10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2022; 18(16):6035-6051. doi:10.7150/ijbs.76573 This issue Cite
Research Paper
Downregulation of SEPTIN5 inhibits prostate cancer progression by increasing CD8+ T cell infiltration
1. School of Medicine, Southern University of Science and Technology, Shenzhen, Guangdong 518055, China.
2. Guangdong Provincial Key Laboratory of Cell Microenvironment and Disease Research, Shenzhen, Guangdong 518055, China.
3. Aurora Discovery Inc, Foshan, Guangdong 528303, China.
Abstract
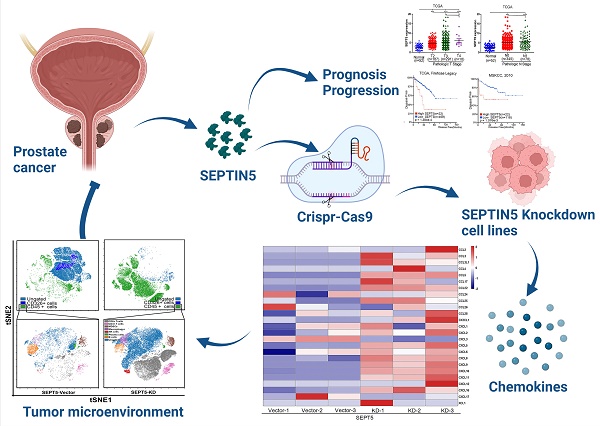
Background: Prostate cancer (PCa) is one of the most common carcinomas in men, and aberrant expression of SEPTIN5 (SEPT5) has been detected in PCa tissues. However, the role of SEPT5 in PCa is still unclear. In this study, we attempted to explore the expression changes, clinical relevance and immunomodulatory function of SEPT5 in PCa.
Methods: The expression and clinical significance of SEPT5 were evaluated based on bioinformatic analysis and were verified by western blotting, real time PCR and IHC. Allograft mouse models were used to assess the role of SEPT5 on PCa tumour formation and immunomodulatory function. Mass cytometry and IHC were used to determine the effects of SEPT5 on immune cell infiltration, especially CD8+ T cell infiltration. Correlations between SEPT5 expression and cytokine gene expression were analyzed based on TCGA and DKFZ datasets. RNA-seq and chemokine array were performed to confirm the effects of SEPT5 on cytokine production.
Results: High SEPT5 expression was found in PCa and was associated with PCa prognosis. Importantly, downregulation of SEPT5 inhibited PCa progression in vivo. In addition, SEPT5 expression was negatively correlated with immune infiltrating cell levels, chemokine and cytokine gene expression associated with CD8+ T cell infiltration and activation. Downregulation of SEPT5 increased the proportion of immune cells, especially CD8+ T cells, in tumour tissue. Both the expression of CCL5, CXCL5, CXCL9, CXCL10 and INFGR1 were elevated in mRNA and protein levels after SEPT5 knockdown.
Conclusions: In summary, downregulation of SEPT5 inhibited PCa progression, which may be mediated by increasing immune cell infiltration levels, especially CD8+ T cells, by promoting the production of IFNG-inducible chemokines and cytokines expression associated with immune cell infiltration. Our findings suggest that SEPT5 may serve as a prognostic biomarker of PCa and may be a target molecule to enhance the efficacy of immunotherapy for PCa in the future.
Keywords: prostate cancer, SEPTIN5, immune infiltrating, CD8+ T cells, chemokine, cytokine.

 Global reach, higher impact
Global reach, higher impact