gao Q, Shi GM, Zhang X, Wang J, Jiang JH. et al. Intratumoral IL-17⁺ cells and neutrophils show strong prognostic significance in intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2012;19:2506-14
84. Mao ZY, Zhu GQ, Xiong M, Ren L, Bai L. Prognostic value of neutrophil distribution in cholangiocarcinoma. World J Gastroenterol. 2015;21:4961-8
85. Wang LX, Zhang SX, Wu HJ, Rong XL, Guo J. M2b macrophage polarization and its roles in diseases. J Leukoc Biol. 2019;106:345-58
86. Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122:787-95
87. Talmadge JE, Donkor M, Scholar E. Inflammatory cell infiltration of tumors: Jekyll or Hyde. Cancer Metastasis Rev. 2007;26:373-400
88. Tu J, Wu F, Chen L, Zheng L, Yang Y, Ying X. et al. Long Non-Coding RNA PCAT6 Induces M2 Polarization of Macrophages in Cholangiocarcinoma via Modulating miR-326 and RhoA-ROCK Signaling Pathway. Front Oncol. 2020;10:605877
89. Locati M, Curtale G, Mantovani A. Diversity, Mechanisms, and Significance of Macrophage Plasticity. Annu Rev Pathol. 2020;15:123-47
90. Sica A, Schioppa T, Mantovani A, Allavena P. Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: potential targets of anti-cancer therapy. Eur J Cancer. 2006;42:717-27
91. Zhang Y, Chen S, Li J, Dai W, Qian Y. Immune infiltrating cells in cholangiocarcinoma may become clinical diagnostic markers: based on bioinformatics analysis. World J Surg Oncol. 2021;19:59
92. Thanee M, Loilome W, Techasen A, Namwat N, Boonmars T, Pairojkul C. et al. Quantitative changes in tumor-associated M2 macrophages characterize cholangiocarcinoma and their association with metastasis. Asian Pac J Cancer Prev. 2015;16:3043-50
93. Sun D, Luo T, Dong P, Zhang N, Chen J, Zhang S. et al. M2-polarized tumor-associated macrophages promote epithelial-mesenchymal transition via activation of the AKT3/PRAS40 signaling pathway in intrahepatic cholangiocarcinoma. J Cell Biochem. 2020;121:2828-38
94. Dwyer BJ, Jarman EJ, Gogoi-Tiwari J, Ferreira-Gonzalez S, Boulter L, Guest RV. et al. TWEAK/Fn14 signalling promotes cholangiocarcinoma niche formation and progression. J Hepatol. 2021;74:860-72
95. Saito Y, Nakaoka T, Muramatsu T, Ojima H, Sukeda A, Sugiyama Y. et al. Induction of differentiation of intrahepatic cholangiocarcinoma cells to functional hepatocytes using an organoid culture system. Sci Rep. 2018;8:2821
96. Chen S, Chen Z, Li Z, Li S, Wen Z, Cao L. et al. Tumor-associated macrophages promote cholangiocarcinoma progression via exosomal Circ_0020256. Cell Death Dis. 2022;13:94
97. Luo C, Xin H, Zhou Z, Hu Z, Sun R, Yao N. et al. Tumor-derived exosomes induce immunosuppressive macrophages to foster intrahepatic cholangiocarcinoma progression. Hepatology. 2022
98. Zhou Z, Wang P, Sun R, Li J, Hu Z, Xin H. et al. Tumor-associated neutrophils and macrophages interaction contributes to intrahepatic cholangiocarcinoma progression by activating STAT3. J Immunother Cancer. 2021 9
99. Ruffolo LI, Jackson KM, Kuhlers PC, Dale BS, Figueroa Guilliani NM, Ullman NA. et al. GM-CSF drives myelopoiesis, recruitment and polarisation of tumour-associated macrophages in cholangiocarcinoma and systemic blockade facilitates antitumour immunity. Gut. 2022;71:1386-98
100. Atanasov G, Dietel C, Feldbrügge L, Benzing C, Krenzien F, Brandl A. et al. Tumor necrosis and infiltrating macrophages predict survival after curative resection for cholangiocarcinoma. Oncoimmunology. 2017;6:e1331806
101. Veglia F, Perego M, Gabrilovich D. Myeloid-derived suppressor cells coming of age. Nat Immunol. 2018;19:108-19
102. Loeuillard E, Yang J, Buckarma E, Wang J, Liu Y, Conboy C. et al. Targeting tumor-associated macrophages and granulocytic myeloid-derived suppressor cells augments PD-1 blockade in cholangiocarcinoma. J Clin Invest. 2020;130:5380-96
103. Zhang Q, Ma C, Duan Y, Heinrich B, Rosato U, Diggs LP. et al. Gut Microbiome Directs Hepatocytes to Recruit MDSCs and Promote Cholangiocarcinoma. Cancer Discov. 2021;11:1248-67
104. da Silva EZ, Jamur MC, Oliver C. Mast cell function: a new vision of an old cell. J Histochem Cytochem. 2014;62:698-738
105. Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiol Rev. 1997;77:1033-79
106. Galli SJ, Tsai M. Mast cells: versatile regulators of inflammation, tissue remodeling, host defense and homeostasis. J Dermatol Sci. 2008;49:7-19
107. Williams CM, Galli SJ. The diverse potential effector and immunoregulatory roles of mast cells in allergic disease. J Allergy Clin Immunol. 2000;105:847-59
108. Redegeld FA, Yu Y, Kumari S, Charles N, Blank U. Non-IgE mediated mast cell activation. Immunol Rev. 2018;282:87-113
109. Johnson C, Huynh V, Hargrove L, Kennedy L, Graf-Eaton A, Owens J. et al. Inhibition of Mast Cell-Derived Histamine Decreases Human Cholangiocarcinoma Growth and Differentiation via c-Kit/Stem Cell Factor-Dependent Signaling. Am J Pathol. 2016;186:123-33
110. Sheu LF, Lee WC, Lee HS, Kao WY, Chen A. Co-expression of c-kit and stem cell factor in primary and metastatic nasopharyngeal carcinomas and nasopharyngeal epithelium. J Pathol. 2005;207:216-23
111. Strijker M, Belkouz A, van der Geest LG, van Gulik TM, van Hooft JE, de Meijer VE. et al. Treatment and survival of resected and unresected distal cholangiocarcinoma: a nationwide study. Acta Oncol. 2019;58:1048-55
112. Jarnagin WR, Fong Y, DeMatteo RP, Gonen M, Burke EC, Bodniewicz BJ. et al. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg. 2001;234:507-17 discussion 17-9
113. Kim Y, Amini N, Wilson A, Margonis GA, Ethun CG, Poultsides G. et al. Impact of Chemotherapy and External-Beam Radiation Therapy on Outcomes among Patients with Resected Gallbladder Cancer: A Multi-institutional Analysis. Ann Surg Oncol. 2016;23:2998-3008
114. Lawrence TS, Robertson JM, Anscher MS, Jirtle RL, Ensminger WD, Fajardo LF. Hepatic toxicity resulting from cancer treatment. Int J Radiat Oncol Biol Phys. 1995;31:1237-48
115. Primrose JN, Fox RP, Palmer DH, Malik HZ, Prasad R, Mirza D. et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019;20:663-73
116. Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK. et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409-13
117. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY. et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. 2020;382:1894-905
118. Xia Y, Tang W, Qian X, Li X, Cheng F, Wang K. et al. Efficacy and safety of camrelizumab plus apatinib during the perioperative period in resectable hepatocellular carcinoma: a single-arm, open label, phase II clinical trial. J Immunother Cancer. 2022 10
119. Oh D-Y, He AR, Qin S, Chen L-T, Okusaka T, Vogel A. et al. A phase 3 randomized, double-blind, placebo-controlled study of durvalumab in combination with gemcitabine plus cisplatin (GemCis) in patients (pts) with advanced biliary tract cancer (BTC): TOPAZ-1. Journal of Clinical Oncology. 2022;40:378 -
120. Chen X, Wu X, Wu H, Gu Y, Shao Y, Shao Q. et al. Camrelizumab plus gemcitabine and oxaliplatin (GEMOX) in patients with advanced biliary tract cancer: a single-arm, open-label, phase II trial. J Immunother Cancer. 2020 8
121. Monge C, Pehrsson EC, Xie C, Duffy AG, Mabry D, Wood BJ. et al. A Phase II Study of Pembrolizumab in Combination with Capecitabine and Oxaliplatin with Molecular Profiling in Patients with Advanced Biliary Tract Carcinoma. Oncologist. 2022;27:e273-e85
122. Kim RD, Chung V, Alese OB, El-Rayes BF, Li D, Al-Toubah TE. et al. A Phase 2 Multi-institutional Study of Nivolumab for Patients With Advanced Refractory Biliary Tract Cancer. JAMA Oncol. 2020;6:888-94
123. Lee SH, Lee HS, Lee SH, Woo SM, Kim DU, Bang S. Efficacy and Safety of Pembrolizumab for Gemcitabine/Cisplatin-Refractory Biliary Tract Cancer: A Multicenter Retrospective Study. J Clin Med. 2020 9
124. Lan C, Kitano Y, Yamashita YI, Yamao T, Kajiyama K, Yoshizumi T. et al. Cancer-associated fibroblast senescence and its relation with tumour-infiltrating lymphocytes and PD-L1 expressions in intrahepatic cholangiocarcinoma. Br J Cancer. 2022;126:219-27
125. Zhang XF, Dong M, Pan YH, Chen JN, Huang XQ, Jin Y. et al. Expression pattern of cancer-associated fibroblast and its clinical relevance in intrahepatic cholangiocarcinoma. Hum Pathol. 2017;65:92-100
126. Sha M, Jeong S, Qiu BJ, Tong Y, Xia L, Xu N. et al. Isolation of cancer-associated fibroblasts and its promotion to the progression of intrahepatic cholangiocarcinoma. Cancer Med. 2018;7:4665-77
127. Nicolás-Boluda A, Vaquero J, Laurent G, Renault G, Bazzi R, Donnadieu E. et al. Photothermal Depletion of Cancer-Associated Fibroblasts Normalizes Tumor Stiffness in Desmoplastic Cholangiocarcinoma. ACS Nano. 2020;14:5738-53
128. Thongchot S, Vidoni C, Ferraresi A, Loilome W, Khuntikeo N, Sangkhamanon S. et al. Cancer-Associated Fibroblast-Derived IL-6 Determines Unfavorable Prognosis in Cholangiocarcinoma by Affecting Autophagy-Associated Chemoresponse. Cancers (Basel). 2021 13
129. Lobe C, Vallette M, Arbelaiz A, Gonzalez-Sanchez E, Izquierdo L, Pellat A. et al. Zinc Finger E-Box Binding Homeobox 1 Promotes Cholangiocarcinoma Progression Through Tumor Dedifferentiation and Tumor-Stroma Paracrine Signaling. Hepatology. 2021;74:3194-212
130. Byrling J, Sasor A, Nilsson J, Said Hilmersson K, Andersson R, Andersson B. Expression of fibroblast activation protein and the clinicopathological relevance in distal cholangiocarcinoma. Scand J Gastroenterol. 2020;55:82-9
131. Lin Y, Cai Q, Chen Y, Shi T, Liu W, Mao L. et al. CAFs shape myeloid-derived suppressor cells to promote stemness of intrahepatic cholangiocarcinoma through 5-lipoxygenase. Hepatology. 2022;75:28-42
132. Vaquero J, Lobe C, Tahraoui S, Clapéron A, Mergey M, Merabtene F. et al. The IGF2/IR/IGF1R Pathway in Tumor Cells and Myofibroblasts Mediates Resistance to EGFR Inhibition in Cholangiocarcinoma. Clin Cancer Res. 2018;24:4282-96
133. Aoki S, Inoue K, Klein S, Halvorsen S, Chen J, Matsui A. et al. Placental growth factor promotes tumour desmoplasia and treatment resistance in intrahepatic cholangiocarcinoma. Gut. 2022;71:185-93
134. Thongchot S, Ferraresi A, Vidoni C, Loilome W, Yongvanit P, Namwat N. et al. Resveratrol interrupts the pro-invasive communication between cancer associated fibroblasts and cholangiocarcinoma cells. Cancer Lett. 2018;430:160-71
135. Kabashima A, Hirsova P, Bronk SF, Hernandez MC, Truty MJ, Rizvi S. et al. Fibroblast growth factor receptor inhibition induces loss of matrix MCL1 and necrosis in cholangiocarcinoma. J Hepatol. 2018;68:1228-38
136. Cadamuro M, Nardo G, Indraccolo S, Dall'olmo L, Sambado L, Moserle L. et al. Platelet-derived growth factor-D and Rho GTPases regulate recruitment of cancer-associated fibroblasts in cholangiocarcinoma. Hepatology. 2013;58:1042-53
137. Utaijaratrasmi P, Vaeteewoottacharn K, Tsunematsu T, Jamjantra P, Wongkham S, Pairojkul C. et al. The microRNA-15a-PAI-2 axis in cholangiocarcinoma-associated fibroblasts promotes migration of cancer cells. Mol Cancer. 2018;17:10
138. Mertens JC, Fingas CD, Christensen JD, Smoot RL, Bronk SF, Werneburg NW. et al. Therapeutic effects of deleting cancer-associated fibroblasts in cholangiocarcinoma. Cancer Res. 2013;73:897-907
139. Carpino G, Cardinale V, Di Giamberardino A, Overi D, Donsante S, Colasanti T. et al. Thrombospondin 1 and 2 along with PEDF inhibit angiogenesis and promote lymphangiogenesis in intrahepatic cholangiocarcinoma. J Hepatol. 2021;75:1377-86
140. Deng M, Li SH, Fu X, Yan XP, Chen J, Qiu YD. et al. Relationship between PD-L1 expression, CD8+ T-cell infiltration and prognosis in intrahepatic cholangiocarcinoma patients. Cancer Cell Int. 2021;21:371
141. Miyazaki K, Morine Y, Imura S, Ikemoto T, Saito Y, Yamada S. et al. Preoperative lymphocyte/C-reactive protein ratio and its correlation with CD8(+) tumor-infiltrating lymphocytes as a predictor of prognosis after resection of intrahepatic cholangiocarcinoma. Surg Today. 2021;51:1985-95
142. Vigano L, Soldani C, Franceschini B, Cimino M, Lleo A, Donadon M. et al. Tumor-Infiltrating Lymphocytes and Macrophages in Intrahepatic Cholangiocellular Carcinoma. Impact on Prognosis after Complete Surgery. J Gastrointest Surg. 2019;23:2216-24
143. Su SB, Zhang JF, Huang FF, Cen Y, Jiang HX. Large numbers of interleukins-22- and -17A-producing T helper cells in cholangiocarcinoma related to liver fluke infection. Microbiol Immunol. 2017;61:345-54
144. Ding GY, Ma JQ, Yun JP, Chen X, Ling Y, Zhang S. et al. Distribution and density of tertiary lymphoid structures predict clinical outcome in intrahepatic cholangiocarcinoma. J Hepatol. 2022;76:608-18
145. Sadeghlar F, Vogt A, Mohr RU, Mahn R, van Beekum K, Kornek M. et al. Induction of cytotoxic effector cells towards cholangiocellular, pancreatic, and colorectal tumor cells by activation of the immune checkpoint CD40/CD40L on dendritic cells. Cancer Immunol Immunother. 2021;70:1451-64
146. Hu ZQ, Zhou ZJ, Luo CB, Xin HY, Li J, Yu SY. et al. Peritumoral plasmacytoid dendritic cells predict a poor prognosis for intrahepatic cholangiocarcinoma after curative resection. Cancer Cell Int. 2020;20:582
147. Thepmalee C, Panya A, Sujjitjoon J, Sawasdee N, Poungvarin N, Junking M. et al. Suppression of TGF-β and IL-10 receptors on self-differentiated dendritic cells by short-hairpin RNAs enhanced activation of effector T-cells against cholangiocarcinoma cells. Hum Vaccin Immunother. 2020;16:2318-27
148. Kulma I, Panrit L, Plengsuriyakarn T, Chaijaroenkul W, Warathumpitak S, Na-Bangchang K. A randomized placebo-controlled phase I clinical trial to evaluate the immunomodulatory activities of Atractylodes lancea (Thunb) DC. in healthy Thai subjects. BMC Complement Med Ther. 2021;21:61
149. Zeng J, Liu Z, Sun S, Xie J, Cao L, Lv P. et al. Tumor-associated macrophages recruited by periostin in intrahepatic cholangiocarcinoma stem cells. Oncol Lett. 2018;15:8681-6
150. Ekuban A, Zong C, Ekuban FA, Kimura Y, Takizawa R, Morikawa K. et al. Role of Macrophages in Cytotoxicity, Reactive Oxygen Species Production and DNA Damage in 1,2-Dichloropropane-Exposed Human Cholangiocytes In vitro. Toxics. 2021 9
151. Yang T, Deng Z, Xu L, Li X, Yang T, Qian Y. et al. Macrophages-aPKC(ɩ)-CCL5 Feedback Loop Modulates the Progression and Chemoresistance in Cholangiocarcinoma. J Exp Clin Cancer Res. 2022;41:23
152. Yuan H, Lin Z, Liu Y, Jiang Y, Liu K, Tu M. et al. Intrahepatic cholangiocarcinoma induced M2-polarized tumor-associated macrophages facilitate tumor growth and invasiveness. Cancer Cell Int. 2020;20:586
153. Raggi C, Correnti M, Sica A, Andersen JB, Cardinale V, Alvaro D. et al. Cholangiocarcinoma stem-like subset shapes tumor-initiating niche by educating associated macrophages. J Hepatol. 2017;66:102-15
154. Hasita H, Komohara Y, Okabe H, Masuda T, Ohnishi K, Lei XF. et al. Significance of alternatively activated macrophages in patients with intrahepatic cholangiocarcinoma. Cancer Sci. 2010;101:1913-9
155. Kunk PR, Dougherty SC, Lynch K, Whitehair R, Meneveau M, Obeid JM. et al. Myeloid Cell Infiltration Correlates With Prognosis in Cholangiocarcinoma and Varies Based on Tumor Location. J Immunother. 2021;44:254-63
156. Zong C, Kimura Y, Kinoshita K, Takasu S, Zhang X, Sakurai T. et al. Exposure to 1,2-Dichloropropane Upregulates the Expression of Activation-Induced Cytidine Deaminase (AID) in Human Cholangiocytes Co-Cultured With Macrophages. Toxicol Sci. 2019;168:137-48
157. Loilome W, Bungkanjana P, Techasen A, Namwat N, Yongvanit P, Puapairoj A. et al. Activated macrophages promote Wnt/β-catenin signaling in cholangiocarcinoma cells. Tumour Biol. 2014;35:5357-67
158. Terada T, Matsunaga Y. Increased mast cells in hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Hepatol. 2000;33:961-6
159. Chen X, Qin S, Gu S, Ren Z, Chen Z, Xiong J. et al. Camrelizumab plus oxaliplatin-based chemotherapy as first-line therapy for advanced biliary tract cancer: A multicenter, phase 2 trial. Int J Cancer. 2021;149:1944-54
160. Oh D-Y, He AR, Qin S, Chen L-T, Okusaka T, Vogel A. et al. A phase 3 randomized, double-blind, placebo-controlled study of durvalumab in combination with gemcitabine plus cisplatin (GemCis) in patients (pts) with advanced biliary tract cancer (BTC): TOPAZ-1. Journal of Clinical Oncology. 2022;40:378 -
161. Goyal L, Sirard C, Schrag M, Kagey MH, Eads JR, Stein S. et al. Phase I and Biomarker Study of the Wnt Pathway Modulator DKN-01 in Combination with Gemcitabine/Cisplatin in Advanced Biliary Tract Cancer. Clin Cancer Res. 2020;26:6158-67
162. Xie C, Duffy AG, Mabry-Hrones D, Wood B, Levy E, Krishnasamy V. et al. Tremelimumab in Combination With Microwave Ablation in Patients With Refractory Biliary Tract Cancer. Hepatology. 2019;69:2048-60
163. Boilève A, Hilmi M, Gougis P, Cohen R, Rousseau B, Blanc JF. et al. Triplet combination of durvalumab, tremelimumab, and paclitaxel in biliary tract carcinomas: Safety run-in results of the randomized IMMUNOBIL PRODIGE 57 phase II trial. Eur J Cancer. 2021;143:55-63
Author contact
![]() Corresponding authors: Dr. Yongxiang Xia, E-mail: yx_xiaedu.cn; Dr. Weiwei Tang, E-mail: 1243773473twwwcom.
Corresponding authors: Dr. Yongxiang Xia, E-mail: yx_xiaedu.cn; Dr. Weiwei Tang, E-mail: 1243773473twwwcom.

 Global reach, higher impact
Global reach, higher impact