Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2022; 18(15):5928-5942. doi:10.7150/ijbs.76924 This issue Cite
Review
Hepatic FGF21: Its Emerging Role in Inter-Organ Crosstalk and Cancers
1. School of Chinese Medicine, The University of Hong Kong, Pokfulam, Hong Kong, China.
2. Shenzhen Institute of Research and Innovation, The University of Hong Kong, Shenzhen, China.
Received 2022-7-7; Accepted 2022-9-18; Published 2022-10-3
Abstract
Fibroblast growth factor (FGF) 21 is one of the FGF members with special endocrine properties. In the last twenty years, it has attracted intense research and development for its physiological functions that respond to dietary manipulation, pharmacological benefits of improving the macronutrient metabolism, and clinical values as a biomarker of various human diseases. Generally, FGF21 can be produced by major metabolic organs, but only the subgroup from the liver shows canonical endocrine properties, which emphasizes the special value of delineating the unique secretory and functional characteristics of hepatic FGF21. There has been a growth in literature to address the extra-hepatic activities of FGF21, and many striking findings have therefore been published. Yet, they are fragmented and scattered, and controversies are raised from divergent findings. For this reason, there is a need for a systematic and critical evaluation of current research in this aspect. In this review, we focus on the current knowledge about the molecular biology of endocrine FGF21, especially present details on the regulation of circulating levels of FGF21. We also emphasize its emerging roles in inter-organ crosstalk and cancer development.
Keywords: Fibroblast growth factor 21, Hepatic FGF21, Endocrine FGF21, Liver, Inter-organ crosstalk, Cancer
Introduction
The mammalian fibroblast growth factor (FGF) superfamily consists of 23 members, which are grouped into 7 subfamilies based on their sequence homology and function. FGF19 (FGF15 for mice) is the only endocrine-acting subfamily that can secrete into the circulation and function as hormones [1]. FGF21 is a member of FGF19 family identified in 2000 by Nishimura, Nakatake [2]. Over the last two decades, FGF21 has attracted great interest due to its pleiotropic metabolic effects in response to diverse physiological and pathological stress (Figure 1). With the expansion of knowledge of FGF21, our understanding of its biology is constantly undergoing modification, especially for the liver-secreted FGF21, which is considered the main source of circulating FGF21 and has been implicated in various diseases (Table 3). As a dominant metabolic organ, the liver can sense the stress from both external and internal environments, and accordingly activate its intra- and extra-organ metabolic activities [3]. The latter is realized partly via a group of proteins named hepatokines which are exclusively or predominantly secreted from the liver [4]. In this regard, there is a growing body of research that points to the role of FGF21, as a newly defined member of hepatokines, in liver-participated inter-organ communications and extra-hepatic diseases. Here, we will discuss current studies and gaps in aberrant expressions and the underlying mechanisms of hepatic FGF21 observed in inter-organ crosstalk and cancer progressions. And in light of the findings summarized in this review, we proposed that FGF21 is a sensitive hormone regulated for adaptive stress response, a potent modulator acting as an endocrine hepatokine in inter-organ crosstalk, and also a promising biomarker with pleiotropic activities in cancer progressions.
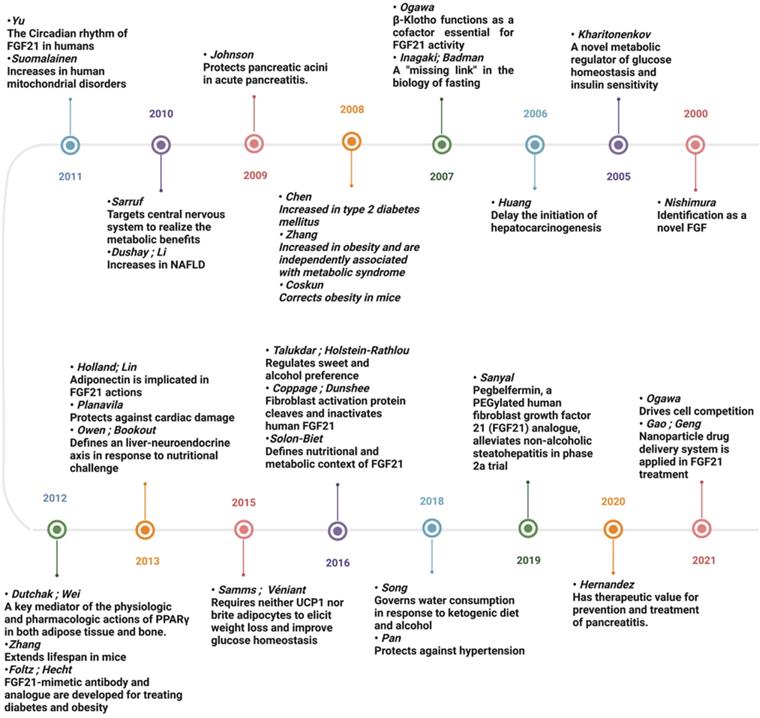
Timeline figure showing the milestones in the history and development of FGF21. Since 2000, FGF21 has attracted great interest due to its pleiotropic effects in response to diverse physiological and pathological stress. With the knowledge of FGF21 expanding exponentially, our understanding of its biology is constantly undergoing modification, and the therapeutic value of FGF21 is being hotly discussed.
FGF21 is a sensitive hormone regulated for adaptive stress response
Mechanically, the expression and secretion of hepatic FGF21 are coordinately regulated by a set of proteins which are partly listed in Table 1. Generally, FGF21 is mainly under the control of peroxisome proliferator activated receptor α (PPARα) as an adaptive response to stress [5]. The trafficking and secretion of FGF21 are regulated by the Yip1 domain family member 6 gene, which sorts and packages FGF21 into COPII vesicles for secretion with the guidance of SEC23A [6]. As a stress-induced factor, the expression of FGF21 is closely connected with a series of biological events, such as circadian rhythm, diet intervention, exercise, and cold exposure.
Circadian rhythm
For both humans and mice, serum FGF21 shows circadian rhythm during fasting with the nocturnal rise and diurnal fall, but this pattern is largely diminished in obese individuals and under standardized meals or cold exposure [7-9]. As a circadian output gene, the FGF21 promoter contains circadian-responsive elements, and the circadian oscillation of FGF21 is therefore controlled directly by several clock proteins (Figure 2) [10-12]. Particularly, PPARα is the major activator of FGF21 via binding to PPRE sites during fasting [13], and insulin-mediated upregulation of E4BP4 suppressed FGF21 transcription via D-box under feeding [10]. In addition, while RORα activated the FGF21 promoter via an upstream RORE site, REV-ERBα negatively regulated FGF21 expression with the cooperation of HNF6 at RORE site [11, 12]. Interestingly, the circadian rhythm of FGF21 seems to be influenced by gender and fed state. Early studies reported that whether under fed or fasting states, both male and female subjects displayed circadian changes [7, 8]. But Foo, Aronis [14] later found that the day-night variation patterns of FGF21 only showed in females with standardized meals, but not in fasting state or in male subjects, which were positively correlated with circulating patterns of free fatty acid (FFA) [9, 14]. The discrepancy may be secondary to the different study designs, the race of the subjects, and the assays used. However, it indicated that sexual dimorphism exists in the circadian rhythm of FGF21.
The direct regulators of hepatic FGF21
| Regulator | Condition | Correlation with FGF21 production | Reference |
|---|---|---|---|
| Peroxisome proliferator activated receptor | Gluconeogenesis; Ketogenesis; Adaptive starvation response | + | [5] |
| Farnesoid X receptor | Ketogenic diet | + | [125] |
| cAMP-responsive element-binding protein H | Fasting; High-fat diet | + | [126] |
| Nuclear receptor subfamily 4 group A member 1/Nur77 | Fasting | + | [127] |
| Activating transcription factor 4 | High-fat diet; Carbon monoxide induction; Glucagon plus insulin induction | + | [128-130] |
| Carbohydrate responsive-element binding protein | High-fructose diets | + | [131] |
| Aryl hydrocarbon receptor | 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced hepatotoxicity | + | [132] |
| Glucocorticoid receptor | Dexamethasone induction; Glucocorticoids induction | + | [45, 133] |
| Liver X receptor | Cholesterol-enriched diet | - | [134] |
| Estrogen-related receptor γ | Hepatic CB1 receptor-mediated induction | + | [135] |
| E4 binding protein 4 | Refeeding | - | [136] |
| Yip1 domain family member 6 gene | Trafficking and secretion | - | [6] |
Exercise
For both rodents and humans, exercise resulted in a significant increase in serum FGF21 levels, which were in line with the elevated lipolysis and decreased glucose levels [15, 16]. Mechanistically, the upregulated glucagon to insulin ratio during exercise contributed to the increased circulating levels of FGF21, and the concomitant blood FFA elevations may act synergistically on hepatic FGF21 secretion [15]. On the other hand, studies found that, under certain conditions, exercise could stimulate FGF21 production in the muscle, which was also associated with the increased circulating FGF21 [17]. However, divarication was observed in both human and animal-based studies [16, 18]. These paradoxical findings suggest that both muscle and liver respond to the exercise-induced elevation of circulating FGF21, but the dominance may change under different conditions. It is noted that the response of FGF21 to exercise is transient, and the sampling time is important. This may explain the lack of difference between the before and 48h after exercise [19]. Moreover, different exercise modes and sex also contributed to different outcomes. Studies found that long-term endurance exercise increased FGF21 while neither shortened time of endurance exercise nor resistance exercise changed serum FGF21 levels in men subjects [20, 21]. For women, long-term endurance training reduced baseline FGF21 levels, while resistance training significantly increased FGF21 levels [22, 23]. Even though these confusing scenarios do lend support to the idea that exercise alters circulating levels of FGF21, further study is warranted to delineate the mechanism explaining the complex patterns of FGF21 facing exercise, and both the study subject and training plan need to be carefully designed to gain more compelling evidence.
Dietary intervention
The sensitivity and tendency of FGF21 to dietary intervention are different between mice and humans. Both hepatic and circulating levels of FGF21 are robustly induced by fasting, high-fat diet, low-carbohydrate, and high protein ketogenic diets in mice [5, 24]. However, humans showed a more complicated response to diet intervention. Circulating FGF21 levels are not altered in humans by either shorter-term fasting or ketogenic diets, but increased by long-term fasting (7 days) or with a low-protein/high-carbohydrate diet [7, 25-27]. Besides, human FGF21 was sharply increased after 3-day overfeeding with high-fat snacks but almost returned to baseline on day 28 [28]. These findings indicated the complex intrinsic variation in FGF21 metabolism, which is regulated by both fasting and feeding signals. Moreover, a sex-dependent difference was also implicated where female mice were less sensitive than males to metabolic improvement observed following the high-carbohydrate diet corresponding with the less significant increase in circulating FGF21 level, but this difference was largely reduced after ovariectomy [27]. In view of the sexual dimorphism observed above, we can speculate that the metabolic benefits of FGF21 for diet intervention among females are regulated by ovarian steroid hormones (or the intact female reproductive system), though further study is required to reveal the underlying mechanisms.
The regulation of hepatic FGF21. The expression of FGF21 is tightly connected with circadian rhythm, diet intervention, exercise, and cold exposure. The circadian rhythm of FGF21 is controlled by several clock proteins. PPARα and RORα positively activate the expression of FGF21 while REV-ERBα and E4BP4 repress its transcription; Exercise stimulates FGF21 expression via the increased plasma glucagon to insulin ratio, and this process positively correlated with the elevated lipolysis and decreased glucose levels; Diet regimen has magnificent impacts on FGF21 levels, both nutrient proportions and food timing are implicated in the complex regulation system of FGF21; Increased FGF21 is also observed under cold exposure which may be cooperated with the thermogenic response of BAT to maintain body temperature.
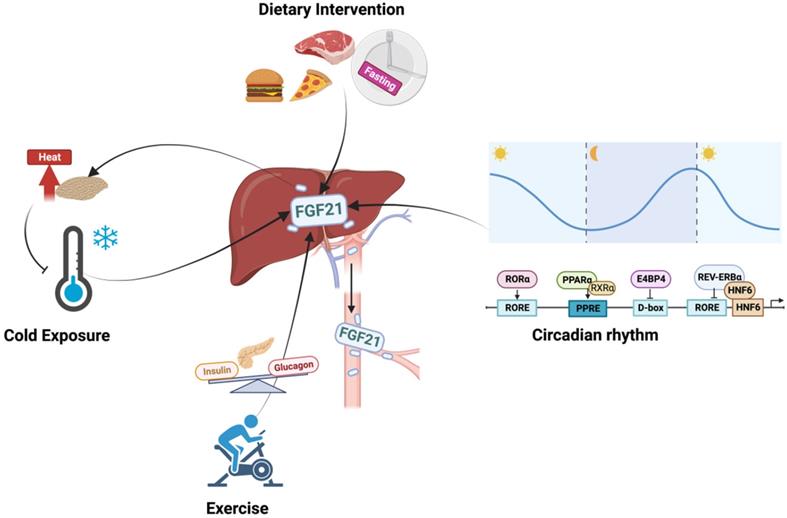
Cold
While increased FGF21 levels, decreased half-life, and retained diurnal rhythm during cold exposure have been consistently observed in mice [29], changes in humans are still under debate. Paul Lee at el. [30] and Hanssen at el. [31] found that mild cold exposure augmented overall FGF21 levels, which were positively associated with improved body core temperature. However, some studies observed decreased plasma FGF21 levels in either short-term mild cold exposure or overweight subjects [32, 33]. It should be noted that the diurnal rhythm of plasma FGF21 was weakened but still retained under mild cold exposure [34].
On the other hand, FGF21 expanded overall thermogenic capacity by stimulating fat browning activity [30]. Therefore, brown adipose tissue (BAT) was also implicated in the regulation of FGF21 under thermogenic activation [29, 35]. Norepinephrine-stimulated increase of FGF21 outputted from interscapular BAT caused marked arteriovenous differences in FGF21 levels under cold exposure [29]. However, counterevidence has also been reported [35, 36]. In FGF21 liver-specific-KO mice, both acute cold exposure and norepinephrine-induced increases in circulating FGF21 levels were completely lost, accompanied by the markedly attenuated BAT sympathetic nerve activity and impaired BAT glucose metabolism [37, 38]. These findings support a more plausible hypothesis that cold exposure evokes the simultaneous elevation of FGF21 in the liver and BAT, wherein the BAT-divided FGF21 shows autocrine and paracrine effects on activating the thermogenic response of BAT, while hepatic FGF21 modulates the circulating FGF21 levels and signals to the central nervous system (CNS) to enhance this adaption function further.
The regulation of FGF21 is complex and still not being fully understood. In this regard, establishing strict eligibility criteria for participant selection, measuring FGF21 levels at multiple time points, and setting paired samples are indispensable for studies to decipher the precise response of FGF21 under certain conditions.
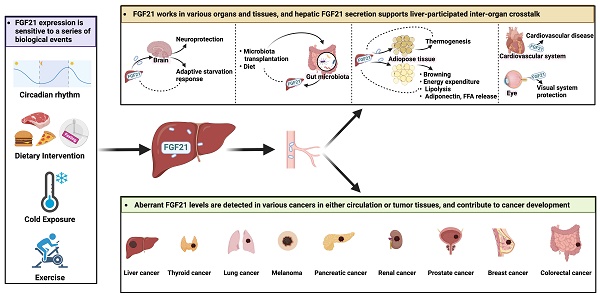
FGF21 is a potential modulator in liver-participated inter-organ crosstalk
FGF21-FGF receptors (FGFR) axis could activate a multitude of signaling cascades, including the RAS-MAPK pathway, the JAK-STAT pathway, the PI3K-AKT pathway, the PLCγ pathway, and JNK pathways [39]. As a pleiotropic metabolic regulator, the activities of FGF21 are complex and vary in different physiological or pathophysiological context [40], and whether the liver is the direct target of FGF21 remains debatable. Hepatocytes express β-Klotho (KLB) but not FGFR1. Therefore, in vivo administration of FGF21 stimulated the response of white adipose tissue (WAT) and breast tissue but not the liver [41]. However, FGF21 enhanced hepatic fatty acid oxidation and tricarboxylic acid cycle flux [42], implicating that FGF21 exerted these hepatic actions via an indirect mechanism that may involve other secretory factors. On the other hand, as a secretory hepatokine, hepatic FGF21 is supposed to integrate liver metabolic status with systemic needs and may contribute to inter-organ communications. The aberrant expression levels of circulating FGF21 in different diseases (Table 3) show its pleiotropic effects. Up to now, the role of FGF21 has been unearthed in several organs/tissues, and the existence of FGF21-based inter-organ crosstalk was implied (Figure 3).
Hepatic FGF21 is a modulator in liver-participated inter-organ crosstalk. The aberrant expressions of FGF21 have been associated with brain, eye, and heart diseases and implicated in various liver-participated inter-organ crosstalk. As a modulator, hepatic FGF21 has displayed an important role in mediating brain-regulated adaptive response and adipose tissue-performed energy metabolism.
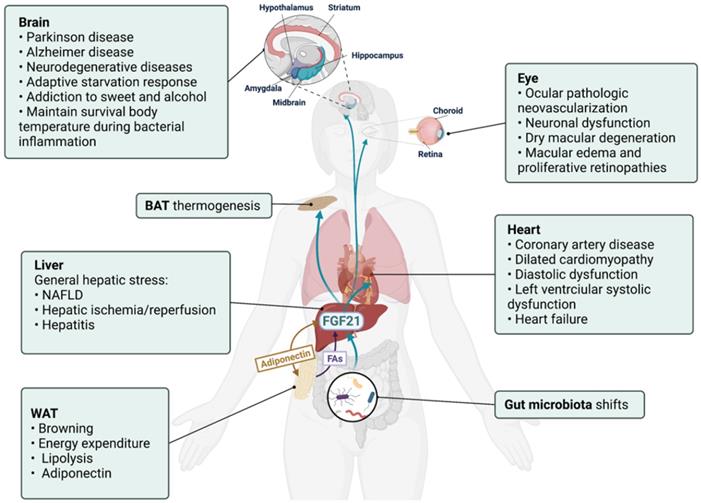
Nervous system
Though undetectable in the nervous system, FGF21 could cross the blood-brain barrier and act in the CNS [43]. Moreover, FGF receptors FGFR1c, 2, and 3c are broadly expressed in the nervous system, and co-receptor KLB is selectively expressed in the suprachiasmatic nucleus [44]. Hepatic FGF21 regulated glucocorticoid production via the CNS [45]. Also, FGF21 has defined an important liver-neuroendocrine axis to modulate the adaptive starvation response, including the regulation of circadian behavior, body weight, and insulin levels via suppressing the output of the suprachiasmatic nucleus [44]. Similarly, the caloric restriction increased hepatic expression and systematic concentration of FGF21, and FGF21 treatment showed neuroprotection effects via activating FGFR1 pathways in primary glial cells [46]. An intriguing study about gut microbiota transplantation found that the increased expressions of hepatic/systemic FGF21 and hippocampal KLB expression were positively connected with improved neurogenesis in recipients, shedding light into the link between a FGF21-based microbial and the neuron system [47].
There has been a focus on neuroprotection activities of FGF21 in brain disorders in recent years. Decreased serum FGF21 levels were detected in patients with Parkinson's disease and Alzheimer's disease [48, 49]. And FGF21 treatment improved related neural pathologies and degeneration by rescuing the astrocyte-neuron lactate shuttle system [50-52]. In this regard, the FGF21-expressing lentiviral vector and FGF21 analogue LY2405319 have been proposed as promising therapy for Alzheimer's disease [53, 54]. However, whether these neuroprotective functions are related to the hepatic secretory FGF21 has not yet been reported. Instead, the hippocampus and cortex could secret FGF21 under neuronal mitochondrial dysfunction [55], and an increased pancreas source of FGF21 was also reported to improve CNS damage [56]. Therefore, the aberrant FGF21 levels detected in neuronal diseases (especially in conditions associated with the neuron mitochondrial and endoplasmic reticulum stress) in both brain and blood should be identified with caution, and the liver-CNS axis may help further explore the neuroprotection activities of FGF21.
Gut microbiome
Gut microbiota (GM) represents a mysterious group of microorganisms living in the digestive tract, and host-GM crosstalk has been discussed in various human diseases [57]. The FGF19-bile acid-GM axis has been established and implicated in the GM-organs crosstalk, especially with the liver [58]. Hepatic FGF21 expression is influenced by GM and involved in the microbiota-gut-brain axis [47]. Moreover, decreased serum FGF21 levels were detected in Clostridioides difficile infection patients receiving fecal microbiota transplantation therapy [59]. Mice supplied with B. adolescentis, the dominant species in the human intestine, showed reduced hepatic FGF21 levels, which were believed as an improvement of the non-alcoholic fatty liver disease (NAFLD)-related FGF21 resistance state [60]. More compelling, it was found that the increased hepatic FGF21 expression of protein restriction relied on the activity of GM, and dietary fiber supplementation influenced hepatic FGF21 stress response via shifting GM composition [61]. However, the significance of GM-induced hepatic FGF21 response to bodily functions, and whether the GM-liver communication is bidirectional requires further exploration.
Adipose tissue
While the liver is the major contributor to circulating levels of FGF21, WAT is the target contributing greatly to the metabolic effects of FGF21. The expression of KLB in conjunction with FGFR1c in adipose tissue confers FGF21 activities there [62, 63]. The FGF21 expression level in human adipose tissue is negligible [25], which indicates that liver-secreted FGF21 is requisite for realizing the metabolic benefits of FGF21 found in adipose tissues. Along this line, studies have revealed the important role of FGF21 in liver-adipose tissue crosstalk. The elevated liver production of FGF21 in hepatic Cpt1a knockout mice contributed to the increased adipose browning and energy expenditure [64]. A recent study found that methionine adenosyltransferase Matla knockdown prevented and reversed obesity, insulin resistance, and hepatosteatosis via stimulating the expression and secretion of hepatic FGF21, which subsequently activated the liver-adipose tissue axis resulting in increased BAT thermogenesis, WAT lipolysis, and secretion of adiponectin [65].
The interactions between FFA and FGF21 create the loop between WAT and the liver. The FFA level in the serum of healthy individuals showed a similar oscillatory pattern with FGF21 under both fasting and feeding conditions, though the pick time was preceded. On the one hand, hepatic p38 stimulated WAT lipolysis and FFA release via upregulating FGF21 secretion from the liver to WAT [66]. On the other hand, a low dosage of FFA treatment stimulated FGF21 secretion in HepG2 cells [8]. And growth hormone-stimulated release of FFA from WAT resulted in the CREBH/PPARα-mediated over-expression of hepatic FGF21, which in turn suppressed WAT lipolysis [67, 68]. Notably, a study found that only unsaturated FFA could induce hepatic FGF21 expression [8]. Considering the FFA composition is different in different sites of WAT [69], it is worth uncovering whether their effects on hepatic FGF21 are also site specific.
Adiponectin is the most abundant adipokine secreted by adipocytes [70]. Intriguingly, recent studies have started to delineate the positive interactions between FGF21 and adiponectin. A study found that hepatic FGF21 induced the biosynthesis and secretion of adiponectin in adipocytes, which in turn conferred FGF21 systematic and hepatic functions through positive feedback [71]. And WAT adiponectin was reduced in FGF21-KO mice but was completely restored after FGF21 replenishment [72]. Furthermore, both acute and chronic administration of FGF21 increased circulating adiponectin levels, and adiponectin deficiency diminished the metabolic effects of FGF21 on glucose tolerance and insulin resistance [71]. Magnificently, the study found that JNK deficiency-induced adipocyte FGF21 upregulation triggered a feed-forward loop in the liver-WAT axis, which promoted hepatocyte FGF21 expression and secretion via regulating adipocyte adiponectin expression and secretion [73]. These findings represent an unfolding story about the interactions between FGF21 and adipokine.
Heart
The evaluated levels of FGF21 were predictive of poor outcomes in cardiac diseases [74, 75] (Table 3). Interestingly, one recent study detected that the cardiac FGF21 gene expression levels in heart failure patients were as low as healthy subjects, but the protein levels of FGF21 in both the serum and cardiac section were aberrantly increased and accompanied by elevated cardiac FGFR3 expression, which indicated the existence of a FGF21-dependent cardiohepatic signaling circuit [74]. Consistently, hepatic FGF21 maintained chronotropy in mice during bacterial inflammation [76]. Mice cardiomyocytes express FGFRs and KLB, and the activated PI3K-Akt and ERK signaling pathways partly contributed to the cardiac benefits of FGF21 [77, 78]. However, the mechanism of FGF21 cardioprotective actions is still under debate, and the existence of the FGF21-based liver-brain axis remains to be proved. For example, cardiomyocytes were not only a target but also a source of FGF21 [78]. KLB expression was diseased in cardiac dysfunction, and in vivo FGF21 treatment failed to activate ERK signaling in the heart [76]. These findings indicated that both systemic and cardiac FGF21 had cardioprotective activities via direct, indirect, or both pathways.
Retina /Choroid
Retinal and choroidal ocular pathologic neovascularization is aggravated in FGF21 knockout mice and suppressed via the FGF21 analog-activated adiponectin pathway [79]. FGF21 depletion also resulted in the increased occurrence of dry macular degeneration-like pathological changes, while exogenous FGF21 administration reversed this phenomenon [80]. A later study found that FGF21 prevented VEGF-induced retinal vascular leakage in mice and strengthened human primary retinal microvascular endothelial cells barrier function [81]. However, it is still unclear whether circulating or local FGF21 contributed to these protective effects. Considering the extremely low/absent expression of FGF21 detected in the visual system [82], it is plausible to postulate that the protective effect of FGF21 on the eye is mainly realized by the circulating FGF21, though further studies are necessary.
The data discussed above indicate that the aberrant expressions of FGF21 observed in circulation and organs in different contexts are likely to be an ex officio action of the liver to realize the pleiotropic effects of FGF21, especially regarding the adaptive starvation response in the CNS system, nutrition metabolism in GM, and energy homeostasis in adipose tissue. A crosstalk network is therefore delineated between the liver and other organs/tissues, which is no doubt striking and should be an area of fruitful future study.
FGF21 is a promising biomarker with pleiotropic activities in cancer progressions
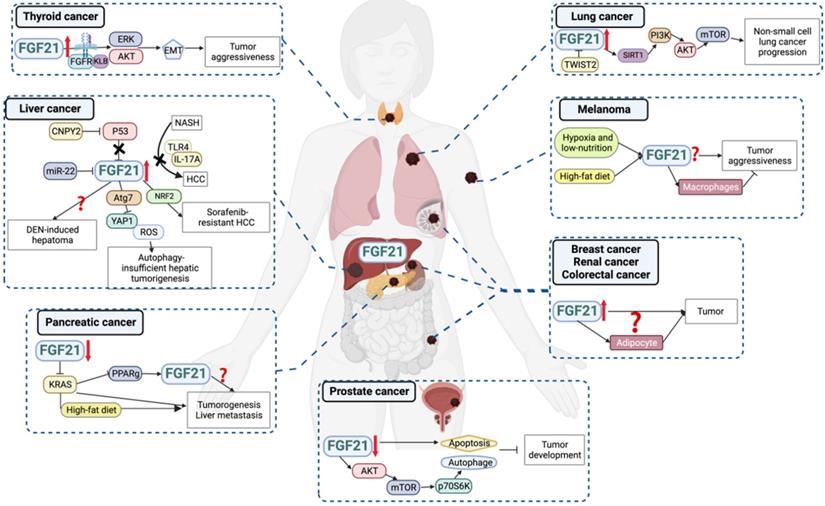
As a stress-responsive hepatokine, the implications of FGF21 in cancers have been focused on in recent years. Overall, overexpression of FGF21 in both circulating and tissue levels has been found in different types and clinical stages of cancers (Figure 4, Table 2), suggesting the biomarker value of FGF21 in cancer diagnosis and treatment. Theoretically, the well-studied findings of the oncogenic FGF-FGFR axis attest to this hypothesis. FGF21-related FGF receptors FGFR1c, 2c, 3c, and 4 are widely expressed in tumor cells and aberrantly activated in cancers [83]. The deregulation of FGF expression drives oncogenic FGFR signaling and activates downstream signaling through MARK-ERK, PI3K-AKT, and JAK-STAT pathways that regulate tumor cell proliferation, differentiation, and survival [84]. Another plausible reason for FGF21 participating in cancers is its high sensitivity to macronutrients. Imbalanced nutrition intake is not only a cause of carcinogenesis but also occurs during cancer progression. In this setting, recruiting FGF21 from either adjacent or distant organs will benefit tumor tissues against adverse environments. This may partially explain the elevated circulating and peritumoral FGF21 levels observed in cancer patients [85, 86]. However, in a low-protein ketogenic diet, FGF21 knockout mice showed a comparable decrease in tumor growth to that of the wild type [87]. Therefore, although the aberrant expression of FGF21 was a sensitive response to diet intervention, whether it benefits anti-cancer activities in low protein diets and ketogenic diets or plays a part in countertrending obese diets requires further study [88].
Liver cancer
The close relationship between the liver and FGF21 aroused researchers' attention to the role of FGF21 in liver cancers. Accumulated data gave us a relatively deeper understanding of the FGF21-liver cancer axis compared to other cancers. Though FGFR4 is the major FGFR present in mature hepatocytes, elevated expression of FGFR1 has been observed in hepatocellular carcinoma (HCC) and contributed to tumor development [89]. This aberrant expression appears to be more favorable for FGF21's binding. Consistently, both liver and serum levels of FGF21 were upregulated in patients with hepatitis, cirrhosis, and liver tumors, and associated with a significantly decreased survival rate in patients with inferior hepatocellular carcinoma [90]. For the carcinogen-induced HCC animal model, increased FGF21 levels were observed in the early and middle stages of tumorigenesis, and also in normal hepatocytes adjacent to the tumor foci, while sharply diminished once cells progressed to malignancy. This change indicated that FGF21 is an independent indicator of genetic hepatocarcinogenesis, but its expression is not a direct genetic marker of hepatoma cells per se [91]. Instead, a study showed that canopy homolog 2 promoted liver oncogenesis via destabilizing p53 and activating hepatic FGF21 expression [92]. Consistently, tumor suppressor miR-22 reduced hepatic FGFR1 by direct targeting and inhibited FGF21 expression by reducing its synthesis process [93]. Moreover, FGF21 showed a positive feedback loop with NRF2 accounting for sorafenib's resistance. Higher FGF21 expression was correlated with more resistance to sorafenib treatment in HCC patients, and intra-tumoral FGF21 knockdown effectively inhibited tumor growth in sorafenib-resistant HCC animal models [94]. These findings indicated that the pathological upregulation of FGF21 promoted tumor development.
On the other hand, some studies have discussed the tumor suppressor effects of FGF21 on HCC under both physiological and pharmacological levels. FGF21 knockout mice were more sensitive to a high fat, high sucrose diet-induced HCC [95], which was later proven through FGF21's negative feedback on hepatocyte-TLR4-IL-17A signaling [96]. And FGF21 knockout accelerated autophagy gene Atg7 deletion induced hepatic tumor process, implicating the suppressive effect of FGF21 on autophagy-insufficient hepatoma [97]. Besides, the overexpression of FGF21 delayed the tumor incidence in the diethylnitrosamine-induced liver cancer mice model, but contrarily accelerated the progression of adenoma to HCC, resulting in similar HCC outcomes between overexpression and wild-type groups [98]. Also, the distinct effects of long-term pharmacological dosage of FGF21 on HCC were observed between rats and mice [99], indicating that the pharmacological effects of FGF21 on hepatoma may be highly susceptible to species and treatment regimens.
The correlations and activities of FGF21 in cancers
| Cancer | Clinical study | In vivo study | In vitro study | Reference | |
|---|---|---|---|---|---|
| Correlation with circulating FGF21 levels | Correlation with intra-tumoral FGF21 levels | ||||
| Liver cancer | + | + | Serum FGF21 levels were different in different stages of tumorigenesis; Intratumoral FGF21 knockdown inhibited sorafenib-resistant HCC; Deficiency of FGF21 promoted obesogenic diet-induced HCC; FGF21 knockout promoted autophagy-deficient hepatoma; Overexpression of FGF21 or exogenous FGF21 treatment delayed carcinogen-induced HCC tumorigenesis; FGF21 treatment promotes carcinogen-induced HCC tumorigenesis in rats. | Overexpression of FGF21 increased sorafenib resistance; FGF21 treatment suppressed carcinogen-induced oxidative stress. | [89-91, 94, 95, 97, 99] |
| Thyroid cancer | + | Undetectable | Unknown | FGF21 treatment promoted tumor cell migration and invasion. | [85] |
| Prostate cancer | Unknown | - | Overexpression of FGF21 inhibits prostate cancer tumorigenesis. | Overexpression of FGF21 inhibits prostate cancer cell viability. | [100] |
| Non-small cell lung cancer | Unknown | + | Unknown | Overexpression of FGF21 promoted tumor cell growth and migration, while down-regulated FGF21 presented opposite effects on lung cancer cells. | [86, 101] |
| Melanoma | Unknown | Unknown | Overexpression of FGF21 enhanced tumorigenesis; Serum FGF21 levels were negatively connected with the metastatic ability. | Overexpression of FGF21 enhanced cell aggressiveness and tumorigenicity; Conditioned medium from exogenous FGF21 treated macrophage inhibited melanoma cell viability. | [102, 103] |
| Pancreatic cancer | Unknown | - | Pharmacological supplementation of FGF21 delayed cancer development under obesogenic diet challenge. | Decreased FGF21 levels in pancreatic cancer cells. | [104] |
| Breast cancer | + | Unknown | [106] | ||
| Renal cancer | + | Unknown | [108] | ||
| Colorectal cancer | + | Unknown | [107-109, 112] | ||
| Gastric cancer | + | Unknown | [113] | ||
| Endometrioid carcinoma | + | Unknown | [114] | ||
| Urothelial carcinoma | + | Unknown | [115] | ||
Hepatic FGF21 is a promising biomarker for cancers. Aberrant expression of FGF21 has been correlated with cancer development. Accumulating data indicated the predictive and diagnostic values of FGF21 as a promising biomarker for human cancers, for some of which its underlying mechanisms have been discussed.
FGF21 is a promising biomarker for risk prediction and diseases progression
| Disease | Correlation with circulating FGF21 | Reference |
|---|---|---|
| NAFLD | + | [137, 138] |
| Hepatic ischemia/reperfusion injury in patients with liver transplantation | + | [139] |
| Genotype-4 chronic hepatitis C | + | [140] |
| Advanced fibrosis/cirrhosis in chronic hepatitis B patients on antiviral treatment. | - | [141] |
| Carotid atherosclerotic diseases | + | [142] |
| Coronary artery disease | + | [75, 143] |
| Heart failure | + | [74] |
| Vascular calcification | + | [144] |
| Adverse cardiovascular events in patients with type 2 diabetes | + | [145] |
| Adverse cardiovascular events in ST-segment elevation myocardial infarction patients | + | [146] |
| Adverse clinical events in patients with myocardial infarction who have undergone coronary artery bypass graft | + | [147] |
| Diabetes | + | [148] |
| Obesity | + | [149] |
| Diabetic nephropathy | + | [150] |
| Renal disease | + | [151] |
| Mitochondrial disease/mitochondrial myopathies | + | [152] |
| Pediatric mitochondrial disease | + | [153] |
| Primary sarcopenia | + | [154] |
| Dravet syndrome | + | [155] |
| Pterygium | - | [156] |
| Missed abortion | + | [157] |
| Mortality of patient with sepsis and acute respiratory distress syndrome. | + | [158] |
Thyroid cancer
Serum FGF21 levels were significantly higher in the papillary thyroid carcinoma patients than in controls and were positively associated with pathological stage, recurrence, and mortality. In vitro FGF21 treatment promoted tumor cell migration and invasion by upregulating FGFR-EMT signaling. It is worth noting that FGF21 expression was detected in neither normal thyroid tissues nor tumor tissues, which indicated that FGF21 might induce thyroid tumor progression in an endocrine way [85].
Prostate cancer
FGF21 expression was decreased in clinic prostate cancer tissues, and overexpression of FGF21 inhibited prostate cancer cell viability [100]. But whether the decreased FGF21 levels in tumor tissues were due to the decreased circulating FGF21 needs to be confirmed. Besides, using high glucose conditions to strengthen cell viability seems uncalled for considering cancer cells' commonality of uncontrolled proliferation and anti-apoptosis abilities. Furthermore, it may lead to the effects of FGF21 on cancer cells easily overshadowed by its outstanding regulation ability of glucose metabolism, which the author also mentioned.
Non-small cell lung cancer
FGF21 was upregulated in non-small cell lung cancer tissues, and the higher expression level was associated with shorter overall survival time and advanced histological type. In vitro study showed that FGF21 was elevated in tumor cells compared with normal lung cell lines, which facilitated tumor progression by promoting cell growth, migration, and defending oxidative stress via Sirtuin 1/PI3K/AKT signaling [86]. Moreover, FGF21 expression was suppressed in TWIST2-overexpressed lung cancer cells, resulting in decreased cell viability, increased oxidative stress, and cell apoptosis [101]. However, current research only focused on the autocrine function of FGF21 in lung cancers. Thus future studies concentrating on the interplay between the endocrine form of FGF21 and lung tumor tissues are encouraged.
Melanoma
Overexpression of FGF21 in mouse melanoma cell line enhanced cell aggressiveness and tumorigenicity under hypoxia and low-nutrition double deprivation stress [102]. However, FGF21 serum levels were negatively connected with the metastatic ability of melanoma cells in animals, and exogenous FGF21 treated macrophage inhibited melanoma cell viability via intercellular crosstalk in a dose-dependent manner [103]. This controversy reflected the pleiotropic actions of FGF21 in melanoma and the tumor microenvironment.
Pancreatic cancer
The circulating levels of FGF21 in pancreatic cancer patients are still unclear, but both pancreatic cancer specimens and cell lines had lower levels of FGF21 than normal human pancreatic tissues and cells, respectively. The pharmacological dosage of FGF21 supplementation effectively inhibited pancreatic intraepithelial neoplasia lesions, inhibited liver metastasis, and prolonged the overall survival of KRAS-mediated pancreatic cancer under the high-fat-diet challenge. Though serum FGF21 level was not influenced by pancreatic KRAS mutation, the effect of pathological levels of circulating FGF21 under a high-fat diet, in turn, on pancreatic tumor development was not addressed [104]. Moreover, KRAS, the most frequently mutated RAS isoform, happens not only in pancreatic cancer but also in lung cancer, multiple myeloma, and colorectal cancer [105]. Whether this KRAS-FGF21 axis exists in other types of cancer and the distinctions between different mutant hotspots deserve further study.
Breast cancer, Renal cancer and Colorectal cancer
As mentioned, adipose tissue is not only a source of autocrine FGF21 but also a direct and predominant target of hepatic FGF21, which may result in a substantial enrichment of FGF21 in the microenvironment of the tumors growing in the anatomical vicinity of adipose tissue, such as breast, renal and colon cancers. Even though the activities of FGF21 in these types of cancers have not been explored yet, elevated serum levels of FGF21 were reported in the early stages of breast cancer [106] but significantly reduced following 12 months of hormonal therapy [107]. Patients with both clear renal cell carcinoma and chromophobe renal cancer had higher levels of serum FGF21 compared with healthy control [108]. Also, FGF21 has shown positive associations with colon site cancer, stage I-II colorectal cancer, advanced colorectal neoplasia, and even in cases collected over 5 years before diagnosis, which indicated that FGF21 is potentially predictive and possibly existing in colorectal cancer etiology [109-111]. Compatible with these findings, higher circulating levels of FGF21 increased the risk of metachronous colorectal adenomas, especially in the older population [112]. These limited data suggest the great suitability of serum FGF21 as a diagnostic biomarker of cancers, and also open the door for the speculation that an FGF21-driven liver-adipose tissue-tumor tissue axis is implicated in cancers.
Other cancers
Increased FGF21 levels were observed in patients with different stages of gastric cancer, suggesting it is a suitable biomarker for early-stage gastric cancer [113]. Also, serum FGF21 level was higher in patients with endometrioid carcinoma, sufficing it to diagnose the cancer stage and grade [114]. Similarly, in urothelial carcinoma, serum FGF21 level was positively associated with the tumor stage, cardiovascular disease, and history of recurrence [115].
So far, there is limited information available to explain the role of FGF21 in tumor initiation and progression. Though the results listed above are promising, they need more detailed and in-depth investigations as it is hard to discern whether the increased level of FGF21 contributes to cancer development or is just a result of carcinogenesis or a stress response for maintaining body system homeostasis. As a potential biomarker, the aberrant expression of FGF21 has been generally observed in different types and stages of cancers, which indicates its low specificity. Instead, FGF21 may be an ideal non-invasive biomarker applied in regular health tests for discovering and preventing cancers at an early stage, though the sensitivity and cancer-specificity for clinical use need to be evaluated critically.
Discussion
Since 2000, the scientific appreciation of FGF21 in various human diseases has been rapidly expanding. As an unfolded area, the increasing roles of FGF21 generate a series of inspiring ideas, such as whether exclusive FGF21 secretion ability confers liver extra-hepatic functions, whether endocrine FGF21 is the holistic hub point for interorgan crosstalk, whether there are concomitant modulators that collaborate with FGF21 or whether FGF21 is an actionable biomarker with therapeutic values for cancers. However, studies in this area are still in their infancy. To boost the research process of FGF21, several factors need to be considered.
The first factor is about distinguishing between pathological and pharmacological doses of FGF21. The physiological actions of FGF21 occur at much lower concentrations and in more restricted organ systems and tissues than its pharmacological actions [40]. Both long-term administrations of pharmacological dosage of FGF21 and transgenic mice over-expressing FGF21 did not develop liver tumor or show evidence of any other tissue hyperplasia, which potently denied the carcinogenic risk of FGF21 therapy [116, 117]. Future studies regarding the role of aberrant pathological levels of FGF21 in cancer progression are therefore recommended. The distinction between pathological and pharmacological levels of FGF21 did not receive adequate attention in previous studies. The highly inconsistent dosage selection was observed in studies that were all focused on the pharmacological actions of FGF21 [99, 104]. Furthermore, converting in vitro concentrations to in vivo doses (and vice versa) is even thornier since it is hard to set a clear line between pathological and pharmacological dosages for in vitro study. In this case, in vivo study is more reliable for observing the biological effects of FGF21 under different conditions.
Secondly, the elimination half-life of FGF21 is only 0.5~2 hours [116, 118]. Thus selecting the appropriate administration dosage and mode is important and highly dependent on the objective of your research. Even though a study indicated that FGF21 action did not require the prolonged presence of the actual protein in circulation, approximately a 10-fold greater dose of FGF21 was needed for a once-daily subcutaneous injection to achieve an equivalent body weight reduction compared with continuous FGF21 administration via osmotic pump [116]. Since continuous infusion allows the stable presence of circulating FGF21 levels throughout the course of the study, it is particularly suitable for studying the long-term effects of FGF21 (such as mimicking the overall increased FGF21 levels in metabolic diseases or testing the chronic therapeutic efficacy of FGF21). Furthermore, the bioactivity of recombinant FGF21 protein should also be evaluated with caution. It should be realized that different purification and activity, combined with the non-standardized selection of dosage and administration mode, will leave non-negligible impacts on the results and further contribute to the inconsistencies between studies.
Besides, the similarities and differences between mice and humans need to be considered. While it may sound hypercritical, the aforementioned distribution and activity difference between human and mouse FGF21 warn us of the existence of species specificity in various contexts. Therefore, the clinical translation of FGF21 activities demonstrated in mice to humans needs to be further investigated. For example, mouse adipose tissues express detectable levels of FGF21, which has been implicated in the magnificent effects of FGF21 on energy metabolism. However, human adipose tissues do not express FGF21, which questions the clinical value of autocrine activities of adipose FGF21 observed in rodent-based studies [119]. Also, studies found that a significant percentage of circulating human FGF21 is not active due to fibroblast activation protein-mediated post-translation and inactivation. In contrast, mouse FGF21 is resistant to cleavage because of the different C-terminus [118, 120]. The conjunction of these overlooked differences may finally contribute to the lower-than-expected translation efficacy of FGF21-based therapeutic benefits from bench to bedside [121].
Last but not least, it is plausible that the physiology and function of FGF21 may be sexual dimorphism considering the substantial difference between genders in sex hormone levels and macronutrient metabolism patterns. Besides the different sensitivity to circadian rhythms [9, 14], exercise [20, 21], and diet [27], pharmacological levels of FGF21 showed stronger metabolic effects in males and were antagonized by estradiol replenishment in ovariectomized females [122, 123]. However, endogenous hepatic FGF21 transcription is positively regulated by the estradiol-Wnt pathway in a PPARα-independent way, which resulted in reduced hepatic FGF21 production in female mice following ovariectomy [124]. These findings suggest a notable role of estrogen in FGF21 activities. Sexual dimorphism is a new frontier in FGF21 research, so future studies concentrating on this difference, especially unearthing the orchestrated interactions between FGF21 and estrogen, will expand our understanding of FGF21 biology.
Conclusion
Focusing on FGF21, the findings discussed in this review indicated its high sensitivity to adaptive stress response, its important role in modulating liver-participated inter-organ crosstalk, and its potential value in cancer diagnosis and treatment. In-depth studies to establish a comprehensive framework of the biology and activities of endocrine FGF21 are called upon.
Abbreviations
FGF: fibroblast growth factor; PPARα: peroxisome proliferator activated receptor α; BAT: brown adipose tissue; CNS: central nervous system; WAT: white adipose tissue; GM: gut microbiota; COPII: coat Protein Complex II; RORα: retinoic acid-related orphan receptor alpha; HNF6: hepatocyte nuclear factor 6; FFA: free fatty acid; FGFR: fibroblast growth factor receptor; MAPK: mitogen-activated protein kinase; JAK: Janus kinase; STAT: signal transducer and activator of transcription protein; PI3K: phosphoinositide 3 kinase; AKT: serine/threonine-protein kinase; PLCγ: phospholipase C gamma; JNK: c-Jun N-terminal kinase; KLB: β-klotho; NAFLD: non-alcoholic fatty liver disease; VEGF: vascular endothelial growth factor; ERK: extracellular signal-regulated kinases; HCC: hepatocellular carcinoma; TLR4: toll-like receptor 4; IL-17A: interleukin 17A; Atg7: autophagy related 7; EMT: epithelial-mesenchymal transition; TWIST2: twist family BHLH transcription factor 2; KRAS: kirsten rat sarcoma virus; RAS: rat sarcoma virus.
Supplementary Material
Supplementary information.
Acknowledgements
All figures were created with BioRender.com.
Funding
This study was supported by the National Science Foundation of China (81573663) and Guangxi Science and Technology Key Research and Development Program (AB16450008).
Ethics approval and consent to participate
This research was based on the review of published/publicly reported literature and did not require ethical approval.
Author contributions
Yue SUI wrote the article. Jianping CHEN designed and reviewed the manuscript. All authors read and approved the final paper.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Dolegowska K, Marchelek-Mysliwiec M, Nowosiad-Magda M, Slawinski M, Dolegowska B. FGF19 subfamily members: FGF19 and FGF21. Journal of Physiology and Biochemistry. 2019;75:229-40
2. Nishimura T, Nakatake Y, Konishi M, Itoh N. Identification of a novel FGF, FGF-21, preferentially expressed in the liver. Biochimica et biophysica acta. 2000;1492:203-6
3. Jensen-Cody SO, Potthoff MJ. Hepatokines and metabolism: Deciphering communication from the liver. Molecular Metabolism. 2021;44:101138
4. Wang F, So KF, Xiao J, Wang H. Organ-organ communication: The liver's perspective. Theranostics. 2021;11:3317-30
5. Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos-Flier E. Hepatic Fibroblast Growth Factor 21 Is Regulated by PPARα and Is a Key Mediator of Hepatic Lipid Metabolism in Ketotic States. Cell metabolism. 2007;5:426-37
6. Wang L, Mazagova M, Pan C, Yang S, Brandl K, Liu J. et al. YIPF6 controls sorting of FGF21 into COPII vesicles and promotes obesity. Proceedings of the National Academy of Sciences. 2019;116:15184-93
7. Andersen B, Beck-Nielsen H, Højlund K. Plasma FGF21 displays a circadian rhythm during a 72-h fast in healthy female volunteers. Clin Endocrinol (Oxf). 2011;75:514-9
8. Yu H, Xia F, Lam KS, Wang Y, Bao Y, Zhang J. et al. Circadian rhythm of circulating fibroblast growth factor 21 is related to diurnal changes in fatty acids in humans. Clin Chem. 2011;57:691-700
9. Foo JP, Aronis KN, Chamberland JP, Mantzoros CS. Lack of Day/Night variation in fibroblast growth factor 21 levels in young healthy men. International Journal of Obesity. 2015;39:945-8
10. Tong X, Muchnik M, Chen Z, Patel M, Wu N, Joshi S. et al. Transcriptional Repressor E4-binding Protein 4 (E4BP4) Regulates Metabolic Hormone Fibroblast Growth Factor 21 (FGF21) during Circadian Cycles and Feeding. Journal of Biological Chemistry. 2010;285:36401-9
11. Chavan R, Preitner N, Okabe T, Strittmatter LM, Xu C, Ripperger JA. et al. REV-ERBα regulates Fgf21 expression in the liver via hepatic nuclear factor 6. Biol Open. 2017;6:1-7
12. Wang Y, Solt LA, Burris TP. Regulation of FGF21 expression and secretion by retinoic acid receptor-related orphan receptor alpha. J Biol Chem. 2010;285:15668-73
13. Inagaki T, Dutchak P, Zhao G, Ding X, Gautron L, Parameswara V. et al. Endocrine Regulation of the Fasting Response by PPARα-Mediated Induction of Fibroblast Growth Factor 21. Cell metabolism. 2007;5:415-25
14. Foo JP, Aronis KN, Chamberland JP, Paruthi J, Moon HS, Mantzoros CS. Fibroblast growth factor 21 levels in young healthy females display day and night variations and are increased in response to short-term energy deprivation through a leptin-independent pathway. Diabetes Care. 2013;36:935-42
15. Hansen JS, Clemmesen JO, Secher NH, Hoene M, Drescher A, Weigert C. et al. Glucagon-to-insulin ratio is pivotal for splanchnic regulation of FGF-21 in humans. Molecular Metabolism. 2015;4:551-60
16. Kim KH, Kim SH, Min Y-K, Yang H-M, Lee J-B, Lee M-S. Acute Exercise Induces FGF21 Expression in Mice and in Healthy Humans. PLOS ONE. 2013;8:e63517
17. Gao Y, Zhang W, Zeng LQ, Bai H, Li J, Zhou J. et al. Exercise and dietary intervention ameliorate high-fat diet-induced NAFLD and liver aging by inducing lipophagy. Redox Biol. 2020;36:101635
18. Parmar B, Lewis JE, Samms RJ, Ebling FJP, Cheng CC, Adams AC. et al. Eccentric exercise increases circulating fibroblast activation protein α but not bioactive fibroblast growth factor 21 in healthy humans. Experimental Physiology. 2018;103:876-83
19. Kruse R, Vienberg SG, Vind BF, Andersen B, Højlund K. Effects of insulin and exercise training on FGF21, its receptors and target genes in obesity and type 2 diabetes. Diabetologia. 2017;60:2042-51
20. Taniguchi H, Tanisawa K, Sun X, Higuchi M. Acute endurance exercise lowers serum fibroblast growth factor 21 levels in Japanese men. Clin Endocrinol (Oxf). 2016;85:861-7
21. Morville T, Sahl RE, Trammell SAJ, Svenningsen JS, Gillum MP, Helge JW. et al. Divergent effects of resistance and endurance exercise on plasma bile acids, FGF19, and FGF21 in humans. JCI insight. 2018 3
22. Pérez-López A, Gonzalo-Encabo P, Pérez-Köhler B, García-Honduvilla N, Valadés D. Circulating myokines IL-6, IL-15 and FGF21 response to training is altered by exercise type but not by menopause in women with obesity. European Journal of Sport Science. 2021:1-10
23. Saeidi A, Jabbour G, Ahmadian M, Abbassi-Daloii A, Malekian F, Hackney AC. et al. Independent and Combined Effects of Antioxidant Supplementation and Circuit Resistance Training on Selected Adipokines in Postmenopausal Women. Frontiers in physiology. 2019;10:484
24. Ueno S, Seino Y, Hidaka S, Maekawa R, Takano Y, Yamamoto M. et al. High Protein Diet Feeding Aggravates Hyperaminoacidemia in Mice Deficient in Proglucagon-Derived Peptides. Nutrients. 2022 14
25. Fisher FM, Chui PC, Nasser IA, Popov Y, Cunniff JC, Lundasen T. et al. Fibroblast Growth Factor 21 Limits Lipotoxicity by Promoting Hepatic Fatty Acid Activation in Mice on Methionine and Choline-Deficient Diets. Gastroenterology. 2014;147:1073-83.e6
26. Gälman C, Lundåsen T, Kharitonenkov A, Bina HA, Eriksson M, Hafström I. et al. The Circulating Metabolic Regulator FGF21 Is Induced by Prolonged Fasting and PPARα Activation in Man. Cell metabolism. 2008;8:169-74
27. Larson KR, Russo KA, Fang Y, Mohajerani N, Goodson ML, Ryan KK. Sex Differences in the Hormonal and Metabolic Response to Dietary Protein Dilution. Endocrinology. 2017;158:3477-87
28. Heilbronn LK, Campbell LV, Xu A, Samocha-Bonet D. Metabolically protective cytokines adiponectin and fibroblast growth factor-21 are increased by acute overfeeding in healthy humans. PLoS One. 2013;8:e78864
29. Hondares E, Iglesias R, Giralt A, Gonzalez FJ, Giralt M, Mampel T. et al. Thermogenic Activation Induces FGF21 Expression and Release in Brown Adipose Tissue. Journal of Biological Chemistry. 2011;286:12983-90
30. Lee P, Linderman Joyce D, Smith S, Brychta Robert J, Wang J, Idelson C. et al. Irisin and FGF21 Are Cold-Induced Endocrine Activators of Brown Fat Function in Humans. Cell metabolism. 2014;19:302-9
31. Hanssen MJW, Broeders E, Samms RJ, Vosselman MJ, van der Lans AAJJ, Cheng CC. et al. Serum FGF21 levels are associated with brown adipose tissue activity in humans. Scientific Reports. 2015;5:10275
32. Hollstein T, Heinitz S, Ando T, Rodzevik TL, Basolo A, Walter M. et al. Metabolic Responses to 24-Hour Fasting and Mild Cold Exposure in Overweight Individuals Are Correlated and Accompanied by Changes in FGF21 Concentration. Diabetes. 2020;69:1382-8
33. Enevoldsen LH, Tindborg M, Hovmand NL, Christoffersen C, Ellingsgaard H, Suetta C. et al. Functional brown adipose tissue and sympathetic activity after cold exposure in humans with type 1 narcolepsy. Sleep. 2018 41
34. Lee P, Brychta RJ, Linderman J, Smith S, Chen KY, Celi FS. Mild Cold Exposure Modulates Fibroblast Growth Factor 21 (FGF21) Diurnal Rhythm in Humans: Relationship between FGF21 Levels, Lipolysis, and Cold-Induced Thermogenesis. The Journal of Clinical Endocrinology & Metabolism. 2013;98:E98-E102
35. Chartoumpekis DV, Habeos IG, Ziros PG, Psyrogiannis AI, Kyriazopoulou VE, Papavassiliou AG. Brown Adipose Tissue Responds to Cold and Adrenergic Stimulation by Induction of FGF21. Molecular Medicine. 2011;17:736-40
36. Sepa-Kishi DM, Ceddia RB. Circulating fibroblast growth factor 21 is reduced, whereas its production is increased in a fat depot-specific manner in cold-acclimated rats. Adipocyte. 2018;7:238-47
37. Ameka M, Markan KR, Morgan DA, BonDurant LD, Idiga SO, Naber MC. et al. Liver Derived FGF21 Maintains Core Body Temperature During Acute Cold Exposure. Scientific Reports. 2019;9:630
38. Markan KR, Naber MC, Ameka MK, Anderegg MD, Mangelsdorf DJ, Kliewer SA. et al. Circulating FGF21 Is Liver Derived and Enhances Glucose Uptake During Refeeding and Overfeeding. Diabetes. 2014;63:4057-63
39. Kilkenny DM, Rocheleau JV. The FGF21 Receptor Signaling Complex: Klothobeta, FGFR1c, and Other Regulatory Interactions. Vitam Horm. 2016;101:17-58
40. Potthoff MJ, Kliewer SA, Mangelsdorf DJ. Endocrine fibroblast growth factors 15/19 and 21: from feast to famine. Genes & Development. 2012;26:312-24
41. Yang C, Jin C, Li X, Wang F, McKeehan WL, Luo Y. Differential Specificity of Endocrine FGF19 and FGF21 to FGFR1 and FGFR4 in Complex with KLB. PLoS ONE. 2012;7:e33870
42. Potthoff MJ, Inagaki T, Satapati S, Ding X, He T, Goetz R. et al. FGF21 induces PGC-1alpha and regulates carbohydrate and fatty acid metabolism during the adaptive starvation response. Proc Natl Acad Sci U S A. 2009;106:10853-8
43. Hsuchou H, Pan W, Kastin AJ. The fasting polypeptide FGF21 can enter brain from blood. Peptides. 2007;28:2382-6
44. Bookout AL, De Groot MHM, Owen BM, Lee S, Gautron L, Lawrence HL. et al. FGF21 regulates metabolism and circadian behavior by acting on the nervous system. Nature medicine. 2013;19:1147-52
45. Patel R, Bookout AL, Magomedova L, Owen BM, Consiglio GP, Shimizu M. et al. Glucocorticoids Regulate the Metabolic Hormone FGF21 in a Feed-Forward Loop. Molecular Endocrinology. 2015;29:213-23
46. Rühlmann C, Wölk T, Blümel T, Stahn L, Vollmar B, Kuhla A. Long-term caloric restriction in ApoE-deficient mice results in neuroprotection via Fgf21-induced AMPK/mTOR pathway. Aging. 2016;8:2777-89
47. Kundu P, Lee HU, Garcia-Perez I, Tay EXY, Kim H, Faylon LE. et al. Neurogenesis and prolongevity signaling in young germ-free mice transplanted with the gut microbiota of old mice. Sci Transl Med. 2019 11
48. Conte M, Sabbatinelli J, Chiariello A, Martucci M, Santoro A, Monti D. et al. Disease-specific plasma levels of mitokines FGF21, GDF15, and Humanin in type II diabetes and Alzheimer's disease in comparison with healthy aging. GeroScience. 2021;43:985-1001
49. Picca A, Guerra F, Calvani R, Marini F, Biancolillo A, Landi G. et al. Mitochondrial Signatures in Circulating Extracellular Vesicles of Older Adults with Parkinson's Disease: Results from the EXosomes in PArkiNson's Disease (EXPAND) Study. Journal of Clinical Medicine. 2020;9:504
50. Yang C, Wang W, Deng P, Li C, Zhao L, Gao H. Fibroblast Growth Factor 21 Modulates Microglial Polarization That Attenuates Neurodegeneration in Mice and Cellular Models of Parkinson's Disease. Front Aging Neurosci. 2021;13:778527
51. Amiri M, Braidy N, Aminzadeh M. Protective Effects of Fibroblast Growth Factor 21 Against Amyloid-Beta(1-42)-Induced Toxicity in SH-SY5Y Cells. Neurotox Res. 2018;34:574-83
52. Sun Y, Wang Y, Chen ST, Chen YJ, Shen J, Yao WB. et al. Modulation of the Astrocyte-Neuron Lactate Shuttle System contributes to Neuroprotective action of Fibroblast Growth Factor 21. Theranostics. 2020;10:8430-45
53. Rühlmann C, Dannehl D, Brodtrück M, Adams AC, Stenzel J, Lindner T. et al. Neuroprotective Effects of the FGF21 Analogue LY2405319. J Alzheimers Dis. 2021;80:357-69
54. Kakoty V, C SK, Yang C-H, Kumari S, Dubey SK, Taliyan R. Neuroprotective Effect of Lentivirus-Mediated FGF21 Gene Delivery in Experimental Alzheimer's Disease is Augmented when Concerted with Rapamycin. Molecular Neurobiology. 2022;59:2659-77
55. Restelli LM, Oettinghaus B, Halliday M, Agca C, Licci M, Sironi L. et al. Neuronal Mitochondrial Dysfunction Activates the Integrated Stress Response to Induce Fibroblast Growth Factor 21. Cell Reports. 2018;24:1407-14
56. Kuroda M, Muramatsu R, Maedera N, Koyama Y, Hamaguchi M, Fujimura H. et al. Peripherally derived FGF21 promotes remyelination in the central nervous system. Journal of Clinical Investigation. 2017;127:3496-509
57. Lynch SV, Pedersen O. The Human Intestinal Microbiome in Health and Disease. The New England journal of medicine. 2016;375:2369-79
58. Jiao N, Baker SS, Chapa-Rodriguez A, Liu W, Nugent CA, Tsompana M. et al. Suppressed hepatic bile acid signalling despite elevated production of primary and secondary bile acids in NAFLD. Gut. 2018;67:1881-91
59. Monaghan T, Mullish BH, Patterson J, Wong GKS, Marchesi JR, Xu H. et al. Effective fecal microbiota transplantation for recurrent Clostridioides difficile infection in humans is associated with increased signalling in the bile acid-farnesoid X receptor-fibroblast growth factor pathway. Gut Microbes. 2019;10:142-8
60. Long X, Liu D, Gao Q, Ni J, Qian L, Ni Y. et al. Bifidobacterium adolescentis Alleviates Liver Steatosis and Steatohepatitis by Increasing Fibroblast Growth Factor 21 Sensitivity. Frontiers in Endocrinology. 2021 12
61. Martin A, Ecklu-Mensah G, Ha CWY, Hendrick G, Layman DK, Gilbert J. et al. Gut microbiota mediate the FGF21 adaptive stress response to chronic dietary protein-restriction in mice. Nature communications. 2021;12:3838
62. Ding X, Boney-Montoya J, Bryn, Angie, Katie, David. et al. βKlotho Is Required for Fibroblast Growth Factor 21 Effects on Growth and Metabolism. Cell metabolism. 2012;16:387-93
63. Adams AC, Yang C, Coskun T, Cheng CC, Gimeno RE, Luo Y. et al. The breadth of FGF21's metabolic actions are governed by FGFR1 in adipose tissue. Mol Metab. 2012;2:31-7
64. Sun W, Nie T, Li K, Wu W, Long Q, Feng T. et al. Hepatic CPT1A Facilitates Liver-Adipose Cross-Talk via Induction of FGF21 in Mice. Diabetes. 2021: db210363.
65. Sáenz De Urturi D, Buqué X, Porteiro B, Folgueira C, Mora A, Delgado TC. et al. Methionine adenosyltransferase 1a antisense oligonucleotides activate the liver-brown adipose tissue axis preventing obesity and associated hepatosteatosis. Nature communications. 2022 13
66. Liu W, Sun C, Yan Y, Cao H, Niu Z, Shen S. et al. Hepatic p38 Activation Modulates Systemic Metabolism Through FGF21-Mediated Interorgan Communication. Diabetes. 2022;71:60-72
67. Chen W, Hoo RL, Konishi M, Itoh N, Lee PC, Ye HY. et al. Growth hormone induces hepatic production of fibroblast growth factor 21 through a mechanism dependent on lipolysis in adipocytes. J Biol Chem. 2011;286:34559-66
68. Park JG, Xu X, Cho S, Hur KY, Lee MS, Kersten S. et al. CREBH-FGF21 axis improves hepatic steatosis by suppressing adipose tissue lipolysis. Sci Rep. 2016;6:27938
69. Li M, Fu W, Li X-A. Differential fatty acid profile in adipose and non-adipose tissues in obese mice. International journal of clinical and experimental medicine. 2010;3:303-7
70. Achari AE, Jain SK. Adiponectin, a Therapeutic Target for Obesity, Diabetes, and Endothelial Dysfunction. Int J Mol Sci. 2017 18
71. Lin Z, Tian H, Lam KS, Lin S, Hoo RC, Konishi M. et al. Adiponectin mediates the metabolic effects of FGF21 on glucose homeostasis and insulin sensitivity in mice. Cell metabolism. 2013;17:779-89
72. Li H, Wu G, Fang Q, Zhang M, Hui X, Sheng B. et al. Fibroblast growth factor 21 increases insulin sensitivity through specific expansion of subcutaneous fat. Nature communications. 2018;9:272
73. Han MS, Perry RJ, Camporez JP, Scherer PE, Shulman GI, Gao G. et al. A feed-forward regulatory loop in adipose tissue promotes signaling by the hepatokine FGF21. Genes Dev. 2021;35:133-46
74. Sommakia S, Almaw NH, Lee SH, Ramadurai DKA, Taleb I, Kyriakopoulos CP. et al. FGF21 (Fibroblast Growth Factor 21) Defines a Potential Cardiohepatic Signaling Circuit in End-Stage Heart Failure. Circulation: Heart Failure. 2022 15
75. Shen Y, Ma X, Zhou J, Pan X, Hao Y, Zhou M. et al. Additive relationship between serum fibroblast growth factor 21 level and coronary artery disease. Cardiovascular diabetology. 2013;12:124
76. Huen SC, Wang A, Feola K, Desrouleaux R, Luan HH, Hogg R. et al. Hepatic FGF21 preserves thermoregulation and cardiovascular function during bacterial inflammation. Journal of Experimental Medicine. 2021 218
77. Liu SQ, Roberts D, Kharitonenkov A, Zhang B, Hanson SM, Li YC. et al. Endocrine Protection of Ischemic Myocardium by FGF21 from the Liver and Adipose Tissue. Scientific Reports. 2013;3:2767
78. Planavila A, Redondo I, Hondares E, Vinciguerra M, Munts C, Iglesias R. et al. Fibroblast growth factor 21 protects against cardiac hypertrophy in mice. Nature communications. 2013;4:2019
79. Fu Z, Gong Y, Liegl R, Wang Z, Liu C-H, Meng SS. et al. FGF21 Administration Suppresses Retinal and Choroidal Neovascularization in Mice. Cell Reports. 2017;18:1606-13
80. Zhao T, Wang W, Gao K, Li S, Jiang Y, Yang Z. et al. Fibroblast growth factor-21 alleviates phenotypic characteristics of dry age-related macular degeneration in mice. Exp Eye Res. 2022;218:109014
81. Tomita Y, Fu Z, Wang Z, Cakir B, Cho SS, Britton W. et al. Long-Acting FGF21 Inhibits Retinal Vascular Leakage in In vivo and In vitro Models. International Journal of Molecular Sciences. 2020;21:1188
82. Smith CM, Hayamizu TF, Finger JH, Bello SM, McCright IJ, Xu J. et al. The mouse Gene Expression Database (GXD): 2019 update. Nucleic Acids Research. 2019;47:D774-D9
83. Katoh M, Nakagama H. FGF Receptors: Cancer Biology and Therapeutics. Medicinal Research Reviews. 2014;34:280-300
84. Babina IS, Turner NC. Advances and challenges in targeting FGFR signalling in cancer. Nature Reviews Cancer. 2017;17:318-32
85. Kang YE, Kim JT, Lim MA, Oh C, Liu L, Jung SN. et al. Association between Circulating Fibroblast Growth Factor 21 and Aggressiveness in Thyroid Cancer. Cancers (Basel). 2019 11
86. Yu X, Li Y, Jiang G, Fang J, You Z, Shao G. et al. FGF21 promotes non-small cell lung cancer progression by SIRT1/PI3K/AKT signaling. Life sciences. 2021;269:118875
87. Stemmer K, Zani F, Habegger KM, Neff C, Kotzbeck P, Bauer M. et al. FGF21 is not required for glucose homeostasis, ketosis or tumour suppression associated with ketogenic diets in mice. Diabetologia. 2015;58:2414-23
88. Mittelman SD. The Role of Diet in Cancer Prevention and Chemotherapy Efficacy. Annual review of nutrition. 2020;40:273-97
89. Huang X, Yu C, Jin C, Kobayashi M, Bowles CA, Wang F. et al. Ectopic Activity of Fibroblast Growth Factor Receptor 1 in Hepatocytes Accelerates Hepatocarcinogenesis by Driving Proliferation and Vascular Endothelial Growth Factor-Induced Angiogenesis. Cancer Research. 2006;66:1481-90
90. Liu ZY, Luo Y, Fang AP, Wusiman M, He TT, Liu XZ. et al. High serum fibroblast growth factor 21 is associated with inferior hepatocellular carcinoma survival: A prospective cohort study. Liver International. 2022;42:663-73
91. Yang C, Lu W, Lin T, You P, Ye M, Huang Y. et al. Activation of Liver FGF21 in hepatocarcinogenesis and during hepatic stress. BMC gastroenterology. 2013;13:67
92. Hong F, Lin CY, Yan J, Dong Y, Ouyang Y, Kim D. et al. CNPY2 Contributes to Liver Oncogenesis by Promoting UPR-dependent destabilization of p53. Hepatology. 2022
93. Hu Y, Liu HX, Jena PK, Sheng L, Ali MR, Wan YY. miR-22 inhibition reduces hepatic steatosis via FGF21 and FGFR1 induction. JHEP Rep. 2020;2:100093
94. Chen J, Jiang S, Shao H, Li B, Ji T, Staiculescu D. et al. CRISPR-Cas9-based genome-wide screening identified novel targets for treating sorafenib-resistant hepatocellular carcinoma: a cross-talk between FGF21 and the NRF2 pathway. Sci China Life Sci. 2022
95. Singhal G, Kumar G, Chan S, Fisher FM, Ma Y, Vardeh HG. et al. Deficiency of fibroblast growth factor 21 (FGF21) promotes hepatocellular carcinoma (HCC) in mice on a long term obesogenic diet. Mol Metab. 2018;13:56-66
96. Zheng Q, Martin RC, Shi X, Pandit H, Yu Y, Liu X. et al. Lack of FGF21 promotes NASH-HCC transition via hepatocyte-TLR4-IL-17A signaling. Theranostics. 2020;10:9923-36
97. Kim J, Lee S, Lee MS. Suppressive Effect of Autocrine FGF21 on Autophagy-Deficient Hepatic Tumorigenesis. Front Oncol. 2022;12:832804
98. Huang X, Yu C, Jin C, Yang C, Xie R, Cao D. et al. Forced expression of hepatocyte-specific fibroblast growth factor 21 delays initiation of chemically induced hepatocarcinogenesis. Mol Carcinog. 2006;45:934-42
99. Xu P, Zhang Y, Wang W, Yuan Q, Liu Z, Rasoul LM. et al. Long-Term Administration of Fibroblast Growth Factor 21 Prevents Chemically-Induced Hepatocarcinogenesis in Mice. Digestive diseases and sciences. 2015;60:3032-43
100. Dai H, Hu W, Zhang L, Jiang F, Mao X, Yang G. et al. FGF21 facilitates autophagy in prostate cancer cells by inhibiting the PI3K-Akt-mTOR signaling pathway. Cell Death & Disease. 2021 12
101. Song Y, Zhang W, Zhang J, You Z, Hu T, Shao G. et al. TWIST2 inhibits EMT and induces oxidative stress in lung cancer cells by regulating the FGF21-mediated AMPK/mTOR pathway. Experimental cell research. 2021;405:112661
102. Osawa T, Muramatsu M, Watanabe M, Shibuya M. Hypoxia and low-nutrition double stress induces aggressiveness in a murine model of melanoma. Cancer Sci. 2009;100:844-51
103. Fonseca M, Soares R, Coelho P. Lower melanoma pulmonary metastatic burden in obese mice: role of FGF-21. Melanoma Research. 2021;31:515-25
104. Luo Y, Yang Y, Liu M, Wang D, Wang F, Bi Y. et al. Oncogenic KRAS Reduces Expression of FGF21 in Acinar Cells to Promote Pancreatic Tumorigenesis in Mice on a High-Fat Diet. Gastroenterology. 2019;157:1413-28 e11
105. Cook JH, Melloni GEM, Gulhan DC, Park PJ, Haigis KM. The origins and genetic interactions of KRAS mutations are allele- and tissue-specific. Nature communications. 2021 12
106. Knott ME, Ranuncolo SM, Nuñez M, Armanasco E, Puricelli LI, De Lorenzo MS. Abstract 1577: Levels of Fibroblast Growth Factor 21 (FGF21) in serum as diagnostic biomarker in patients with breast cancer. Cancer Research. 2015;75:1577 -
107. Akyol M, Alacacioglu A, Demir L, Kucukzeybek Y, Yildiz Y, Gumus Z. et al. The alterations of serum FGF-21 levels, metabolic and body composition in early breast cancer patients receiving adjuvant endocrine therapy. Cancer biomarkers: section A of Disease markers. 2017;18:441-9
108. Knott ME, Minatta JN, Roulet L, Gueglio G, Pasik L, Ranuncolo SM. et al. Circulating Fibroblast Growth Factor 21 (Fgf21) as Diagnostic and Prognostic Biomarker in Renal Cancer. Journal of molecular biomarkers & diagnosis. 2016 1
109. Harlid S, Harbs J, Myte R, Brunius C, Gunter MJ, Palmqvist R. et al. A two-tiered targeted proteomics approach to identify pre-diagnostic biomarkers of colorectal cancer risk. Sci Rep. 2021;11:5151
110. Qian J, Tikk K, Weigl K, Balavarca Y, Brenner H. Fibroblast growth factor 21 as a circulating biomarker at various stages of colorectal carcinogenesis. British Journal of Cancer. 2018;119:1374-82
111. Harlid S, Myte R, Van Guelpen B. The Metabolic Syndrome, Inflammation, and Colorectal Cancer Risk: An Evaluation of Large Panels of Plasma Protein Markers Using Repeated, Prediagnostic Samples. Mediators Inflamm. 2017;2017:4803156
112. Florea A, Harris RB, Klimentidis YC, Kohler LN, Jurutka PW, Jacobs ET. Circulating Fibroblast Growth Factor-21 and Risk of Metachronous Colorectal Adenoma. Journal of Gastrointestinal Cancer. 2021;52:940-6
113. Kimak E, Nurczyk K, Skoczylas T, Duma D, Gieroba R, Solski J. Fibroblast growth factor 21, epidermal growth factor receptor, interleukin 6, myeloperoxidase, lipid hydroperoxide, apolipoproteins A-I and B, as well as lipid and lipoprotein ratios as diagnostic serum biomarkers for gastric cancer. Polish archives of internal medicine. 2019;129:559-62
114. Cymbaluk-Płoska A, Gargulińska P, Chudecka-Głaz A, Kwiatkowski S, Pius-Sadowska E, Machaliński B. The Suitability of FGF21 and FGF23 as New Biomarkers in Endometrial Cancer Patients. Diagnostics. 2020;10:414
115. Li J, Chiu K, Ou Y, Wang S, Chen C, Yang C. et al. Alteration in serum concentrations of FGF19, FGF21, and FGF23 in patients with urothelial carcinoma. BioFactors (Oxford, England). 2019;45:62-8
116. Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ. et al. FGF-21 as a novel metabolic regulator. Journal of Clinical Investigation. 2005;115:1627-35
117. Zhu L, Zhao H, Liu J, Cai H, Wu B, Liu Z. et al. Dynamic folding modulation generates FGF21 variant against diabetes. EMBO reports. 2021;22:e51352
118. Dunshee DR, Bainbridge TW, Kljavin NM, Zavala-Solorio J, Schroeder AC, Chan R. et al. Fibroblast Activation Protein Cleaves and Inactivates Fibroblast Growth Factor 21. J Biol Chem. 2016;291:5986-96
119. Papatheodorou I, Moreno P, Manning J, Fuentes AM-P, George N, Fexova S. et al. Expression Atlas update: from tissues to single cells. Nucleic Acids Research. 2019;48:D77-D83
120. Zhen EY, Jin Z, Ackermann BL, Thomas MK, Gutierrez JA. Circulating FGF21 proteolytic processing mediated by fibroblast activation protein. The Biochemical journal. 2016;473:605-14
121. Shao W, Jin T. Hepatic hormone FGF21 and its analogues in clinical trials. Chronic Diseases and Translational Medicine. 2021
122. Makarova E, Kazantseva A, Dubinina A, Denisova E, Jakovleva T, Balybina N. et al. Fibroblast Growth Factor 21 (FGF21) Administration Sex-Specifically Affects Blood Insulin Levels and Liver Steatosis in Obese Ay Mice. Cells. 2021;10:3440
123. Jakovleva TV, Kazantseva AY, Dubinina AD, Balybina NY, Baranov KO, Makarova EN. et al. Estradiol-dependent and independent effects of FGF21 in obese female mice. Vavilovskii Zhurnal Genet Selektsii. 2022;26:159-68
124. Badakhshi Y, Shao W, Liu D, Tian L, Pang J, Gu J. et al. Estrogen-Wnt signaling cascade regulates expression of hepatic fibroblast growth factor 21. American journal of physiology Endocrinology and metabolism. 2021;321:E292-e304
125. Cyphert HA, Ge X, Kohan AB, Salati LM, Zhang Y, Hillgartner FB. Activation of the farnesoid X receptor induces hepatic expression and secretion of fibroblast growth factor 21. J Biol Chem. 2012;287:25123-38
126. Kim H, Mendez R, Zheng Z, Chang L, Cai J, Zhang R. et al. Liver-enriched transcription factor CREBH interacts with peroxisome proliferator-activated receptor α to regulate metabolic hormone FGF21. Endocrinology. 2014;155:769-82
127. Min A-K, Bae K-H, Jung Y-A, Choi Y-K, Kim M-J, Kim J-H. et al. Orphan Nuclear Receptor Nur77 Mediates Fasting-Induced Hepatic Fibroblast Growth Factor 21 Expression. Endocrinology. 2014;155:2924-31
128. Zarei M, Barroso E, Leiva R, Barniol-Xicota M, Pujol E, Escolano C. et al. Heme-Regulated eIF2α Kinase Modulates Hepatic FGF21 and Is Activated by PPARβ/δ Deficiency. Diabetes. 2016;65:3185-99
129. Alonge KM, Meares GP, Hillgartner FB. Glucagon and Insulin Cooperatively Stimulate Fibroblast Growth Factor 21 Gene Transcription by Increasing the Expression of Activating Transcription Factor 4. J Biol Chem. 2017;292:5239-52
130. Joe Y, Kim S, Kim HJ, Park J, Chen Y, Park HJ. et al. FGF21 induced by carbon monoxide mediates metabolic homeostasis via the PERK/ATF4 pathway. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2018;32:2630-43
131. Fisher FM, Kim M, Doridot L, Cunniff JC, Parker TS, Levine DM. et al. A critical role for ChREBP-mediated FGF21 secretion in hepatic fructose metabolism. Mol Metab. 2017;6:14-21
132. Cheng X, Vispute SG, Liu J, Cheng C, Kharitonenkov A, Klaassen CD. Fibroblast growth factor (Fgf) 21 is a novel target gene of the aryl hydrocarbon receptor (AhR). Toxicology and applied pharmacology. 2014;278:65-71
133. Vispute SG, Bu P, Le Y, Cheng X. Activation of GR but not PXR by dexamethasone attenuated acetaminophen hepatotoxicities via Fgf21 induction. Toxicology. 2017;378:95-106
134. Uebanso T, Taketani Y, Yamamoto H, Amo K, Tanaka S, Arai H. et al. Liver X receptor negatively regulates fibroblast growth factor 21 in the fatty liver induced by cholesterol-enriched diet. The Journal of nutritional biochemistry. 2012;23:785-90
135. Jung YS, Lee JM, Kim DK, Lee YS, Kim KS, Kim YH. et al. The Orphan Nuclear Receptor ERRγ Regulates Hepatic CB1 Receptor-Mediated Fibroblast Growth Factor 21 Gene Expression. PLoS One. 2016;11:e0159425
136. Tong X, Zhang D, Buelow K, Guha A, Arthurs B, Brady HJ. et al. Recruitment of histone methyltransferase G9a mediates transcriptional repression of Fgf21 gene by E4BP4 protein. J Biol Chem. 2013;288:5417-25
137. Li H, Dong K, Fang Q, Hou X, Zhou M, Bao Y. et al. High serum level of fibroblast growth factor 21 is an independent predictor of non-alcoholic fatty liver disease: a 3-year prospective study in China. J Hepatol. 2013;58:557-63
138. Dushay J, Chui PC, Gopalakrishnan GS, Varela-Rey M, Crawley M, Fisher FM. et al. Increased fibroblast growth factor 21 in obesity and nonalcoholic fatty liver disease. Gastroenterology. 2010;139:456-63
139. Ye D, Li H, Wang Y, Jia W, Zhou J, Fan J. et al. Circulating Fibroblast Growth Factor 21 Is A Sensitive Biomarker for Severe Ischemia/reperfusion Injury in Patients with Liver Transplantation. Sci Rep. 2016;6:19776
140. El Sagheer G, Ahmad A, Abd-Elfattah A, Saad Z, Hamdi L. A study of the circulating fibroblast growth factor 21 as a novel noninvasive biomarker of hepatic injury in genotype-4 chronic hepatitis C: Egyptian patients and their response to direct-acting antiviral agents. Clinical and Experimental Gastroenterology. 2018 Volume 11: 415-22
141. Mak LY, Lee CH, Cheung KS, Wong DK, Liu F, Hui RW. et al. Association of adipokines with hepatic steatosis and fibrosis in chronic hepatitis B patients on long-term nucleoside analogue. Liver international: official journal of the International Association for the Study of the Liver. 2019;39:1217-25
142. Chow WS, Xu A, Woo YC, Tso AW, Cheung SC, Fong CH. et al. Serum fibroblast growth factor-21 levels are associated with carotid atherosclerosis independent of established cardiovascular risk factors. Arterioscler Thromb Vasc Biol. 2013;33:2454-9
143. Lee CH, Woo YC, Chow WS, Cheung CYY, Fong CHY, Yuen MMA. et al. Role of Circulating Fibroblast Growth Factor 21 Measurement in Primary Prevention of Coronary Heart Disease Among Chinese Patients With Type 2 Diabetes Mellitus. J Am Heart Assoc. 2017 6
144. Jiang L, Yin Q, Yang M, Li M, Pan M, Han Y. et al. Fibroblast Growth Factor 21 Predicts and Promotes Vascular Calcification in Haemodialysis Patients. Kidney Diseases. 2021;7:227-40
145. Ong KL, Januszewski AS, O'Connell R, Jenkins AJ, Xu A, Sullivan DR. et al. The relationship of fibroblast growth factor 21 with cardiovascular outcome events in the Fenofibrate Intervention and Event Lowering in Diabetes study. Diabetologia. 2015;58:464-73
146. Gu L, Jiang W, Qian H, Zheng R, Li W. Elevated serum FGF21 predicts the major adverse cardiovascular events in STEMI patients after emergency percutaneous coronary intervention. PeerJ. 2021;9:e12235
147. Xie W, Li D, Shi Y, Yu N, Yan Y, Zhang Y. et al. Serum FGF21 Levels Predict the MACE in Patients With Myocardial Infarction After Coronary Artery Bypass Graft Surgery. Front Cardiovasc Med. 2022;9:850517
148. Xiao Y, Xu A, Law LS, Chen C, Li H, Li X. et al. Distinct changes in serum fibroblast growth factor 21 levels in different subtypes of diabetes. The Journal of clinical endocrinology and metabolism. 2012;97:E54-8
149. Zhang X, Yeung DC, Karpisek M, Stejskal D, Zhou ZG, Liu F. et al. Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes. 2008;57:1246-53
150. Chang LH, Chu CH, Huang CC, Lin LY. Fibroblast Growth Factor 21 Levels Exhibit the Association With Renal Outcomes in Subjects With Type 2 Diabetes Mellitus. Front Endocrinol (Lausanne). 2022;13:846018
151. Stein S, Bachmann A, Lössner U, Kratzsch J, Blüher M, Stumvoll M. et al. Serum levels of the adipokine FGF21 depend on renal function. Diabetes Care. 2009;32:126-8
152. Lehtonen JM, Forsström S, Bottani E, Viscomi C, Baris OR, Isoniemi H. et al. FGF21 is a biomarker for mitochondrial translation and mtDNA maintenance disorders. Neurology. 2016;87:2290-9
153. Riley LG, Nafisinia M, Menezes MJ, Nambiar R, Williams A, Barnes EH. et al. FGF21 outperforms GDF15 as a diagnostic biomarker of mitochondrial disease in children. Mol Genet Metab. 2022;135:63-71
154. Bag Soytas R, Suzan V, Arman P, Emiroglu Gedik T, Unal D, Cengiz M. et al. Association of FGF-19 and FGF-21 levels with primary sarcopenia. Geriatr Gerontol Int. 2021;21:959-62
155. Kwong AK, Wong VC, Wong SS, Chu VL, Koene S, Smeitink J. et al. High FGF-21 level in a cohort of 22 patients with Dravet Syndrome-Possible relationship with the disease outcomes. Epilepsia Open. 2021;6:685-93
156. Yaghoobi G-H, Shokoohi-Rad S, Jafarzadeh H, Abodollahi E. Serum Fibroblast Growth Factor 21 in Patients with and without Pterygia. Journal of Ophthalmic and Vision Research. 2020
157. Yang Y, Wu J, Wang X, Yao J, Lao KS, Qiao Y. et al. Circulating fibroblast growth factor 21 as a potential biomarker for missed abortion in humans. Fertil Steril. 2021
158. Li X, Shen H, Zhou T, Cao X, Chen Y, Liang Y. et al. Does an increase in serum FGF21 level predict 28-day mortality of critical patients with sepsis and ARDS? Respiratory Research. 2021;22:182
Author Biography
Dr. Jianping CHEN is with the School of Chinese Medicine, the University of Hong Kong. She has coauthored over 80 publications and holds four patents. The current research interests in Dr. CHEN's group include: (1) Prevention and treatment of breast cancer with Traditional Chinese Medicine; (2) The implication of liver dysfunction and metabolic disorder in breast cancer development.
Yue SUI is currently a Ph.D. student at the University of Hong Kong. Her research is centered on the pathogenesis and treatment of breast cancer.
![]() Corresponding author: Jianping CHEN, School of Chinese Medicine, The University of Hong Kong, Pokfulam, Hong Kong, China. Work Telephone Numbers +852-39176479; Fax Numbers 21684259; E-mail: abchenhk.
Corresponding author: Jianping CHEN, School of Chinese Medicine, The University of Hong Kong, Pokfulam, Hong Kong, China. Work Telephone Numbers +852-39176479; Fax Numbers 21684259; E-mail: abchenhk.

 Global reach, higher impact
Global reach, higher impact