10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2023; 19(3):789-810. doi:10.7150/ijbs.79328 This issue Cite
Review
Advanced strategies for nucleic acids and small-molecular drugs in combined anticancer therapy
1. Artemisinin Research Center, and Institute of Chinese Materia Medica, China Academy of Chinese Medical Sciences, Beijing 100700, China.
2. Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing 100730, China.
3. School of Traditional Chinese Medicine, Southern Medical University, Guangzhou 510515, China.
4. College of Integrative Medicine, Laboratory of Pathophysiology, Key Laboratory of Integrative Medicine on Chronic Diseases, Fujian University of Traditional Chinese Medicine, Fuzhou 350122, China.
5. Department of Nephrology, Shenzhen key Laboratory of Kidney Diseases, and Shenzhen Clinical Research Centre for Geriatrics, Shenzhen People's Hospital, The First Affiliated Hospital, Southern University of Science and Technology, Shenzhen 518020, China.
#Equal contribution
Received 2022-9-27; Accepted 2022-12-27; Published 2023-1-1
Abstract
Cancer has been considered as complex malignant consequence of genetic mutations that control the cellular proliferation, differentiation and homeostasis, thus making tumor treatment extremely challenging. To date, a variety of cargo molecules, including nucleic acids drugs (pDNA, miRNA and siRNA), therapeutic drugs (doxorubicin, paclitaxel, daunomycin and gefitinib) and imaging agents (radioisotopes, fluorescence dyes, and MRI contrast agents) have been regarded as the potential medicines in clinical application. However, non-single therapeutic drug could induce the satisfied clinical results because of tumor heterogeneity and multiple drug resistance and the nanotechnology-based combined therapy is becoming an advanced important mode for enhanced anticancer effects. The review gathers the current advanced development to co-deliver small-molecular drugs and nucleic acids for the anticancer therapy with nanomedicine-based combination. Furthermore, the superiority is definitely presented and the barriers are detail discussed to surmount the clinical challenges. In final, future perspectives in rational direction for combined tumor therapy of drugs and nucleic acids are exhibited.
Keywords: anticancer, nucleic acids, small-molecular drugs, combination, synergistic therapy
Introduction
Cancer is a malignant type of genetic disease in which not one, but several, mutations are required. It continues to be main factors of death with increasing incidence throughout the world, accounting for over eight million people worldwide annually according to the worldwide scientific report published in 2022 [1], which equated to the entire population of New York. Therefore, it has been regarded as the extremely urgent that exploring the reasonable treatments to ameliorate or even surmount the current situation.
Significant advances in anticancer therapy have been made in recent decades. Of the various therapeutic agents, chemotherapy has become a preferred choice for most managements of cancers due to its high cytotoxicity of chemo agents against cancer cells [2]. However, these conventional small-molecular anticancer drugs usually show inherent defects (such as the poor bioavailability, non-specific distribution, rapid blood clearance and multiple drug resistance in physiological conditions), often resulting in severe side effects in patients (sudden death, gastric bleeding, alopecia and heart failure). To improve these limitations, several nano-delivery approaches have been introduced, such as liposomes, nanoparticles and so on [3]. Some of these studies indeed showed an increase in drug accumulation and a decrease in cytotoxic side effects in tumor tissues, some of which have been already in clinical trials [4]. Nevertheless, the major of additional materials may inevitably bring out adverse impacts, such as, the drug loading capacities were relatively low, most delivery materials which were just as excipients may cause added toxicities or undesirable immune response [5, 6].
As genetic aberrations involved in tumor cell signaling are further explored, nucleic acids have been probed as functional bio-macromolecules applied to the treatment of cancer [7]. Gene drugs (such as siRNA, miRNA etc.), compared to the small-molecular drugs, could effectively augment down-regulated the target mRNA or knockdown overexpressed proteins, which have been regarded as the attractive approach for suppressing tumor cell growth and invasion [8, 9]. However, these bio-macromolecules still bear several non-negligible shortcomings to hinder the utilization in clinic. For example, nucleic acids were opted to be degraded by RNase families; have rapid clearance and poor cellular uptake etc. Appropriate nano-delivery materials have been emerged to accelerate their clinic development, which displayed that the loaded nucleic acids could be effectively protected and released at the desired intracellular site [10].
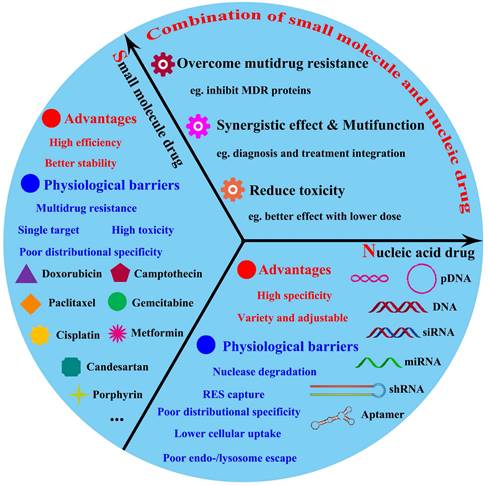
Up to present, it has been indeed achieved in substantive progress for cancer treatment by either chemotherapy small-molecular drugs or nucleic drugs; nevertheless, this malignant disease was still obstinate to be eliminated. This predicament may be attributed from the molecular and structural complexity of tumor, which cancers were regarded as various genetic disorders involved in multiple cells signaling [11-13]. So, it is possible that a single therapeutic strategy is insufficient to halt the progression of most cancers. Therefore, nanomedicine-based combination anticancer therapy between nucleic acids drugs and small-molecular drugs, which targeted at different molecular levels of dysregulated tumor cellular signaling pathways, could be expected to break through the current dilemma in tumor treatment field. Actually, the co-therapy of small-molecular drugs and nucleic acids has displayed the promoted anticancer effects and become the promising potential for tumor treatment [14-16]. As shown in Figure 1, this review firstly will cover an overview of advanced developments of combined-therapy that simultaneously deliver both chemotherapy drugs and nucleic acids to tumor targets; subsequently, elaborate these significant design parameters that were essential for the co-administration of small-molecular drugs and nucleic acids drugs (pDNA, siRNA and miRNA); besides, the potential merits and shortcomings will be respectively discussed; lastly, future perspectives in rational direction for combined tumor therapy of drugs and nucleic acids will be exhibited.
The major advantages and physiological barriers of small molecule drugs/nucleic acid drugs therapy and the advantages offered by combined them.
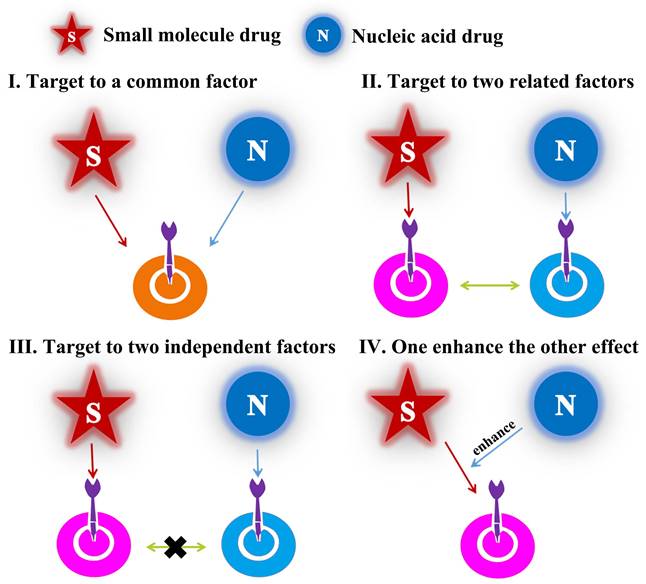
Different modes of drug combination
As mentioned above, the emergence of cancer was generally accompanied of multiple mutations of numerous key proteins which regulated the growth and metastasis of cancer cells. Therefore, in combination with small-molecular drugs, nucleic acids could act another prominent role by down-regulation/knockdown of targeted RNA/proteins in cancer cells. Over the last two decades, combined-anticancer therapy between chemo-and gene drugs have become an increasingly strategy either in preclinic or clinic, which about 1340 articles have been reported according to incomplete statistics. In this review, we covered almost researches and generalized the different synergistic mechanisms. There were divided into four categories (Figure 2): (1) the chemotherapy drugs and nucleic acids shared the same target point, such as Figure 2I, which may importantly enhance the therapeutic effect, for example, anti-neovascularization, apoptosis induction or anti-angiogenesis; (2) the two drugs not acted a target but cooperatively associated in one signaling pathway, like as Figure 2II, which may obtain the unexpected results by similar to “Butterfly Effect”; (3) the two ones coped with different targets, for example, Figure 2III; (4) one drug could have an auxiliary reinforcement for the other, for instance, Figure 2IV. Among the four classifications, the second one, which attempted to actualize multiple-targeted anticancer therapy, occupied most of the proportion (~60%) in recent studies; besides, the third class mainly involved to the integration of diagnosis and treatment for cancer. Actually, all these synergistic mechanisms embodied the unique advantages of combined-therapy, which gave the credit to the assembly structures/modes of nano-medicines encapsulated by nucleic acids drugs and small-molecular drugs.
Structural illustration of four synergetic mechanisms of combined-therapy.
Different types for drug encapsulation
In the past two decades, on account of the emergence of systemic toxicity and multiple drug resistance (MDR) for small-molecular drugs [17], the development of nanomedicine-based combined anticancer therapy between nucleic acids and chemotherapeutic drugs has paralleled the growth of gene nanotechnology. The combined treatment could importantly increase its therapeutic efficacy in many kinds of cancer. The disadvantages associated with chemotherapy could be mitigated by the addition of gene drive drugs; furthermore, the efficacy of gene therapy could also be strengthened by small molecule drugs with more potent effects. All of which led to the significant increase in treatment for aggressive cancers, where the progression and invasion involved various physiologic or pathologic factors [18]. Therefore, construction for a nano-medicine should be prerequisite for perfectly exerting the synergistic effects, which could not only effectively load different drugs but also safely delivered these cargoes into the targeted sites. There were multiple nano-devices applied in the combined anticancer therapy, which could be divided into two categories according to different administration modes (Graphical abstract): (1) “1+1” type: separated encapsulation for combination therapy that free chemotherapeutics drug + gene loaded by nanocarrier or loading two agents into two individual nanocarrier; (2) “2 in 1” type: co-encapsulation for combination therapy including the corporate drug pairs (CDP), “co-assembling drug” with independent agents and drug-carrier conjugates delivering drug (DCDD).
“1+1” type: separated encapsulation for combination therapy
One of the greatest challenges in the process of co-delivery of chemotherapeutics and gene drive agents is optimizing the timing/location of delivery of two drugs into tumor cells. In some cases, the addition of drugs will affect the pharmacokinetics of nano carriers and require major modifications to the design of nanocarriers, which will lead to the difficulty of delivering nucleic acids and small molecule drugs together in a single nanocarrier. Therefore, differences in toxicity profiles or optimal routes of administration often require the two agents to be formulated individually for separate administration so as to achieve the therapeutic better effects for combination anticancer therapy.
Based on this concern or principle, the sequential delivery of therapeutic drugs and nucleic acids to cancers has been increasingly adopted by researchers with great success in recent years, as shown in Figure 3A. One main manner of sequential delivery is combination of free chemotherapeutics drug and gene loaded by nanocarrier and it has the advantage of easily optimizing doses of both therapeutic agents. In the earliest works, Zhang et al. [19] performed a study in which rats were given a single injection of chloroquine two to three hours prior to delivery of polylysine-molossin/DNA via the portal vein and bile duct pathways to increase transfection efficiency. On the basis of that the hTNF-α could sensitize the cancer cells to chemotherapeutic drugs, SR Murugesan and co-workers [20] used gemcitabine to deal with Human pancreatic cancer cells after three hours indications of AdEgr.TNF.11D. The combination treatment was well tolerated, highly active, and resulted in marked delays in the growth of human pancreatic xenograft tumors compared to either agent alone in the in vitro experiments. However, the combination of free chemotherapeutic drug and gene loaded nanocarrier has some weaknesses: 1) it is difficult to deliver controllable ratios of drug to nucleic acid in the same target cells; 2) difficulty in synchronizing pharmacokinetics and biodistribution; and 3) unwanted toxicity in normal tissues caused by free drugs, leading almost to its conclusion.
Compared to the first manner, the other manner for sequential delivery that loading two agents into two individual nanocarrier (same or different) obtains more advantages: 1) independent controllable releasing time and rate of drug action; 2) achieve multiple therapeutic targets; and 3) avoid the toxicity of free agents, and it has been widely used to achieve the combination of two drugs, particularly for overcoming the tumor MDR under the fourth collaboration mechanism (Figure 2IV). Hence, Su et al. sequential inject G3-HD-OEI/TNFα gene vector and liposomal doxorubicin (DOX) into the tumor-bearing mice in order to delay the tumor growth in subcutaneous murine neuroblastoma Neuro2A [21]. Huang et al. [22] fabricated a dual sequential delivery system composed of two independent nanosystems: cyclooxygenase-2 siRNA in poly-d-arginine (9R)/2-deoxyglucose (DG)-loaded gold nanostar (GNS) and paclitaxel-loaded thermosensitive liposome (PTSL), which has been proposed to overcome MDR mediated by hypoxia in tumors (Figure 3B).
A) Structural illustration of co-delivery systems of “1+1” type. B) The sequential dual delivery systems based on siCOX-2@RDG and PTSL to overcome the hypoxia-induced MDR [22]; C) The “2-in-1” integrated nanocarrier constructed from Dox-loaded NOCC/HA-SH NPs and SH-HA-Dopa-coated CaP/siRNA NPs linked through disulfide bond (HA-ss-HA) [23].
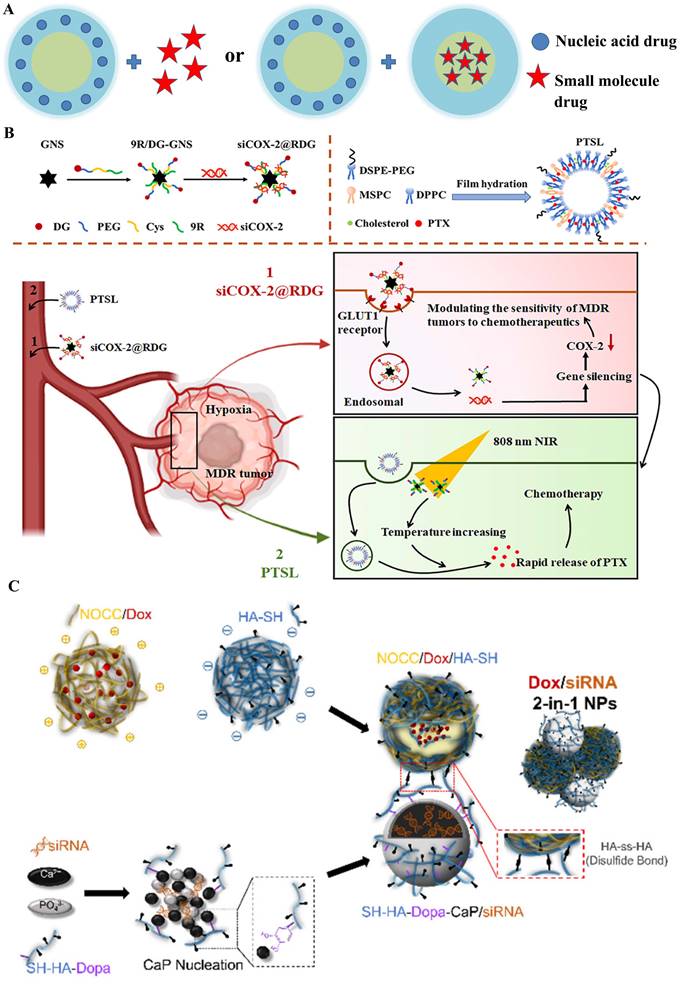
Similarly, Wu et al. [23] designed a redox/pH-sensitive chimeric nanoparticles for co-delivering doxorubicin (DOX) and siBcl-2 in the HeLa cells. The integrated nanosystem was formed with two individual components through a redox-responsive thiol-disulfide bond: DOX loaded N, O-carboxymethyl chitosan (NOCC) complex with a thiolated hyaluronic acid (HA-SH) nanocarrier and dopamine (Dopa)-conjugated thiolated hyaluronic acid (SH-HA-Dopa)-coated calcium phosphate (CaP)-siRNA nanocarrier (Figure 3C). A key to the clinical realization of these treatments would be the development of a nanocarrier system allowing the simultaneous delivery of chemotherapeutic agents and nucleic acids to cancer, in which "co-delivery" would take place of combinatorial delivery.
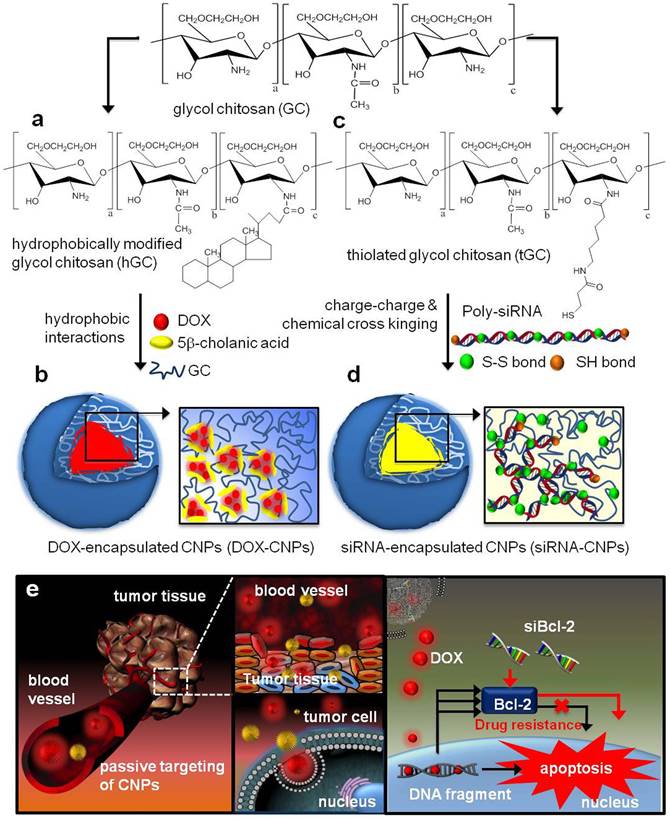
Moreover, encapsulation of different kinds of drugs into the same nanocarriers often possess more advantages: 1) could overcome different instability and in vivo behaviors (pharmacokinetics, tumor accumulation tendency); 2) lower the influence of the intrinsic physicochemical properties from different agents. Wang and co-workers developed a sequential treatment strategy with RGD-modified liposomes containing P-gp siRNA or DOX in order to improve MDR cancer therapy [24]. In vivo studies of drug resistant MCF7/A tumor demonstrated significantly greater tumor growth inhibition followed by sequential treatment of RGD modified liposomes containing siRNA or DOX when compared to liposomal DOX treatment alone. Similarly, Shanthi Ganesh et al. [25] adopted the combination treatment of cisplatin and siRNAs encapsulated in CD44-targeting hyaluronic acid (HA)-based self-assembling nanocarriers. After the last 3 days of intra-articular HA-PEI/PEG/survivin siRNA injection, the HA-ODA/PEG/cisplatin was injected, which reversed cisplatin resistance and significantly retarded tumor growth (growth inhibition increased from 30% to 60%) in cisplatin resistant tumors. As shown in Figure 4, Hong et al. designed the DOX loaded CNPs (DOX-CNPs) by hydrophobic interactions and Bcl-2 siRNA loaded CNPs (siRNA-CNPs) by charge-charge interactions [26]. Significantly, sequential treatment of DOX-CNPs and siRNA-CNPs resulted in significantly longer-term inhibition of tumor growth than each treatment alone. In addition, various nanocarriers also developed to overcome MDR, such as siRNA and paclitaxel co-delivered by triblock copolymer [27] or multifunctional nanocomplex [28], respectively, self-assembling nanoparticle system of siRNA and cisplatin [29, 30], and some other inorganic nanoparticles, such as graphene oxide for DOX and siRNA [31].
The basis of many drug-nucleic acid combinations is to first modify signaling pathways in cancer cells to render them sensitive to chemotherapeutic drugs, so the most important thing is the need of sequential release rather than the must of separate systems. Li et al. formed the sequential drug delivery system SiO2@AuNP, which featured step-by-step controlled and sequential delivery of siRNA, DOX, and HCPT in order to maximize its anticancer efficacy [32]. This suggested that the sustained SiO2 release characteristics made the difference in Tmax between HCPT and DOX about 8 to 12 h, and this increased the sensitizing efficacy of HCPT on DOX compared to co-administration (~10-fold). Similarly, the triblock copolymer (PCL-PEG-PHIS) was synthesized with folate-PEG-PHIS in order to construct a multifunctional targeted (PTX/siVEGF-CPPs/TMPM) polymer micelle for the sequential delivery of siVEGF-CPPs (disulfide bond-linked siVEGF and cell-penetrating peptides) and paclitaxel [33]. Other sequential therapy, such as redox-sensitive glucolipid nanoparticle separately delivering siRNA and DOX to overcome MDR [34], PEI-grafted graphene oxide delivering siRNA and anticancer drugs sequentially [35] and so on. Above all, these sequential delivery nanocarriers show potential as a better effective co-delivery method for nucleic acids drugs and chemo drugs to enhance the efficacy of anticancer therapy.
However, under no circumstance can we ignore the fact that distinct assembly methods may produce the diverse bio-behaviors (like as drug release rate, onset time and biodistribution etc.) of small-molecular drugs and nucleic acids. Briefly, in order to maximize combined therapeutic efficacy, a proper co-delivery way and the proper combination of several drugs should be key factors for anticancer therapy. Meaningfully, co-load of therapeutic chemo drugs and nucleic acids drugs into one single nanocarrier (“2 in 1”) will offer various benefits based on synchronized pharmacokinetics, convenience and delivery of controllable relative amounts of both agents to target cells, which often is what the type “1+1” couldn't possess.
“2 in 1” type: co-encapsulation for combination therapy
The concept of “co-delivery” implied that all different drugs were encapsulated in one nanocarrier. As we mentioned before, compared to the co-administrations by separate vectors, the “2 in 1” strategy could provide a guarantee that both the small-molecular drug and nucleic acids were synergistically introduced into the same tumor cells to promote the anticancer therapy; besides, it ensured that these drugs have a synchronous pharmacokinetics and tuned the reasonable proportions of administration dosage. Three subdivisions were presented and discussed their characteristics respectively in the next context.
Co-encapsulation following drug-drug interaction
Several researches displayed that both the small-molecular drug and nucleic acid were jointly encapsulated in the core of nano-delivery systems [36], which gave a more protection drugs in delivering progress, especially nucleic acids, and were synchronously released into cells. Generally, the formation of these nanomedicines was firstly constructed the corporate drug pairs (CDP) (Figure 5A), which required certain interactions between small-molecular drug and nucleic acid, like as electrostatic adsorption, coordination bond or covalent coupling; then a biocompatible skeleton molecule (e.g., polymers, lipids) were covered on the CDP surfaces to obtain the nano-system loading drugs.
Structural illustration of co-deliver DOX and Bcl-2 siRNA by tumor-homing and biocompatible glycol chitosan (GC)-based nanocarriers (CNPs) to achieve optimal efficacy [26].
A) Structural illustration of co-encapsulation following drug-drug interaction; B) Ternary HMplexation containing benzethonium chloride (BZT) and siRNA [37]; C) Nuclease-resistant synthetic drug-DNA adducts as a platform for anticancer therapy [38]; D) The preparation method of GMP- and/or VEGF siRNA-loaded LCP nanoparticles [39].
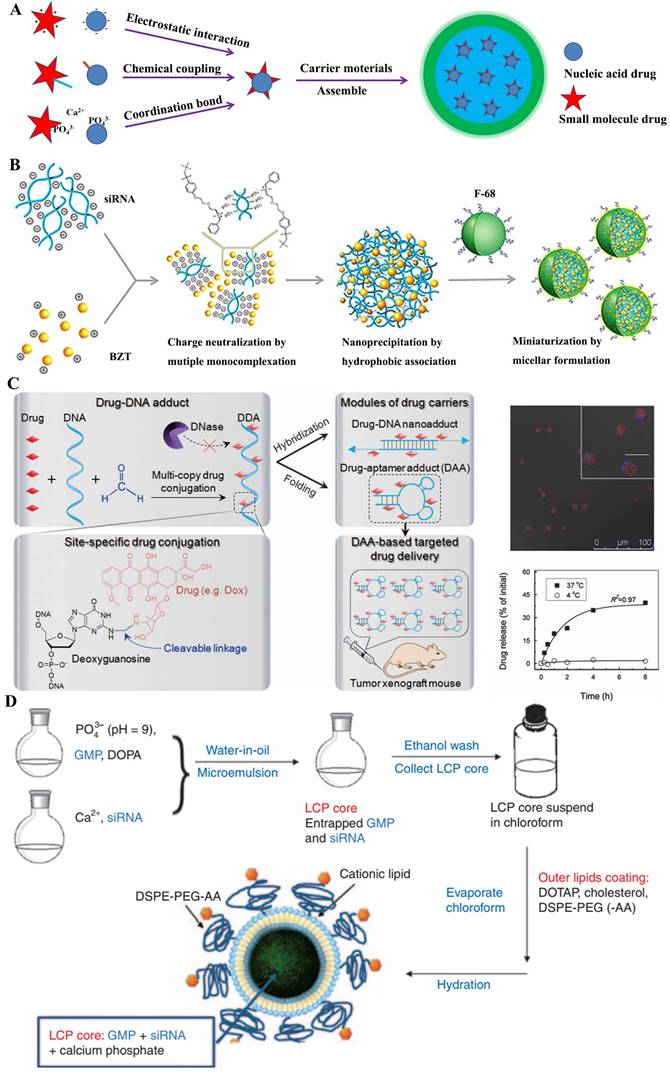
Some traditional anticancer drugs comprised of the amino groups, like as doxorubicin hydrochloride (DOX), benzethonium chloride (BZT) and gemcitabine hydrochloride (GEM), electrostatically coupled with nucleic acids (siRNA or miRNA) by their negative phosphate backbone. Actually, employing the functional nucleic acids could boost the chemo-sensitization of cancer treatment with anticancer drugs. As was known, the overexpression of apoptotic genes NF-kB and Bcl-2 were observed in tumor invasion and metastasis, which has been regarded as the potential target for cancer treatment [40]. M. Yousefie and colleague constructed a chitosan-based nanoparticle where co-delivery of doxorubicin and IL17RB-targeted siRNA for enhanced anticancer efficacy in breast cancer cells [41]. Utilized the negatively charged carboxymethyl dextran (CMD), they designed chitosan-based nanoparticles containing CMD to effectively compress and encapsulate the DOX/oligonucleotide pairs, which significantly inhibited the growth and proliferation of cancer cells. Similarly, combining Bcl-2-targeted siRNA with anticancer drugs could promote the synergistic effect for tumor treatment. S. Kim and colleague [37] attempted to develop a more clinically relevant formation for small-molecular drugs and nucleic acids. Using a single complexation preparation, a colloidal system of hydrophobically associated multiple monocomplex (HMplex) was designed to co-delivery between Bcl-2 targeting siRNA and a monocationic anticancer drug (benzethonium chloride, BZT) (Figure 5B). Chosen the Bcl-2 protein overexpressed cells (MDA-MB-231) as the model, the assay data showed a dramatic reduction of Bcl-2 expression (down to 20%) with these co-delivery nanomedicines; remarkably, the level of early apoptosis was significantly increased from 6% to 18%, which implied that the siRNA could sensitized the resistant cancer cells and then enhanced the anticancer effect of BZT. In addition, R. Paulmurugan et. al. [42] selected the gemcitabine hydrochloride and antisense-miRNA-21 as the CDP encapsulated in a PLGA-PEG-based nano-device to treat hepatocellular carcinoma by the double emulsion method. In their research, the co-delivery system was shown to result in the 14% apoptotic cells in both Hep3B and HepG2 cells, and efficiently blocked endogenous miRNA-21 function and promoted the expression level of the PTEN target protein [39].
In fact, fluorescent tracers were customarily coupled with nucleic acids by covalent conjunction. These CDPs could be applied to both diagnosis and treatment for cancer, which was recently advocated by clinicians. As known, the magnetic resonance imaging (MRI), which was widely used to diagnosis in clinic, had the innate limitation of spatial resolution (ca. 100 mm) to be insatiable to monitor the intracellular transfection of nucleic acid drugs [43]. J. Cheon and his colleague designed a multi-functional probe for simultaneous siRNA delivery and multimodal image in tumor treatment [44]. In this nano-device structures, manganese-doped magnetism engineered iron oxide covered by bovine serum albumin (BSA) was selected to core section, where the CDP of fluorescent dyes (Cy5)/siRNA was conjugated by redox-responsive disulfide bonds; besides, the PEGylated RGD was also incorporated onto the surfaces to enhance special uptake by the ανβ3 integrin recognition. Several assay results showed that these nanomedicines could be clearly visualized in MDAMB-435 cells, which expressed ανβ3 integrin as breast-cancer cells; moreover, these loaded siRNAs displayed an important decline (~ 40%) of targeted GFP proteins at the low dose of 4 and 6 pmol. Similarly, Zhu et al. [38] reported the synthetic drug-DNA adducts (DDAs) for anticancer therapy (Figure 5C). Site-specific conjugation of multiple copies of anthracycline drugs was performed on each DNA, allowing programmable DNA and drug design for DDA preparation. DDAs were resistant to nucleases and stable for storage, but could release drugs gradually at physiological temperature. DOX-aptamer adducts significantly inhibited the xenograft tumor growth, which indicated the potential of DDAs for scale-up production and clinical application in anticancer therapy. Apart from this, aptamer-drug conjugates (ApDCs) also were potential application [45].
Except the methods mentioned above, ion coordination was also a driving force for CDP formation between small-molecular drug and nucleic acid. In general, the chelated phenomenon was based on the special recognition of non-toxic mrtal ions (e.g., Ca2+, Zn2+ ion) with phosphate bonds [46]. Lipid/calcium/phosphate (LCP) nanoparticle could effectively deliver gene drugs into targeted cells [47]. Based on this preparation mechanism, L. Huang and colleague [39] explored the co-delivery strategy of gemcitabine monophosphate (GMP) and VEGF-targeted siRNA for non-small-cell lung cancer (NSCLC) therapy (Figure 5D). They tried to utilize the co-delivery to act the different mechanisms for comprehensively proliferating tumor cells. As we know, GMP could induce apoptosis and the VEGF siRNA could block the tumor neovasculature by inhibition of the VEGF-VEGFR signaling. Their results showed that the combined-nanomedicine triggered a significant amount (~30%) of apoptotic cells, compared to the GMP nanoparticles (~12%). Zinc was another coordinate ion as key element for binding to nucleic acids [48, 49]. For instance, Zn2+-dipicolylamine (Zn-DPA) derivatives, which bear the special selectivity for phosphate-containing molecules, were usually regarded as special probes for the membrane surfaces of necrotic cells. X. Chen and colleague [50] designed a nanoplatform to co-deliver small-molecular drugs and siRNA for cancer therapy, by transplanting the distinct property of Zn-DPA covered by hyaluronic acid, which owned low toxicity and specific accumulation.
Co-encapsulation without drug interaction
The combination of delivering nucleic acid and small-molecular drugs always were regarded as a challenging barrier due to the defined differences of the two types of drugs, especially in their physicochemical properties, including the molecular weight, electrical properties, hydrophilicity or hydrophobicity, and metabolic stability, all of which greatly affect their biodistribution and pharmacokinetic properties [17, 51, 52]. Most recent strategies were developed based on these differences and often the best choice was introducing a module for loading the chemotherapeutic agent into the existing carrier for nucleic acid drug. In addition, some vectors were specifically developed to meet the demand of both drugs and nucleic acids.
Regular distribution
In recent decades, gene therapy has been hailed as an important weapon for combating a variety of diseases, especially cancer. Thence, a variety of materials typified by cationic carriers have been developed to overcome physiological barriers, such as systemic stability, cellular targetability, immunogenicity and safety, for the delivery of genetic drugs, primarily including polyplexes and lipoplexes formed by cationic polymers or lipids with nucleic acids [51, 58, 59]. Much of the research on the respective gene and drug systems provided positive direct principles for designing the co-delivery nanocarriers. Coincidentally, most hydrophobic drugs for cancer chemotherapy are easily loaded into the hydrophobic area of polyplexes or lipoplexes and the hydrophilic area carries the nucleic drugs through electrostatic interaction, which results in the layered or regular distribution in the entire system and the two drugs often appear to a serial release in the focus of infection (Figure 6A). The sequential release contributes to the collaboration of two drugs with different target factors, especially under the second and the fourth mechanism (as shown in Figure 2 II and IV).
In the earlier period, Tamara Minko et al. [60] developed a cationic liposome prepared from DOTAP to co-delivery DOX and two siRNAs targeted to MRP1 mRNA (pump resistance) and Bcl2 mRNA (non-pump resistance), respectively. In the simple co-delivery liposome, the siRNAs absorbed on the outer layer tended to release firstly to knocked the expression of MRP1 and Bcl2 that were related to muti-drug resistant, which paved for the later release of DOX and reduced its efflux. Thus, the system led to the efficient induction of apoptosis and killing of MDR lung cancer cells to a level that cannot be achieved by separate treatment with an anti-cancer drug or siRNA alone. As the manner shown in Figure 6B, based on PDMAEMA-PCL-PDMAEMA triblock copolymers, Zhong and his co-workers [53] constructed the biodegradable cationic micelles to co-deliver siVEGF and paclitaxel into PC-3 cancer cells, which revealed a further improved apoptosis efficiency. In addition to the organic vehicle based on other similar materials, like linear adamantane-terminated octadecane (C18-AD) and Tris(2-aminoethyl) amine-attached β-cyclodextrin-centered hyperbranched polyglycerol (CD-HPG-TAEA) [46], mPEG-PCL-graft-PDMAEMA [61], PLA-b-PDMAEMA [62] and PEI-β-cyclodextrin [63], various inorganic materials also were synthesized for co-delivering two drugs [64]. By functionalizing the surface of mesoporous silica nanoparticles with a phosphonate group, it may be possible to electrostatically bind DOX to the porous interior, where the drug would be released through acidification of the media under both abiotic and biotic conditions. Hence, it was developed to co-deliver nucleic drugs (Pgp siRNA or p53 gene) and DOX with the modification of PEI [65, 66], PDMAEMA/PMPDSAH [67], respectively. Similarly, functionalized magnetic graphene nanoparticle (CMG) [68], QDs [69] and Se nanoparticles [70] platform was used for tumor treatment.
However, the cationic nanocarriers with poor security often remain in the in vitro evaluation stage and couldn't meet the needs of in vivo delivery. So as to improve the stability, the addition of PEG on the outer of nanocarriers became fashionable for system construction, such as mPEG45-b-PCL80-b-PPEEA10 for paclitaxel and siPlk1 [71], PEG-PLL-PLLeu co-delivering docetaxel and Bcl-2 siRNA [72] or cSLN for co-delivering of paclitaxel and siMCL1 [73] for better anti-cancer treatment. Cationic nanocarriers coated by anionic materials also could prolong the circulation time of co-delivery systems. Based on this, Zhang et al. [54] co-delivered miRNA-34a and docetaxel with core-shell nanoparticles for the metastatic breast cancer therapy (Figure 6C). Similarly, Nie et al. [74] designed an ε-polylysine cationic co-polymer to efficiently take up negatively charged si-HIF1a on the surface and encapsulate gemcitabine at the hydrophilic core, then further coating with PEGylated lipid bilayer to reverse the surface charge, which demonstrated excellent ability to inhibit tumor metastasis in orthotopic tumor models.
A) Structural illustration of co-encapsulation without drug interaction. Regular distribution: B) cationic PDMAEMA-PCL-PDMAEMA triblock copolymers to co-deliver paclitaxel and siRNA [53]; C) core-shell nanocarriers to co-deliver DTX and miRNA-34a [54] and D) targeted core-shell type nanoparticles for the co-delivery of paclitaxel and siRNA [55]. Mix-point distribution: E) nano-assembled HBPO(OEI600-PBA)10 for the co-delivery of DOX and siRNA [56]; F) γ-cyclodextrin and γ-CD-OEI-SS-FA co-deliver paclitaxel and p53 gene for anticancer therapy [57].
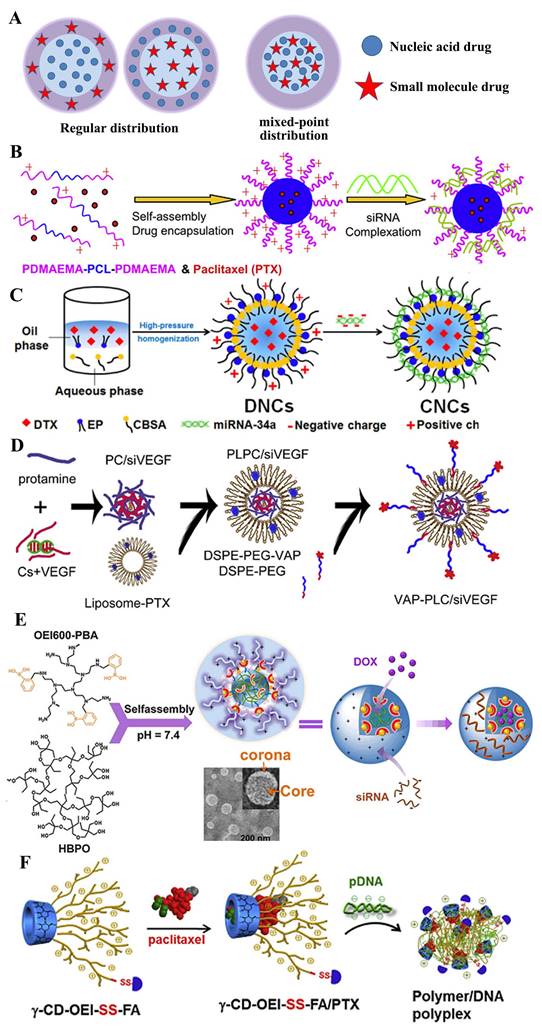
The problem always, the progress on the road. The PEG dilemma dragged the hind legs of the greatest effect of co-administration, and in order to circumvent these salient weak points, two main strategies were developed, one of which was to introduce the targeting ligands, such as the modified synthetic analog of Luteinizing Hormone-Releasing Hormone (LHRH) decapeptide [75], hyaluronic acid [76], TAT [77] and folic acid [78]. Paula T. Hammond and co-workers [79] constructed a layer-by-layer nanoparticle (ie. CML/PLA/siRNA/PLA/HA) to co-deliver siRNA and chemotherapeutic drug to treat the triple negative breast cancer. In addition, the other strategy to overcome the PEG dilemma is the modification of a cleavable PEG. Li et al. [80] designed the nanoparticle to co-deliver PTX and siRNA, which consisted of the pH-responsive core, the cationic shell, and the matrix metalloproteinase (MMP)-cleavable PEG corona conjugated via a peptide linker. Once in tumor-microenvironment, the responsive shedding of PEG would contribute into the cellular uptake of two drugs. Similar systems also structured in other works, such as pH-trigger [66] and redox-sensitive [81] dissociation of protective outer layer. Chen et al. [82] developed a stepwise cleavable composite lipid nanocarrier (PTX/miR124-NP) composed of calcium phosphate to co-deliver PTX and miR124. It exhibited a superior ability to respond to the tumor microenvironment, in which the surface PEG layer was shed in the mildly acidic environment of the tumor tissues and the exposed oligomeric hyaluronic acid could facilitate the cellular uptake by targeting the CD44 receptor to the surface of the cancer cells. Once into cells, o-HA would detach from the core due to the reduction of disulfide bonds by glutathione and to inhibit tumor metastasis. PTX and miR124 were then released sequentially and exerted synergistic anti-tumor effects by reversing the process of the epithelial-mesenchymal transition in MDA-MB 231 cells.
Overall, small molecule drugs and nucleic acid drugs disperse in nanocarrier by a layered or regular manner, but nucleic acid drugs absorbed in the outer layer mostly, which is unfavorable for its protection in the process of circulation in vivo. A better method to avoid this shortcoming is encapsulate it into inner layer, such as the nanoparticles of multi-layers [83-85] or core-shell [86]. As Figure 6D shown, Feng et al. [55] successfully constructed a core-shell nanoparticle to co-deliver PTX and siVEGF for the inhibition of breast cancer. In this study, siRNA was encapsulated in the negative-charged core to form the ternary complex composed of siRNA, chondroitin sulfate (CS) and protamine, which was coated by vapreotide-PEG modified cationic liposome. Meaningfully, the VAP-PCL/siVEGF obtained a stronger inhibition of tumor growth and better safety compared to the formulation with the single one drug.
Mixed-point distribution
Generally, during the construction of the vector, nucleic acid drugs and small molecule drugs are added sequentially and specifically loaded into the hydrophilic area and hydrophobic area or holes, respectively, so they appear to a layered or regular distribution in the co-nanocarriers. Sometimes, the boundary of the hydrophilic/hydrophobic or positive/negative area is not obvious and the areas tend to a state of staggered mixing distribution. Hence, the two drugs also co-loaded in the manner of “mixed-point distribution”, like the bright or dark stars dotted in the night sky. Compared to the system of layered or regular distribution, it often releases two drugs almost simultaneously and it is helpful to enhance the collaboration of two drugs with the same one target factor, especially under the first mechanism (as shown in Figure 2I).
Patil et al. [87] fabricated biotin-PEG-functionalized PLGA nanocarriers loaded with PTX and siRNA targeting multidrug resistance gene 1 (MDR1). The NPs were prepared by the dual emulsion method whereby the mixture of the PEI/siRNA complex and PTX was encapsulated by the PLGA and PLA layer. Additional results indicated that these dual-agent loaded nanocarriers were significantly more cytotoxic, suggesting that silencing MDR1 expression increased PTX accumulation in drug resistant JC cells and PTX/P-gp siRNA-loaded nanocarriers inhibited significant tumor growth than that loaded with PTX only. However, the double-emulsion solvent evaporation has failed in effectively removing the organic solvent, which limited its medical application. Furthermore, the supercritical CO2 technology has been employed for drug nanocarriers due to its mild operating conditions, lower solvent residue and lower cost [88]. Chen et al. [89] fabricated the siRNA-PTX-CMPs by a supercritical process to co-load siP-gp and PTX for the treatment of drug-resistance Bcap-37 cells. The chitosan NPs containing siRNA prepared by ionic gelation and PTX were as core materials and were followed by encapsulation with poly (L-lactide)-poly (ethylene glycol)-poly(L-lactide) triblock copolymer (PLLA-PEG-PLLA) as a shell material. Furthermore, the results demonstrated that the siRNA-PTX-CMPs achieved a much better anti-tumor effect than the CMP encapsulated with PTX alone.
In the development of drugs, the complexity of preparation is often not favored, like the double-emulsion solvent evaporation or its improved proposals. People tend to develop a direct hybrid approach for the nanomedicine with two drugs. Deng et al. [90] constructed the nano-complexes by the one-step mixing method, which prepared using hyaluronic acid and chitosan that simultaneously encapsulate positive charged doxorubicin and negative charged miR-34a mimics, which not only resulted in an effective reduction of drug resistance and side effects of DOX, but also enhanced the therapeutic outcome of DOX, finally obtained the simultaneous inhibition of tumor growth and migration. Similarly, Jia et al. developed a hyperbranched-hyperbranched polymeric (HBPO(OEI600-PBA)10) nano-assembly with pH-dependent stability to co-deliver antitumor DOX and autophagy-inhibitory Beclin1 siRNA, which could silence the Becline1 expression and thereby suppress the DOX-induced cellular autophagy, leading to remarkably enhanced in vitro and in vivo antitumor efficacy [56], as shown in Figure 6E. In addition, various co-deliver system of mixed-point distribution were developed, such as Tf-modified co-encapsulated DOX- and pEGFP-loaded SLN for targeting lung cancer [91], the first use of nanoscale metal-organic frameworks (UiO-NMOFs) for the co-delivery of cisplatin and MDR gene-silencing siRNAs (Bcl-2, P-glycoprotein and survivin) to enhance therapeutic efficacy [92] and γ-cyclodextrin and multiple oligoethylenimine arms with folic acid (γ-CD-OEI-SS-FA) co-deliver paclitaxel PTX and p53 gene for potential cancer therapy [57] (Figure 6F).
Drug-carrier conjugates delivering drug (DCDD)
The functionalization of nanocarriers has been exploited in the last two decades. Actually, the synergic bioactivities of gene delivery systems have been increasingly highlighted by covalently inserting the small-molecular drugs into materials. By the stimuli-broken or non-broken junctions [96, 97], these modified materials could not only transport the nucleic acids into targeted sites but also sequentially produce the synergy effects, which reduced the leakage of drugs in the delivery process.
The important one is delivering genes by the materials comprised of small-molecular drugs. Nanoparticles for delivering gene drugs have been emerged over the last decades, where the auxiliary material was designed for biosafety and biocompatibility [98-100]. Moreover, recent studies have covalently inserted several anticancer drugs into delivery materials [101-106], which was aimed to realize the bio-functionalization of carriers, thus, promoting the synergistic effect [107-112]. In general, the hydrophobic anticancer small-molecular drugs (e.g. doxorubicin [113, 114], camptothecin [93], oleic acid [115] etc.) were grafted onto hydrophilic marcomolecules which could bind to gene. Three subdivisions were classified by the type of gene delivery systems, for instance, polysaccharide-modified macromolecules, cationic polymers, lipid-based nanoparticles and so on (Figure 7A). In addition, nucleic acids could take as a carrier for chemotherapy drugs delivery. According to the kinds of delivery materials and encapsulation methods, they were classified and detailed discussed respectively.
Polysaccharide-derived prodrug
Natural polysaccharides have been regarded as the ideal materials for construing drugs delivery systems [116]. An unexpected result may occur that small-molecular drugs were jointed into their chemical skeletons. H.L. Jiang and colleague designed a hierarchical targeted delivery system loading siRNA and lonidamine (LND) drug sequentially to tumor cells and mitochondria [117]. As known, LND could inhibit hexokinase and directly impact mitochondrial adenine nucleotide translocase to trigger the opening of the mitochondrial permeability transition pore and then induce tumor cell apoptosis [118]. In this nanostructures, two parts were defined as TCPL and PPF. The former was obtained by linking LND and TPP (mitochondria targeting ligand) to the chitosan-g-PEI polymer with Schiff base bonds; and the latter is the PEG-b-poly (acrylic acid) copolymer comprised of folic acid. Through an easy mixture preparation, the hybrid nanoparticles could enhance the tumor cell uptake and promote the co-drugs release. These results showed that treated with the nanomedicine, more cytochrome C (up to two times) were released from mitochondria in Hela cell cytosols; moreover, the quantity of both activated caspase 9 and caspase 3 was sharply increased in HeLa cells; furthermore, a significant decrease (down to ~20%) of Bcl-2 mRNA and Bcl-2 protein were also observed by the assays of qPCR and western blotting. All these results demonstrated that the co-delivery of effective hierarchical system could synergistically activate mitochondria-mediated apoptosis. Similarly, T. Chen and colleague embedded the adriamycin (ADM) into the dextran-graft-PEI (DEX-g-PEI) copolymer, which delivered the plasmid for osteosarcoma disease [113]. This research presented that the DEX-ADM-PEI nanoparticles could efficiently deliver both pEGFP-N1 and ADM to osteosarcoma cells with low cytotoxicity. In addition, J. Li doxorubicin with amantadine-modification was through the “host-guest” hydrophobic force to be linked to the polyethylenimine-co-cyclodextrins (PEI-co-CD) polymers [119], where plasmid DNA was encapsulated to form the hybrid co-delivery nanoparticles (denoted as SNP). These results displayed that a remarkable decrease of cytotoxicity was seen in murine melanoma B16F10 cells; moreover, the intracellular release from nano-system dissociation and siRNA transfection were simultaneously observed; furthermore, the formation inhibited the tumor growth in vivo.
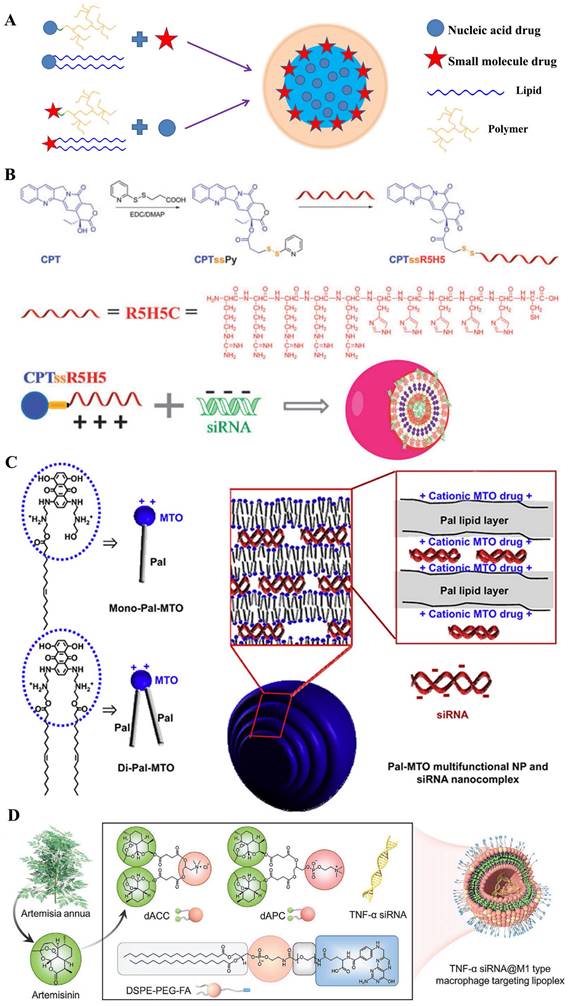
A) Structural illustration of co-encapsulation with prodrug-based carriers; B) Co-delivery system CPTssR5H5 to deliver CPT and MAP3K7-targeted siRNA simultaneously to MDR cancer cells for enhanced chemotherapy [93]; C) Pal-MTO and siRNA nanocomplexes where cationic surface charges of MTO moieties of Pal-MTO could allow electrostatic interaction with siRNA. [94]; D) Schematic illustration of amphipathic artesunate prodrug-hydrogel lipoplexes delivering TNF-α siRNA for rheumatoid arthritis therapy [95].
Cationic polymers-derived prodrug
Prodrug-like gene carriers could be obtained by combination of hydrophobic drugs with cationic hydrophilic polymers [120, 121]. In the research of G. Cheng and his colleagues [93], anticancer drug camptothecin (CPT) was conjugated to a hydrophilic cationic peptide (R5H5) using disulfide bond as a linker; then the synthesized material of CPTssR5H5 was developed in siRNA delivery for MDR cancer therapy (Figure 7B). These co-delivery nanopolymers displayed a suitable parameter (size ~60 nm and zeta ~6 mV); moreover, CPTssR5H5 could carry MAP3K7 siRNA into MDA-MB-231 cells. Furthermore, the result of RT-PCR assay showed an important decrease (~30%) of MAP3K7 mRNA expression in MDA-MB-231 cells at siRNA concentrations of 100 nM. Compared with cationic peptide, another classic cationic hydrophilic polymer (PEI) was also polymerized by an AT1 receptor blocker drug named eprosartan (ES) for DNA delivery. Currently, many reports verified that AT1 receptor blockers (ARBs) exert beneficial effects on tumor progression, vascularization, and metastasis [122]. J.P. Zhou and colleagues [123] designed a reticular eprosartan-g-PEI (ESP) copolymer mediated targeted ES drug and p53 gene co-delivery system. The ESP loading pDNA was a spheroidal particle shared a 150 nm in size and ~16 mV in zeta potential; in PANC-1 cells, the data of MTT assay showed almost non-cytotoxicity (Over 90% cell viability) at the concentration of ESP used dose. Subsequent assays implied that the nanomedicine could significantly inhibit the expression of Ang II-induced angiogenesis related gene VEGF, which the inhibition rate achieved 83%; moreover, anticancer efficacy in vivo displayed an important tumor growth inhibition efficacy and the final tumor volume was below 500 mm3, far less than that in other groups. All these results demonstrated it an effective co-delivery strategy for cancer therapy. Similarly, L. Huang and his colleague [124] polymerized the pharmacophores of Metformin into PEI polymers. On account of the anticancer efficacy of Metformin in many cancers, a co-delivery system named LPH-PolyMet was developed to human non-small-cell lung cancer (NSCLC) treatment. Several results displayed the LPH-PolyMet could high efficiently silence the expression of VEGF mRNA (down to 5%) in NSCLC tumor cell H460, and then induced apoptosis in about 40% of cells, which implied that PolyMet play dual roles as both a gene carrier and an antitumor drug to achieve combinational therapeutic efficacies against cancer.
Lipid-derived prodrug
There was a major of studies involved in amphiphilic where the drug was modified by hydrophilic or hydrophobic groups [125, 126], thus, several drug-inserted lipids could be reasonably applied in DCDD. Mitoxantrone (MTO), as an anticancer chemical drug with cationic property, was conjugated to hydrophobic palmitoleic acid to form the drug-modified lipid for delivering siRNA [94] (Figure 7C). These hybrid nanoparticles were ~220 nm in size and had a narrow PDI index. These results presented that it significantly down-regulated the expression of red fluorescence protein in B16F10-RFP cells (down to ~40%); moreover, antitumor assay in vivo displayed that these nanoparticles loading with Mcl-1-specific siRNA (siMcl-1) could be internalized into human KB tumor cells; and demonstrated a cooperative effect which reduced tumor size by 83.4%. In general, the only cationic drug-modified lipid was not satisfied for development of nucleic acids in clinic. Therefore, some prodrug-hybrid nanoparticles were designed for co-delivery with gene drugs. X. Zhang et al. [127] designed a dual-responsible camptothecin-poly(carboxybetaine) conjugate (PCB-CPT), which could effectively release the CPT based pH and esterase-sensitive cleavage. With the cationic lipid named DDAB, the nano-complexes loaded with siRNA (siPlk1) were prepared to targeting the polo-like kinase 1 in HeLa cells. Results showed that no evident initial burst release was observed in pH 7.4 medium; moreover, the release rate of CPT drugs was accelerated to ~57% with 120 h in a sustained manner at the acidic conditions. Additionally, the amphipathic cisplatin prodrug has also been integrated into the nanoparticles with siRNA co-delivery. Chosen the PLGA-PEG and the cationic lipid named as G0-C14, O.C. Farokhzada and colleague [128] constructed the co-delivery system which simultaneously encapsulated cisplatin prodrug and siRNAs against REV1/REV3L to promote chemo-sensitivity. The hybrid nanoparticles achieved greater than 95% luciferase silence in luciferase-expressing HeLa-derived cells (Dual-Luc HeLa), while no cytotoxicity was evidently observed. Similarly, Li et al [95] synthetized two novel artemisinin derivatives (dAPC and dACC) which possessed mimic phospholipids and cationic lipids, respectively (Figure 7D). Then, they prepared a cationic multicomponent drug-embedded liposome and the TNF-α siRNA/artemisinin co-delivery nano-microplex (MTAsi@MG) by immobilization of TNF-α siRNA/lipoplex on porous microfluidic HA microspheres. The results indicated that it might be one potential gene/drug co-delivery systems for rheumatoid arthritis therapy.
Structural illustration of co-deliver microRNA with gemcitabine-oleic acid conjugate (GOA) based carriers to inhibit the growth of tumor [129].
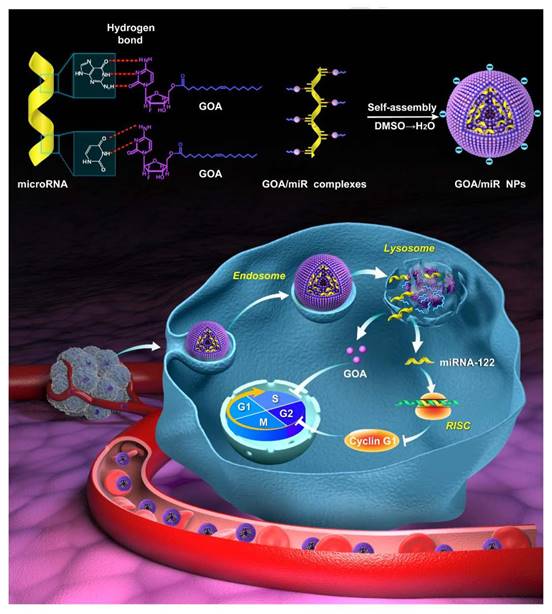
Gemcitabine, a widely used chemotherapeutic drug, also be designed as a prodrug carrier because the cytosine of gemcitabine could bind to miRNA by hydrogen bond interaction of nucleobases. As shown in Figure 8, Zhang et al. [129] synthesized an amphiphilic gemcitabine prodrug (gemcitabine-oleic acid conjugate, GOA) and it was used to bind miRNAs with hydrogen bond forces between nucleobases in dimethyl sulfoxide via a denaturation-annealing procedure. The results indicated that the GOA/miR nanoparticles would be efficiently internalized into cancer cells after intravenous administration and possessed significant tumor growth inhibition effects.
Nucleic acids-derived prodrug
Theoretically, siRNA was apt to be degraded by a series of ribozymes, leading to the instability in the transport process [132]. Thus, the siRNA conjugates could ameliorate this dilemma [133-135]; therein, amphiphilic siRNA-lipids could as a carrier or auxiliary for co-delivering small-molecular drugs [136] (Figure 9A). S.S. Feng and his colleague [130] constructed a co-delivery nanomedicine of docetaxel which was comprised of TPGS-siRNA conjugates and herceptin-conjugated vitamin E TPGS for tumor treatment (Figure 9B). In this system, siRNA conjugate, which was linked by the redox-responsive disulfide, was targeted for the polo-like kinase 1 (Plk1) protein. Results displayed that the ~190 nm micelles could release ~70% of docetaxel drugs in HeLa cells after 120 h; moreover, at the concentration of 125 nM, an important knockdown (88.8%) of Plk1 mRNA expression could be clearly observed. Interestingly, bis-siRNA-phospholipid conjugate played an auxiliary role in co-delivery of DOX drugs. In this study [137], the nanoparticles (siRNA-PCNPs) loading with DOX was composed of siRNA-phospholipids, cationic lipids DDAB and DSPE-PEG2000 fuse around PLGA for targeting Polo-like kinase 1 protein in HeLa cells. Dual functions of siRNA conjugates were designed that gene silence and as a component of nanoparticles to embed hydrophobic DOX drugs. Subsequent results showed that the nanoparticles importantly down-regulated Plk1 mRNA to ~30% in HeLa cells and induced 76.4% apoptosis cells, which effectively inhibited the tumor growth. Besides, siPlk1-phospholipids sharply enhanced the encapsulation of siPlk1 (three times), which was auxiliary for loading drugs.
A) Structural illustration of nucleic acids-derived prodrug; B) Formulation of the docetaxel loaded TPGSesiPlk1/TPGS micelles (micSD) [130]; C) Schematic design of the triangle-shaped DNA origami for doxorubicin intercalation [131].
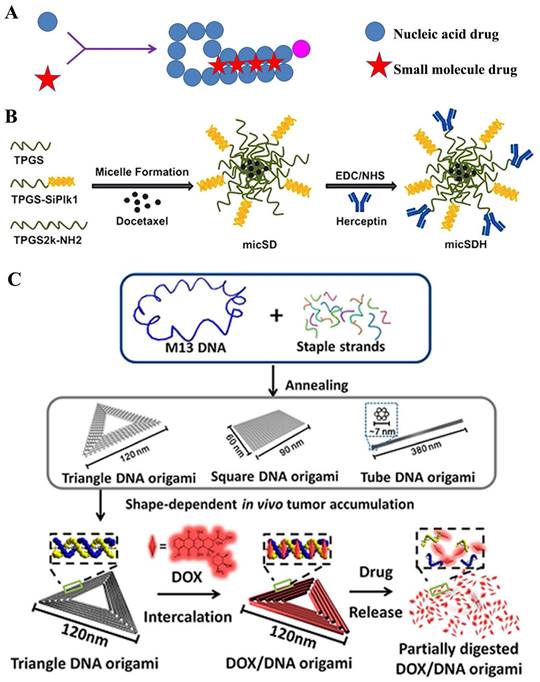
Several double-stranded DNA or aptamer, among the nucleic acids, presented a relatively high stability to meet the in vivo drug delivery [45, 138-140]. Therein, the small-molecular drug, such as DOX, intercalate into DNA base pairs, with a preference for GC/CG regions [141]. H. Mok and colleague [142] synthesized multivalent aptamer-siRNA conjugates as material for co-delivering doxorubicin. This conjugate contained multiple mucin-1 aptamers and Bcl2-specific siRNA, which were attempted to transfect into mucin-1 overexpressing MCF-7 breast cancer cells for combination cancer therapy. Assay data displayed that the DOX-Apt-siRNA gradually released DOX≈80% during one day incubation; moreover, high cellular uptake was observed in both wild-type and MDR MCF-7 cells; furthermore, the conjugates loading with DOX presented two-fold higher of caspase-3/7 activity than that in free DOX group, which may exert important cytotoxic effects for MDR cancer therapy. Recently, DNA origami techniques provided a potential platform for novel drug delivery [143]. Y. Du and his colleague [131] designed the triangular-shaped DNA origami vehicles to load DOX through intercalation. Therein, DNA origami carriers were obtained through the self-assembly between M13mp18 phage DNA the complementary DNA helper strands, where the doxorubicin was subsequently non-covalently intercalated into (Figure 9C). A series of assays showed that triangle DNA origami was ~120 nm, and loaded ~50% DOX; moreover, the release slowly progressed, which reached to ~20% under physiological conditions at 48 h. Furthermore, in cytotoxicity assay, these nanomedicines displayed an important antitumor efficacy in the MDA-MB-231-GFP cells. All these results implied that DNA origami had great potential in clinic for safely and efficiently delivering antitumor drugs. Similar strategy was also used for DOX co-delivery by microRNA [140].
Conclusion and Future Direction
Recent developments in the nanotechnology field have provided avenues for the delightful nanomedicine-based combination anticancer therapy between nucleic acids and small-molecular drugs. This review covers new trends in combination therapy and discusses three key issues in detail: 1) different types of collaboration mechanisms; 2) categorized preparation methods; and 3) relatively little reported multiple delivery systems based on the combination of chemotherapeutic and gene agents. So as to achieve the satisfied therapeutic effects of anticancer therapy, three approaches to combination therapy were concisely concluded as follows: 1) different drugs in one nano-system (all-in-one) could synchronously escort the drugs into the same targeted sites; 2) the multiple stimuli-responsive nanocarriers of co-delivery with chemotherapy and gene drugs could be constructed for promoting their effective uptake and release in tumor cells; 3) co-administration of different drugs in separate nanocarriers could verify the kinetics of their pharmacologic activity, respectively (Table 1).
Summary of representative small molecule drug-nucleic acid drug combinations.
| Drug encapsulation | Delivery method | Synergistic mechanism | Small molecule drug | Nucleic acid drug | Cell line | Overcome problems | Ref | |
|---|---|---|---|---|---|---|---|---|
“1+1” type | Separate systems | Different carriers | IV | Doxorubicin | TNFα pDNA | Neuro2A | Proliferation | [21] |
| IV | Paclitaxel | Cyclooxygenase-2 siRNA | HepG2 U87MG | Hypoxia-Induced MDR | [19] | |||
| IV | Doxorubicin | Bcl-2 siRNA | HeLa | MDR | [23] | |||
| Same carrier | IV | Doxorubicin | P-gp siRNA | MCF-7/A | MDR | [24] | ||
| IV | Doxorubicin | Bcl-2; MDR1 siRNA | HeLa; MCF-7 | MDR | [26] | |||
“2 in 1” type | “Corporate drug pairs (CDP)” encapsulated in core | Electrostatic interaction | III | Benzethonium | Bcl-2 siRNA | MDA-MB231 MRC-5 NIH3T3 | Aggressive MDR | [37] |
| II | Gemcitabine | antisense- miRNA-21 | Hep3B; HepG2 | ProliferationTotoxicity | [39] | |||
| II | Doxorubicin | IL17RB siRNA | MDA-MB361 | Apoptosis; migration | [41] | |||
Chemical coupling | IV | Doxorubicin | DNA aptamer sgc8 | CEM Ramos | Resistant to nuclease degradation | [38] | ||
| IV | 5-FU | MT-II and LIB aptamer | HCT116 | Photo- controllable release | [45] | |||
| Coordination bond | III | Gemcitabine | VEGF siRNA | H460 | Anti-growth | [39] | ||
| III | Zn2+-DPA | Luciferase siRNA | 4T1 | Targeting | [50] | |||
Co-encapsulation without drug interaction | Regular distribution | I | Paclitaxel | VEGF siRNA | PC-3 | Apoptosis and anti-growth | [53] | |
| IV | Docetaxel | miRNA-34a | 4T1 | Apoptosis | [54] | |||
| II | Gemcitabine | HIF1α siRNA | Panc-1 | Proliferation; MDR | [74] | |||
| II | Paclitaxel | MCL1 siRNA | MDA-MB231 | Proliferation; MDR | [73] | |||
| III | Camptothecin | Raf-1 siRNA | C6 rat glioma | Proliferation and apoptosis | [93] | |||
| Mix-point distribution | II | Doxorubicin | Beclin1 siRNA | HeLa | Apoptosis | [56] | ||
| I | Paclitaxel | P53 pDNA | A549 | Apoptosis | [57] | |||
| IV | Cisplatin | P-gp siRNA | SKOV-3 | MDR | [92] | |||
Drug-carrier conjugates delivering drug (DCDD) | Small molecule drug-based carrier | II | Mitoxantron | MCL1 siRNA | B16-F10 | Apoptosis | [94] | |
| I | Captopril | GFP, VEGF siRNA | MDA-MB435 | Anti-angiogenesis | [105] | |||
| II | Porphyrin, Docetaxel | MMP-9 shRNA | HNE-1 | PDT; apoptosis | [120] | |||
| I | Eprosartan | P53 pDNA | PANC-1 | Anti-angiogenesis | [123] | |||
| III | Metformin | VEGF siRNA | H460 1205Lu | Apoptosis; proliferation | [124] | |||
| IV | Camptothecin | PLK1 siRNA | HeLa | MDR | [127] | |||
| IV | Cisplatin | REV1, REV3L siRNA | MDA-MB231 | MDR | [128] | |||
| II | Pheophorbide A | PD-L1 siRNA | B16-F10 | Diagnosis and treatment | [144] | |||
Nucleic acid drug-based carrier | II | Doxorubicin | pORF-hTRAIL pDNA | Bel-7402 | Apoptosis; proliferation | [114] | ||
| II | Doxorubicin | PLK1 siRNA | HeLa SK-BR-3 | Apoptosis; proliferation | [130, 137] | |||
| III | Doxorubicin | PLK1 siRNA miRNA-21 | HeLa | Apoptosis and anti-growth | [140] | |||
As the aforementioned descriptions, it was observed that the strategy of “co-assembling drugs” in independent zones was mainly reported in the field of the nanomedicine-based combining therapy, which generally presented an amphiphilic nano-system where anticancer drug was encapsulated in hydrophobic sections; while the design of drug-carrier conjugates delivering drug (DCDD) would be increasingly potential in synergistic treatment of cancers. By the stimuli-broken bond as linkers, these prodrug-modified carriers could not only effectively deliver genes into targeted cells but also sequentially produce the synergy effects of tumor treatment. Besides, the “corporate drug pairs (CDP)” suggested both gene and anticancer drugs were encapsulated in the core area of the nanocarriers, which should be considered as a promising approach where all the drugs had the capability of resistance against degradation and synchronous release.
The combined delivery strategy could importantly enhance anticancer effects by several aspects, for instance, accumulating drugs to tumor sites in vivo, surmounting the multiple drug resistance (MDR) or inducing different apoptosis pathways etc. However, there were still numerous technical barriers that hampered the development of the combination approach in the clinic. Therein, a deeper understanding of the nature of tumor heterogeneity should be the prerequisite; besides, the clear relationship was also needed between the pharmacodynamic response and associated toxicity/immune response in the simultaneous administration of gene and chemo drugs. In addition, the high developmental costs of designing the combined-complex systems were still an extreme challenge in the clinical development. Therefore, as the potential strategy, the combined nanomedicines need to overcome a variety of efforts to further clinical application between small-molecular drugs and nucleic acids drugs.
Acknowledgements
This work was supported by the Fundamental Research Funds for the National Natural Science Foundation (No. 82204322, 82141001, 82074098, 82274182), the Beijing Natural Science Foundation (No. 7214287) and the Central Public Welfare Research Institutes (Grant Nos.: ZZ14-YQ-050, ZZ14-YQ-051, ZZ14-YQ-055, ZZ14-YQ-058, ZZ14-YQ-059, ZZ14-YQ-061, ZZ14-ND-010, ZZ15-ND-10, and ZZ14-FL-002); National Key Research and Development Program of China (2020YFA0908000); National Key R&D Program of China Key projects for international cooperation on science, technology and innovation (2020YFE0205100); the Innovation Team and Talents Cultivation Program of the National Administration of Traditional Chinese Medicine (No: ZYYCXTD-C-202002); the CACMS Innovation Fund (CI2021A05101 and CI2021A05104); Scientific and Technological Innovation Project of China Academy of Chinese Medical Sciences (CI2021B014). There are no conflicts to declare.
Author Contributions
Writing-original manuscript, Chong Qiu, Yanyan Wu; writing-review & editing, Chong Qiu, Yanyan Wu, Jigang Wang, Fei Xia, Qiaoli Shi, Qiuyan Guo, Junzhe Zhang, Yuqing Meng, Chen Wang and Chengchao Xu. All authors have read and agree to the published version of the manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Znaor A, Skakkebaek NE, Rajpert-De Meyts E, Kulis T, Laversanne M, Gurney J. et al. Global patterns in testicular cancer incidence and mortality in 2020. Int J Cancer. 2022;151:692-8
2. Pan ST, Li ZL, He ZX, Qiu JX, Zhou SF. Molecular mechanisms for tumour resistance to chemotherapy. Clin Exp Pharmacol Physiol. 2016;43:723-37
3. Yao Y, Zhou Y, Liu L, Xu Y, Chen Q, Wang Y. et al. Nanoparticle-Based Drug Delivery in Cancer Therapy and Its Role in Overcoming Drug Resistance. Front Mol Biosci. 2020;7:193
4. Mundekkad D, Cho WC. Nanoparticles in Clinical Translation for Cancer Therapy. Int J Mol Sci. 2022;23:1685
5. Lu Y, Wang S, Wang Y, Li M, Liu Y, Xue D. Current Researches on Nanodrug Delivery Systems in Bladder Cancer Intravesical Chemotherapy. Front Oncol. 2022;12:879828
6. Tian L, Pei R, Zhang X, Li K, Zhong Y, Luo Y. et al. Tumor Cell-Specific and Lipase-Responsive Delivery of Hydrogen Sulfide for Sensitizing Chemotherapy of Pancreatic Cancer. Front Bioeng Biotechnol. 2022;10:934151
7. McErlean EM, McCrudden CM, McCarthy HO. Delivery of nucleic acids for cancer gene therapy: overcoming extra- and intra-cellular barriers. Ther Deliv. 2016;7:619-37
8. Subhan MA, Attia SA, Torchilin VP. Advances in siRNA delivery strategies for the treatment of MDR cancer. Life Sci. 2021;274:119337
9. Liu T, Li T, Zheng Y, Xu X, Sun R, Zhan S. et al. Evaluating adipose-derived stem cell exosomes as miRNA drug delivery systems for the treatment of bladder cancer. Cancer Med. 2022
10. Sharma P, Dando I, Strippoli R, Kumar S, Somoza A, Cordani M. et al. Nanomaterials for Autophagy-Related miRNA-34a Delivery in Cancer Treatment. Front Pharmacol. 2020;11:1141
11. de Souza PS, Biba GCC, Melo E, Muzitano MF. Chalcones against the hallmarks of cancer: a mini-review. Nat Prod Res. 2022;36:4809-26
12. Zhou L, Li Y, Liang Q, Liu J, Liu Y. Combination therapy based on targeted nano drug co-delivery systems for liver fibrosis treatment: a review. J Drug Target. 2022;30:577-88
13. Li B, Shao H, Gao L, Li H, Sheng H, Zhu L. Nano-drug co-delivery system of natural active ingredients and chemotherapy drugs for cancer treatment: a review. Drug Deliv. 2022;29:2130-61
14. Chung SL, Yee MS, Hii LW, Lim WM, Ho MY, Khiew PS. et al. Advances in Nanomaterials Used in Co-Delivery of siRNA and Small Molecule Drugs for Cancer Treatment. Nanomaterials (Basel). 2021 11
15. Li Y, Maciel D, Rodrigues J, Shi X, Tomas H. Biodegradable Polymer Nanogels for Drug/Nucleic Acid Delivery. Chem Rev. 2015;115:8564-608
16. Yalamarty SSK, Filipczak N, Li X, Pathrikar TV, Cotter C, Torchilin VP. Co-Delivery of siRNA and Chemotherapeutic Drug Using 2C5 Antibody-Targeted Dendrimer-Based Mixed Micelles for Multidrug Resistant Cancers. Pharmaceutics. 2022 14
17. Majernik M, Jendzelovsky R, Vargova J, Jendzelovska Z, Fedorocko P. Multifunctional Nanoplatforms as a Novel Effective Approach in Photodynamic Therapy and Chemotherapy, to Overcome Multidrug Resistance in Cancer. Pharmaceutics. 2022 14
18. Zhao Z, Liu W. Pancreatic Cancer: A Review of Risk Factors, Diagnosis, and Treatment. Technol Cancer Res Treat. 2020;19:1533033820962117
19. Zhang X, Collins L, Sawyer GJ, Dong X, Qiu Y, Fabre JW. In vivo gene delivery via portal vein and bile duct to individual lobes of the rat liver using a polylysine-based nonviral DNA vector in combination with chloroquine. Hum Gene Ther. 2001;12:2179-90
20. Murugesan SR, King CR, Osborn R, Fairweather WR, O'Reilly EM, Thornton MO. et al. Combination of human tumor necrosis factor-alpha (hTNF-alpha) gene delivery with gemcitabine is effective in models of pancreatic cancer. Cancer Gene Ther. 2009;16:841-7
21. Su B, Cengizeroglu A, Farkasova K, Viola JR, Anton M, Ellwart JW. et al. Systemic TNFalpha gene therapy synergizes with liposomal doxorubicine in the treatment of metastatic cancer. Mol Ther. 2013;21:300-8
22. Huang H, Shao L, Chen Y, Han W, Zhou Y, Liu T. et al. Sequential Dual Delivery System Based on siCOX-2-Loaded Gold Nanostar and Thermal-Sensitive Liposomes Overcome Hypoxia-Mediated Multidrug Resistance in Tumors. Mol Pharm. 2022;19:2390-405
23. Wu HC, Kuo WT. Redox/pH-Responsive 2-in-1 Chimeric Nanoparticles for the Co-Delivery of Doxorubicin and siRNA. Polymers (Basel). 2021 13
24. Jiang J, Yang SJ, Wang JC, Yang LJ, Xu ZZ, Yang T. et al. Sequential treatment of drug-resistant tumors with RGD-modified liposomes containing siRNA or doxorubicin. Eur J Pharm Biopharm. 2010;76:170-8
25. Ganesh S, Iyer AK, Weiler J, Morrissey DV, Amiji MM. Combination of siRNA-directed Gene Silencing With Cisplatin Reverses Drug Resistance in Human Non-small Cell Lung Cancer. Mol Ther Nucleic Acids. 2013;2:e110
26. Yoon HY, Son S, Lee SJ, You DG, Yhee JY, Park JH. et al. Glycol chitosan nanoparticles as specialized cancer therapeutic vehicles: sequential delivery of doxorubicin and Bcl-2 siRNA. Sci Rep. 2014;4:6878
27. Shi L, Feng H, Li Z, Shi J, Jin L, Li J. Co-Delivery of Paclitaxel and siRNA with pH-Responsive Polymeric Micelles for Synergistic Cancer Therapy. J Biomed Nanotechnol. 2021;17:322-9
28. Luo K, Gao Y, Yin S, Yao Y, Yu H, Wang G. et al. Co-delivery of paclitaxel and STAT3 siRNA by a multifunctional nanocomplex for targeted treatment of metastatic breast cancer. Acta Biomater. 2021;134:649-63
29. Alemohammad H, Motafakkerazad R, Asadzadeh Z, Farsad N, Hemmat N, Najafzadeh B. et al. siRNA-mediated silencing of Nanog reduces stemness properties and increases the sensitivity of HepG2 cells to cisplatin. Gene. 2022;821:146333
30. Patel V, Lalani R, Vhora I, Bardoliwala D, Patel A, Ghosh S. et al. Co-delivery of cisplatin and siRNA through hybrid nanocarrier platform for masking resistance to chemotherapy in lung cancer. Drug Deliv Transl Res. 2021;11:2052-71
31. Sun Q, Wang X, Cui C, Li J, Wang Y. Doxorubicin and anti-VEGF siRNA co-delivery via nano-graphene oxide for enhanced cancer therapy in vitro and in vivo. Int J Nanomedicine. 2018;13:3713-28
32. Fan L, Zhang Y, Wang F, Yang Q, Tan J, Grifantini R. et al. Multifunctional all-in-one drug delivery systems for tumor targeting and sequential release of three different anti-tumor drugs. Biomaterials. 2016;76:399-407
33. Yang Y, Meng Y, Ye J, Xia X, Wang H, Li L. et al. Sequential delivery of VEGF siRNA and paclitaxel for PVN destruction, anti-angiogenesis, and tumor cell apoptosis procedurally via a multi-functional polymer micelle. J Control Release. 2018;287:103-20
34. Meng T, Lu B, Shao S, Yuan M, Liu X, Yuan H. et al. Sequential therapy with redox-responsive glucolipid nanocarrier separately delivering siRNA and doxorubicin to overcome multidrug resistance. Int J Pharm. 2017;534:368-77
35. Zhang L, Lu Z, Zhao Q, Huang J, Shen H, Zhang Z. Enhanced chemotherapy efficacy by sequential delivery of siRNA and anticancer drugs using PEI-grafted graphene oxide. Small. 2011;7:460-4
36. Lee JH, Lee K, Moon SH, Lee Y, Park TG, Cheon J. All-in-one target-cell-specific magnetic nanoparticles for simultaneous molecular imaging and siRNA delivery. Angew Chem Int Ed Engl. 2009;48:4174-9
37. Lee E, Oh C, Kim IS, Kwon IC, Kim S. Co-delivery of chemosensitizing siRNA and an anticancer agent via multiple monocomplexation-induced hydrophobic association. J Control Release. 2015;210:105-14
38. Sanyakamdhorn S, Agudelo D, Tajmir-Riahi HA. Review on the targeted conjugation of anticancer drugs doxorubicin and tamoxifen with synthetic polymers for drug delivery. J Biomol Struct Dyn. 2017;35:2497-508
39. Zhang Y, Schwerbrock NM, Rogers AB, Kim WY, Huang L. Codelivery of VEGF siRNA and gemcitabine monophosphate in a single nanoparticle formulation for effective treatment of NSCLC. Mol Ther. 2013;21:1559-69
40. Xiao J, Gong L, Xiao M, He D, Xiang L, Wang Z. et al. LINC00467 Promotes Tumor Progression via Regulation of the NF-kb Signal Axis in Bladder Cancer. Front Oncol. 2021;11:652206
41. Alinejad V, Hossein Somi M, Baradaran B, Akbarzadeh P, Atyabi F, Kazerooni H. et al. Co-delivery of IL17RB siRNA and doxorubicin by chitosan-based nanoparticles for enhanced anticancer efficacy in breast cancer cells. Biomed Pharmacother. 2016;83:229-40
42. Devulapally R, Foygel K, Sekar TV, Willmann JK, Paulmurugan R. Gemcitabine and Antisense-microRNA Co-encapsulated PLGA-PEG Polymer Nanoparticles for Hepatocellular Carcinoma Therapy. ACS Appl Mater Interfaces. 2016;8:33412-22
43. Aung W, Hasegawa S, Koshikawa-Yano M, Obata T, Ikehira H, Furukawa T. et al. Visualization of in vivo electroporation-mediated transgene expression in experimental tumors by optical and magnetic resonance imaging. Gene Ther. 2009;16:830-9
44. Feng Y, Zhou S, Li G, Hu C, Zou W, Zhang H. et al. Nuclear factor-kappaB-dependent microRNA-130a upregulation promotes cervical cancer cell growth by targeting phosphatase and tensin homolog. Arch Biochem Biophys. 2016;598:57-65
45. Wang R, Zhu G, Mei L, Xie Y, Ma H, Ye M. et al. Automated modular synthesis of aptamer-drug conjugates for targeted drug delivery. J Am Chem Soc. 2014;136:2731-4
46. Yang B, Dong X, Lei Q, Zhuo R, Feng J, Zhang X. Host-Guest Interaction-Based Self-Engineering of Nano-Sized Vesicles for Co-Delivery of Genes and Anticancer Drugs. ACS Appl Mater Interfaces. 2015;7:22084-94
47. Zhang Y, Kim WY, Huang L. Systemic delivery of gemcitabine triphosphate via LCP nanoparticles for NSCLC and pancreatic cancer therapy. Biomaterials. 2013;34:3447-58
48. Liu G, Choi KY, Bhirde A, Swierczewska M, Yin J, Lee SW. et al. Sticky nanoparticles: a platform for siRNA delivery by a bis(zinc(II) dipicolylamine)-functionalized, self-assembled nanoconjugate. Angew Chem Int Ed Engl. 2012;51:445-9
49. Zheng M, Yang Z, Chen S, Wu H, Liu Y, Wright A. et al. Bioreducible Zinc(II)-Dipicolylamine Functionalized Hyaluronic Acid Mediates Safe siRNA Delivery and Effective Glioblastoma RNAi Therapy. ACS Appl Bio Mater. 2019;2:362-9
50. Smith BA, Akers WJ, Leevy WM, Lampkins AJ, Xiao S, Wolter W. et al. Optical imaging of mammary and prostate tumors in living animals using a synthetic near infrared zinc(II)-dipicolylamine probe for anionic cell surfaces. J Am Chem Soc. 2010;132:67-9
51. Kanvinde S, Kulkarni T, Deodhar S, Bhattacharya D, Dasgupta A. Non-Viral Vectors for Delivery of Nucleic Acid Therapies for Cancer. BioTech (Basel). 2022 11
52. Avci-Adali M, H AS. Current trends in delivery of non-viral nucleic acid-based therapeutics for improved efficacy. Adv Drug Deliv Rev. 2022;185:114297
53. Zhu C, Jung S, Luo S, Meng F, Zhu X, Park TG. et al. Co-delivery of siRNA and paclitaxel into cancer cells by biodegradable cationic micelles based on PDMAEMA-PCL-PDMAEMA triblock copolymers. Biomaterials. 2010;31:2408-16
54. Zhang L, Yang X, Lv Y, Xin X, Qin C, Han X. et al. Cytosolic co-delivery of miRNA-34a and docetaxel with core-shell nanocarriers via caveolae-mediated pathway for the treatment of metastatic breast cancer. Sci Rep. 2017;7:46186
55. Feng Q, Yu MZ, Wang JC, Hou WJ, Gao LY, Ma XF. et al. Synergistic inhibition of breast cancer by co-delivery of VEGF siRNA and paclitaxel via vapreotide-modified core-shell nanoparticles. Biomaterials. 2014;35:5028-38
56. Jia HZ, Zhang W, Zhu JY, Yang B, Chen S, Chen G. et al. Hyperbranched-hyperbranched polymeric nanoassembly to mediate controllable co-delivery of siRNA and drug for synergistic tumor therapy. J Control Release. 2015;216:9-17
57. Zhao F, Yin H, Li J. Supramolecular self-assembly forming a multifunctional synergistic system for targeted co-delivery of gene and drug. Biomaterials. 2014;35:1050-62
58. Khan OF. Nucleic acid delivery differences across species. Nat Nanotechnol. 2022;17:223-5
59. Chen K, Zhang Y, Zhu L, Chu H, Shao X, Asakiya C. et al. Insights into nucleic acid-based self-assembling nanocarriers for targeted drug delivery and controlled drug release. J Control Release. 2022;341:869-91
60. Saad M, Garbuzenko OB, Minko T. Co-delivery of siRNA and an anticancer drug for treatment of multidrug-resistant cancer. Nanomedicine (Lond). 2008;3:761-76
61. Cheng Q, Du L, Meng L, Han S, Wei T, Wang X. et al. The Promising Nanocarrier for Doxorubicin and siRNA Co-delivery by PDMAEMA-based Amphiphilic Nanomicelles. ACS Appl Mater Interfaces. 2016;8:4347-56
62. Qian X, Long L, Shi Z, Liu C, Qiu M, Sheng J. et al. Star-branched amphiphilic PLA-b-PDMAEMA copolymers for co-delivery of miR-21 inhibitor and doxorubicin to treat glioma. Biomaterials. 2014;35:2322-35
63. Mousazadeh H, Bonabi E, Zarghami N. Stimulus-responsive drug/gene delivery system based on polyethylenimine cyclodextrin nanoparticles for potential cancer therapy. Carbohydr Polym. 2022;276:118747
64. Chen Y, Chen H, Shi J. Inorganic nanoparticle-based drug codelivery nanosystems to overcome the multidrug resistance of cancer cells. Mol Pharm. 2014;11:2495-510
65. Meng H, Liong M, Xia T, Li Z, Ji Z, Zink JI. et al. Engineered design of mesoporous silica nanoparticles to deliver doxorubicin and P-glycoprotein siRNA to overcome drug resistance in a cancer cell line. ACS Nano. 2010;4:4539-50
66. Han L, Tang C, Yin C. Dual-targeting and pH/redox-responsive multi-layered nanocomplexes for smart co-delivery of doxorubicin and siRNA. Biomaterials. 2015;60:42-52
67. Li Y, Xu B, Bai T, Liu W. Co-delivery of doxorubicin and tumor-suppressing p53 gene using a POSS-based star-shaped polymer for cancer therapy. Biomaterials. 2015;55:12-23
68. Wang C, Ravi S, Garapati US, Das M, Howell M, MallelaMallela J. et al. Multifunctional Chitosan Magnetic-Graphene (CMG) Nanoparticles: a Theranostic Platform for Tumor-targeted Co-delivery of Drugs, Genes and MRI Contrast Agents. J Mater Chem B. 2013;1:4396-405
69. Li JM, Wang YY, Zhao MX, Tan CP, Li YQ, Le XY. et al. Multifunctional QD-based co-delivery of siRNA and doxorubicin to HeLa cells for reversal of multidrug resistance and real-time tracking. Biomaterials. 2012;33:2780-90
70. Zheng W, Yin T, Chen Q, Qin X, Huang X, Zhao S. et al. Co-delivery of Se nanoparticles and pooled SiRNAs for overcoming drug resistance mediated by P-glycoprotein and class III beta-tubulin in drug-resistant breast cancers. Acta Biomater. 2016;31:197-210
71. Tian-Meng Sun, Jin-Zhi Du, Yao Y-D, Cheng-Qiong Mao, Shuang Dou, Song-Yin Huang. et al. Simultaneous Delivery of siRNA and Paclitaxel via a "Two-in-One" Micelleplex Promotes Synnergisitic Tumor Suppression ACS Nano. 2011; 5: 1483-94.
72. Zheng C, Zheng M, Gong P, Deng J, Yi H, Zhang P. et al. Polypeptide cationic micelles mediated co-delivery of docetaxel and siRNA for synergistic tumor therapy. Biomaterials. 2013;34:3431-8
73. Yu YH, Kim E, Park DE, Shim G, Lee S, Kim YB. et al. Cationic solid lipid nanoparticles for co-delivery of paclitaxel and siRNA. Eur J Pharm Biopharm. 2012;80:268-73
74. Zhao X, Li F, Li Y, Wang H, Ren H, Chen J. et al. Corrigendum to "Co-delivery of HIF1alpha siRNA and gemcitabine via biocompatible lipid-polymer hybrid nanoparticles for effective treatment of pancreatic cancer" [Biomaterials 46 (2015) 13-25]. Biomaterials. 2022;280:121296
75. Taratula O, Kuzmov A, Shah M, Garbuzenko OB, Minko T. Nanostructured lipid carriers as multifunctional nanomedicine platform for pulmonary co-delivery of anticancer drugs and siRNA. J Control Release. 2013;171:349-57
76. Yin T, Wang L, Yin L, Zhou J, Huo M. Co-delivery of hydrophobic paclitaxel and hydrophilic AURKA specific siRNA by redox-sensitive micelles for effective treatment of breast cancer. Biomaterials. 2015;61:10-25
77. Han K, Chen S, Chen WH, Lei Q, Liu Y, Zhuo RX. et al. Synergistic gene and drug tumor therapy using a chimeric peptide. Biomaterials. 2013;34:4680-9
78. Seo SJ, Lee SY, Choi SJ, Kim HW. Tumor-Targeting Co-Delivery of Drug and Gene from Temperature-Triggered Micelles. Macromol Biosci. 2015;15:1198-204
79. Deng ZJ, Morton SW, Ben-Akiva E, Dreaden EC, Shopsowitz KE, Hammond PT. Layer-by-layer nanoparticles for systemic codelivery of an anticancer drug and siRNA for potential triple-negative breast cancer treatment. ACS Nano. 2013;7:9571-84
80. Tang S, Meng Q, Sun H, Su J, Yin Q, Zhang Z. et al. Tumor-Microenvironment-Adaptive Nanoparticles Codeliver Paclitaxel and siRNA to Inhibit Growth and Lung Metastasis of Breast Cancer. Advanced Functional Materials. 2016;26:6033-46
81. Cui C, Yu P, Wu M, Zhang Y, Liu L, Wu B. et al. Reduction-sensitive micelles with sheddable PEG shells self-assembled from a Y-shaped amphiphilic polymer for intracellular doxorubicine release. Colloids Surf B Biointerfaces. 2015;129:137-45
82. Chen C, Shen M, Liao H, Guo Q, Fu H, Yu J. et al. A paclitaxel and microRNA-124 coloaded stepped cleavable nanosystem against triple negative breast cancer. J Nanobiotechnology. 2021;19:55
83. Ochs CJ, Such GK, Yan Y, van Koeverden MP, Caruso F. Biodegradable click capsules with engineered drug-loaded multilayers. ACS Nano. 2010;4:1653-63
84. Yan Y, Ochs CJ, Such GK, Heath JK, Nice EC, Caruso F. Bypassing multidrug resistance in cancer cells with biodegradable polymer capsules. Adv Mater. 2010;22:5398-403
85. Becker AL, IvanOrlotti, N.; Folini M, Cavalieri F, Zelikin AN, Johnston APR. et al. Redox-Active Polymer Microcapsules for the Delivery of a Survivin-Specific siRNA in Prostate Cancer Cells. ACS Nano. 2011;5:1335-44
86. Ding J, Xiao C, He C, Li M, Li D, Zhuang X. et al. Facile preparation of a cationic poly(amino acid) vesicle for potential drug and gene co-delivery. 2011; 22: 494012-20.
87. Patil YB, Swaminathan SK, Sadhukha T, Ma L, Panyam J. The use of nanoparticle-mediated targeted gene silencing and drug delivery to overcome tumor drug resistance. Biomaterials. 2010;31:358-65
88. Chen A-Z, Zhao C, Wang S-B, Liu Y-G, Lin D-L. Generation of porous poly-l-lactide microspheres by emulsion-combined precipitation with a compressed CO2 antisolvent process. Journal of Materials Chemistry B. 2013;1:2967
89. Chen AZ, Kang YQ, Wang SB, Tang N, Su XQ. Preparation and antitumor effect evaluation of composite microparticles co-loaded with siRNA and paclitaxel by a supercritical process. Journal of Materials Chemistry B. 2015;3:6439-47
90. Deng X, Cao M, Zhang J, Hu K, Yin Z, Zhou Z. et al. Hyaluronic acid-chitosan nanoparticles for co-delivery of MiR-34a and doxorubicin in therapy against triple negative breast cancer. Biomaterials. 2014;35:4333-44
91. Han Y, Zhang P, Chen Y, Sun J, Kong F. Co-delivery of plasmid DNA and doxorubicin by solid lipid nanoparticles for lung cancer therapy. Int J Mol Med. 2014;34:191-6
92. He C, Lu K, Liu D, Lin W. Nanoscale metal-organic frameworks for the co-delivery of cisplatin and pooled siRNAs to enhance therapeutic efficacy in drug-resistant ovarian cancer cells. J Am Chem Soc. 2014;136:5181-4
93. Tang Q, Cao B, Cheng G. Co-delivery of small interfering RNA using a camptothecin prodrug as the carrier. Chem Commun (Camb). 2014;50:1323-5
94. Chang RS, Suh MS, Kim S, Shim G, Lee S, Han SS. et al. Cationic drug-derived nanoparticles for multifunctional delivery of anticancer siRNA. Biomaterials. 2011;32:9785-95
95. Li C-c, Du Y, Lv H, Zhang J, Zhuang P, Yang W. et al. Injectable Amphipathic Artesunate Prodrug-Hydrogel Microsphere as Gene/Drug Nano-Microplex for Rheumatoid Arthritis Therapy. Advanced Functional Materials. 2022 32
96. Karimi M, Ghasemi A, Sahandi Zangabad P, Rahighi R, Moosavi Basri SM, Mirshekari H. et al. Smart micro/nanoparticles in stimulus-responsive drug/gene delivery systems. Chemical Society reviews. 2016;45:1457-501
97. Wang F, Shen Y, Zhang W, Li M, Wang Y, Zhou D. et al. Efficient, dual-stimuli responsive cytosolic gene delivery using a RGD modified disulfide-linked polyethylenimine functionalized gold nanorod. Journal of controlled release: official journal of the Controlled Release Society. 2014;196:37-51
98. Giger EV, Castagner B, Raikkonen J, Monkkonen J, Leroux JC. siRNA transfection with calcium phosphate nanoparticles stabilized with PEGylated chelators. Adv Healthc Mater. 2013;2:134-44
99. Nam K, Nam HY, Kim PH, Kim SW. Paclitaxel-conjugated PEG and arginine-grafted bioreducible poly (disulfide amine) micelles for co-delivery of drug and gene. Biomaterials. 2012;33:8122-30
100. Qiu C, Wei W, Sun J, Zhang HT, Ding JS, Wang JC. et al. Systemic delivery of siRNA by hyaluronan-functionalized calcium phosphate nanoparticles for tumor-targeted therapy. Nanoscale. 2016;8:13033-44
101. Chen M, Zhang Y, Chen Z, Xie S, Luo X, Li X. Synergistic antitumor efficacy of redox and pH dually responsive micelleplexes for co-delivery of camptothecin and genes. Acta Biomater. 2016
102. Dong Y, Zhu Y, Li J, Zhou QH, Wu C, Oupicky D. Synthesis of bisethylnorspermine lipid prodrug as gene delivery vector targeting polyamine metabolism in breast cancer. Mol Pharm. 2012;9:1654-64
103. Hongmei Liu, Chenmeng Qiao, Jun Yang, Weng J, Zhang X. Self-assembling doxorubicin-prodrug nanoparticles as siRNA drug delivery system for cancer treatment: in vitro and in vivo. J Mater Chem B. 2014;2:5910-24
104. Kaneshiro TL, Lu ZR. Targeted intracellular codelivery of chemotherapeutics and nucleic acid with a well-defined dendrimer-based nanoglobular carrier. Biomaterials. 2009;30:5660-6
105. Li M, Li Y, Huang X, Lu X. Captopril-polyethyleneimine conjugate modified gold nanoparticles for co-delivery of drug and gene in anti-angiogenesis breast cancer therapy. J Biomater Sci Polym Ed. 2015;26:813-27
106. Liu C, Liu F, Feng L, Li M, Zhang J, Zhang N. The targeted co-delivery of DNA and doxorubicin to tumor cells via multifunctional PEI-PEG based nanoparticles. Biomaterials. 2013;34:2547-64
107. Liu T, Xue W, Ke B, Xie MQ, Ma D. Star-shaped cyclodextrin-poly(l-lysine) derivative co-delivering docetaxel and MMP-9 siRNA plasmid in cancer therapy. Biomaterials. 2014;35:3865-72
108. Mittal A, Chitkara D, Behrman SW, Mahato RI. Efficacy of gemcitabine conjugated and miRNA-205 complexed micelles for treatment of advanced pancreatic cancer. Biomaterials. 2014;35:7077-87
109. Ouyang Q, Duan Z, Jiao G, Lei J. A Biomimic Reconstituted High-Density-Lipoprotein-Based Drug and p53 Gene Co-delivery System for Effective Antiangiogenesis Therapy of Bladder Cancer. Nanoscale Res Lett. 2015;10:965
110. Ren Y, Kang CS, Yuan XB, Zhou X, Xu P, Han L. et al. Co-delivery of as-miR-21 and 5-FU by poly(amidoamine) dendrimer attenuates human glioma cell growth in vitro. J Biomater Sci Polym Ed. 2010;21:303-14
111. Xu M, Qian J, Suo A, Liu T, Liu X, Wang H. A reduction-dissociable PEG-b-PGAH-b-PEI triblock copolymer as a vehicle for targeted co-delivery of doxorubicin and P-gp siRNA. Polym Chem. 2015;6:2445-56
112. Wang D, Wang T, Liu J, Yu H, Jiao S, Feng B. et al. Acid-Activatable Versatile Micelleplexes for PD-L1 Blockade-Enhanced Cancer Photodynamic Immunotherapy. Nano Letters. 2016;16:5503-13
113. Sun K, Wang J, Zhang J, Hua M, Liu C, Chen T. Dextran-g-PEI nanoparticles as a carrier for co-delivery of adriamycin and plasmid into osteosarcoma cells. Int J Biol Macromol. 2011;49:173-80
114. Han L, Huang R, Li J, Liu S, Huang S, Jiang C. Plasmid pORF-hTRAIL and doxorubicin co-delivery targeting to tumor using peptide-conjugated polyamidoamine dendrimer. Biomaterials. 2011;32:1242-52
115. Lesheng Teng, Jing Xie, Teng L, Lee RJ. Enhanced siRNA Delivery Using Oleic Acid Derivative of Polyethylenimine. Anticancer Research. 2012;32:1267-72
116. Malmo J, Sorgard H, Varum KM, Strand SP. siRNA delivery with chitosan nanoparticles: Molecular properties favoring efficient gene silencing. J Control Release. 2012;158:261-8
117. Zhang BF, Xing L, Cui PF, Wang FZ, Xie RL, Zhang JL. et al. Mitochondria apoptosis pathway synergistically activated by hierarchical targeted nanoparticles co-delivering siRNA and lonidamine. Biomaterials. 2015;61:178-89
118. Kim JH, Kim SH, Alfieri A, Young CW, Silvestrini B. Lonidamine: a hyperthermic sensitizer of HeLa cells in culture and of the Meth-A tumor in vivo. Oncology. 1984;41(Suppl 1):30-5
119. Hu QD, Fan H, Ping Y, Liang WQ, Tang GP, Li J. Cationic supramolecular nanoparticles for co-delivery of gene and anticancer drug. Chem Commun (Camb). 2011;47:5572-4
120. Ma D, Lin QM, Zhang LM, Liang YY, Xue W. A star-shaped porphyrin-arginine functionalized poly(L-lysine) copolymer for photo-enhanced drug and gene co-delivery. Biomaterials. 2014;35:4357-67
121. Kanazawa T, Morisaki K, Suzuki S, Takashima Y. Prolongation of life in rats with malignant glioma by intranasal siRNA/drug codelivery to the brain with cell-penetrating peptide-modified micelles. Mol Pharm. 2014;11:1471-8
122. Ino K, Shibata K, Kajiyama H, Nawa A, Nomura S, Kikkawa F. Manipulating the angiotensin system-new approaches to the treatment of solid tumours. Expert Opin Biol Ther. 2006;6:243-55
123. Ding X, Wang W, Wang Y, Bao X, Wang Y, Wang C. et al. Versatile reticular polyethylenimine derivative-mediated targeted drug and gene codelivery for tumor therapy. Mol Pharm. 2014;11:3307-21
124. Zhao Y, Wang W, Guo S, Wang Y, Miao L, Xiong Y. et al. PolyMetformin combines carrier and anticancer activities for in vivo siRNA delivery. Nat Commun. 2016;7:11822
125. Huang P, Wang D, Su Y, Huang W, Zhou Y, Cui D. et al. Combination of small molecule prodrug and nanodrug delivery: amphiphilic drug-drug conjugate for cancer therapy. J Am Chem Soc. 2014;136:11748-56
126. Tang H, Murphy CJ, Zhang B, Shen Y, Sui M, Van Kirk EA. et al. Amphiphilic curcumin conjugate-forming nanoparticles as anticancer prodrug and drug carriers: in vitro and in vivo effects. Nanomedicine (Lond). 2010;5:855-65
127. Li Y, Liu R, Yang J, Ma G, Zhang Z, Zhang X. Dual sensitive and temporally controlled camptothecin prodrug liposomes codelivery of siRNA for high efficiency tumor therapy. Biomaterials. 2014;35:9731-45
128. Xiaoyang Xua, Kun Xiec, Xue-Qing Zhanga, Eric M. Pridgend, Ga Young Parke, Danica S. Cuia, et al. Enhancing tumor cell response to chemotherapy through nanoparticle-mediated codelivery of siRNA and cisplatin prodrug. PNAS. 2013;11:18638-43
129. Zhang HT, Sun J, Yan Y, Cui SH, Wang H, Wang CH. et al. Encapsulated microRNA by gemcitabine prodrug for cancer treatment. J Control Release. 2019;316:317-30
130. Zhao J, Mi Y, Feng SS. Targeted co-delivery of docetaxel and siPlk1 by herceptin-conjugated vitamin E TPGS based immunomicelles. Biomaterials. 2013;34:3411-21
131. Qian Zhang, Qiao Jiang, Na Li, Dai L, Qing Liu, Linlin Song. et al. DNA Origami as an In Vivo Drug Delivery Vehicle for Cancer Therapy. ACS Nano. 2014;8:6633-43
132. Ichihara M, Murakumo Y, Masuda A, Matsuura T, Asai N, Jijiwa M. et al. Thermodynamic instability of siRNA duplex is a prerequisite for dependable prediction of siRNA activities. Nucleic Acids Res. 2007;35:e123
133. Park K, Yang JA, Lee MY, Lee H, Hahn SK. Reducible hyaluronic acid-siRNA conjugate for target specific gene silencing. Bioconjug Chem. 2013;24:1201-9
134. Sugo T, Terada M, Oikawa T, Miyata K, Nishimura S, Kenjo E. et al. Development of antibody-siRNA conjugate targeted to cardiac and skeletal muscles. J Control Release. 2016;237:1-13
135. Takemoto H, Miyata K, Hattori S, Ishii T, Suma T, Uchida S. et al. Acidic pH-responsive siRNA conjugate for reversible carrier stability and accelerated endosomal escape with reduced IFNalpha-associated immune response. Angew Chem Int Ed Engl. 2013;52:6218-21
136. Zhu QL, Zhou Y, Guan M, Zhou XF, Yang SD, Liu Y. et al. Low-density lipoprotein-coupled N-succinyl chitosan nanoparticles co-delivering siRNA and doxorubicin for hepatocyte-targeted therapy. Biomaterials. 2014;35:5965-76
137. Liu H, Li Y, Mozhi A, Zhang L, Liu Y, Xu X. et al. SiRNA-phospholipid conjugates for gene and drug delivery in cancer treatment. Biomaterials. 2014;35:6519-33
138. Dai B, Hu Y, Duan J, Yang XD. Aptamer-guided DNA tetrahedron as a novel targeted drug delivery system for MUC1-expressing breast cancer cells in vitro. Oncotarget. 2016;7:38257-69
139. Xing H, Wong NY, Xiang Y, Lu Y. DNA aptamer functionalized nanomaterials for intracellular analysis, cancer cell imaging and drug delivery. Curr Opin Chem Biol. 2012;16:429-35
140. Zhang P, Wang C, Zhao J, Xiao A, Shen Q, Li L. et al. Near Infrared-Guided Smart Nanocarriers for MicroRNA-Controlled Release of Doxorubicin/siRNA with Intracellular ATP as Fuel. ACS Nano. 2016;10:3637-47
141. Pérez-Arnaiz C, Busto N, Leal JM, B. G. New insights into the mechanism of the DNA/doxorubicin interaction. J Phys Chem B. 2014;118:1288-95
142. Jeong H, Lee SH, Hwang Y, Yoo H, Jung H, Kim SH. et al. Multivalent Aptamer-RNA Conjugates for Simple and Efficient Delivery of Doxorubicin/siRNA into Multidrug-Resistant Cells. Macromol Biosci. 2017 17
143. Pinheiro AV, Han D, Shih WM, Yan H. Challenges and Opportunities for Structural DNA Nanotechnology. Nat Nanotechnol. 2011;6:763-72
144. Wang D, Wang T, Liu J, Yu H, Jiao S, Feng B. et al. Acid-Activatable Versatile Micelleplexes for PD-L1 Blockade-Enhanced Cancer Photodynamic Immunotherapy. Nano Lett. 2016;16:5503-13
Author contact
![]() Corresponding authors: Fei Xia, Ph.D, Tel/Fax: 86-10-62554449, Email: fxiaac.cn; Jigang Wang, Ph.D, Tel/Fax: 86-10-62554449, Email: jgwangac.cn; Chaocheng Xu, Ph.D, Tel/Fax: 86-10-62554449, Email: ccxuac.cn
Corresponding authors: Fei Xia, Ph.D, Tel/Fax: 86-10-62554449, Email: fxiaac.cn; Jigang Wang, Ph.D, Tel/Fax: 86-10-62554449, Email: jgwangac.cn; Chaocheng Xu, Ph.D, Tel/Fax: 86-10-62554449, Email: ccxuac.cn

 Global reach, higher impact
Global reach, higher impact