Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2023; 19(3):811-828. doi:10.7150/ijbs.79928 This issue Cite
Review
Novel insight into metabolic reprogrammming in cancer radioresistance: A promising therapeutic target in radiotherapy
Department of Ophthalmology, Shanghai Key Laboratory of Orbital Diseases and Ocular Oncology, Shanghai Ninth People's Hospital, Shanghai JiaoTong University School of Medicine, Shanghai, P.R. China.
# These authors contributed equally to this report.
Received 2022-10-16; Accepted 2022-12-9; Published 2023-1-1
Abstract
Currently, cancer treatment mainly consists of surgery, radiotherapy, chemotherapy, immunotherapy, and molecular targeted therapy, of which radiotherapy is one of the major pillars. However, the occurrence of radioresistance largely limits its therapeutic effect. Metabolic reprogramming is an important hallmark in cancer progression and treatment resistance. In radiotherapy, DNA breakage is the major mechanism of cell damage, and in turn, cancer cells are prone to increase the metabolic flux of glucose, glutamine, serine, arginine, fatty acids etc., thus providing sufficient substrates and energy for DNA damage repair. Therefore, studying the linkage between metabolic reprogramming and cancer radioresistance may provide new ideas for improving the efficacy of tumor therapy. This review mainly focuses on the role of metabolic alterations, including glucose, amino acid, lipid, nucleotide and other ion metabolism, in radioresistance, and proposes possible therapeutic targets to improve the efficacy of cancer radiotherapy.
Keywords: Cancer, Radioresistance, Metabolic Reprogrammming, Therapeutic Targets
Background
Cancer is currently a leading cause of death worldwide, killing millions of people every year [1]. As one of the most important treatments for cancer, radiotherapy kills cancer cells mainly through reactive oxygen species (ROS) production and DNA damage [2]. However, tumor cells can develop radioresistance through multiple mechanisms including DNA damage repair, antioxidation, cell cycle arrest, accumulation of cancer stem cells (CSCs), and immunosuppressive microenvironment formation [2-4], leading to cancer recurrence and metastasis after radiotherapy. The resistance of cancer cells to radiotherapy is a huge obstacle to the effective control of cancer development.
Metabolic reprogramming, the adaptive alteration of metabolic pathways in tumor cells, is considered one of the hallmarks of malignancy [5]. The exploration of tumor metabolism dates back to the “Warburg effect” proposed by German biochemist Dr. Warburg in the 1920s [6], which indicated the importance of glycolysis in cancer cells and pioneered the study of tumor metabolism [6, 7]. Afterward, scientists gradually discovered other metabolic reprogramming pathways in cancer cells, such as nucleotide metabolism [5]. More than 70 years ago, American pediatric pathologist Sidney Farber discovered that the folic acid antagonist aminopterin could induce complete remission in children with acute lymphoblastic leukemia (ALL) [8]. This landmark research provided a foundation for cancer chemotherapy based on inhibiting the biosynthesis of nucleotides [8, 9]. Today, many antimetabolites, such as 5-fluorouracil/pemetrexed and methotrexate, have been approved and used in cancer treatment [9]. In particular, the approval of ivosidenib and enasidenib, which target to mutant isocitrate dehydrogenase (IDH) in acute myeloid leukemia (AML), has become another milestone in precision tumor therapy that targets aberrant metabolism [10]. Metabolic reprogramming can be induced by aberrant activation of oncogenes, signaling pathways and epigenetic modifications, influencing the progression of cancer cells [5, 11, 12]. Previous studies have discovered that MYC, AKT, ERK are associated with enhanced glycolysis and nucleotide synthesis in cancer cells [5, 11, 13, 14]. Histone lactylation, elevated in tumors, is associated with poor prognosis of ocular melanoma as well [15]. Apart from tumor cells, noncancer cells in the TME including endothelial cells, fibroblasts, and immune cells are also involved in cancer development through metabolic reprogramming [16-18]. For instance, activation of p38α-MAPK in cancer-associated fibroblasts mobilizes glycogen in tumor cells and promotes its metastasis [18]. Furthermore, metabolic reprogramming is also linked to chemo- and immune-therapy resistance. Van Gastel N et al. found that after chemotherapy, AML cells developed chemoresistance by activating glutamine metabolism to promote pyrimidine synthesis [19]. Effector Treg cells take up lactate through MCT1 to enhance PD-1 expression and remodel its function and phenotype, which may be one of the reasons for resistance to PD-1 immunotherapy [16].
In radiotherapy DNA breakage is the main mechanism of radiation-induced cell damage, in turn, cancer cells upregulate the metabolic flux of glucose, amino acids, fatty acids etc., providing sufficient substrates and energy for DNA damage repair. Similarly, in response to radiation-induced ROS, tumor cells develop a set of antioxidant strategies. For example, radiation-induced hypoxia stimulates serine metabolism through HIF-1/2 to increase NAPDH/GSH levels, enhancing tumor antioxidant capacity [20]. By integrating of machine learning and experimental validation, Joshua E. Lewis et al. demonstrated that many of the metabolites involving glucose metabolites (e.g., fructose 1,6-bisphosphate, 3-phosphoglyceric acid, succinyl-CoA, succinate), lipids and fatty acid metabolites were all positively correlated with radioresistance [21], suggesting that metabolic reprogramming plays a vital role in radiation resistance. Therefore, targeting tumor metabolism may provide a promising therapeutic strategy for radiosensitization. In this review, we elaborate on the role of several basic metabolic alterations (e g. glucose metabolism, amino acid metabolism, lipid metabolism and nucleotide metabolism) in tumor radioresistance, and propose therapeutic prospects for targeting metabolic reprogramming in radiotherapy.
Glucose metabolism and cancer radioresistance
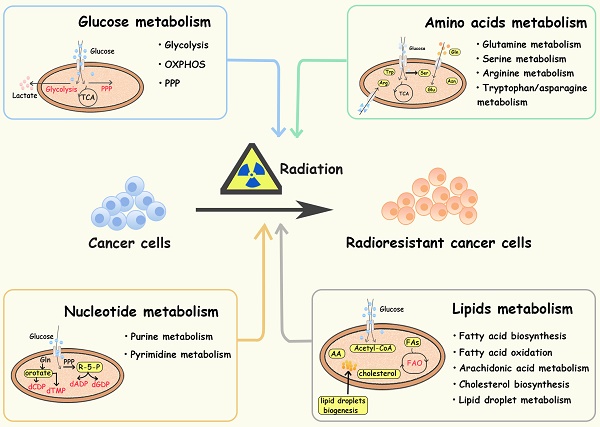
Glucose provides energy and a carbon skeleton for biosynthesis. Cancer cells have elevated glucose uptake, which can be detected by FDG-PET (fluorodeoxyglucose-positron tomography) and used for cancer identification and treatment assessment [22, 23]. Active aerobic glycolysis is one of the hallmarks of metabolic reprogramming in several cancers, contributing to treatment resistance and recurrence [5, 24, 25]. In addition to glycolysis, the pentose phosphate pathway (PPP), and oxidative phosphorylation pathways (OXPHOS) are also reprogrammed to protect tumor cells from radiation (Figure 1).
Aerobic glycolysis
Aerobic glycolysis is a crucial alteration in cancer glucose metabolism reprogramming, and is characterized by an increased glucose uptake rate and high lactic acid production. Tumor cells take up glucose through glucose transporters (GLUTs) and convert it to glucose-6-phosphate(G-6-P) via hexokinase 2 (HK2). Then, G-6-P is converted to pyruvate through a series of enzymatic reactions, in which the M2 isoform of pyruvate kinase (PKM2) plays a key role. Finally, lactic acid is generated by lactate dehydrogenase (LDHA). Lactate and pyruvate are transported by monocarboxylate transporters (MCTs) and mitochondrial pyruvate carriers (MPCs) in the cell membrane and mitochondrial membrane. Glycolysis plays an important role in tumor initiation and progression. For instance, Liu Y et al. found that tumor cells regulate glycolytic metabolism through FTO-mediated m6A modification to promote the activation and function of T cells, protecting tumor cells from immune surveillance [26]. Recently, scientists have been seeking the precise treatment of cancer by inhibiting the activity of key enzymes in the glycolysis pathway [9].
Recent studies have indicated a positive correlation between glycolysis and radioresistance in multiple malignant tumors including pancreatic cancer, prostate cancer and laryngeal carcinoma [23, 25, 27]. GLUT1 overexpression is associated with radioresistance and poor prognosis in patients with oral and head and neck squamous cell carcinomas [28]. Upregulation of GULT1 expression was also observed in radioresistant tumor cells, which was related to oncogene activation, hypoxia stimulation, and regulation of signaling pathways such as PI3K/AKT [25]. Radiotherapy combined with GLUT1 inhibition sensitized tumor cells [29-31]. For example, in subcutaneous glioma cells treated with radiotherapy, the GLUT1 inhibitor apigenin reduced cell stemness and DNA damage repair by inhibiting the NF-κB/hif-1α/GLUT1 pathway, facilitating radiosensitivity [30]. WZB117, a specific inhibitor of GLUT1 sensitized radioresistant breast cancer cells to irradiation [31]. Moreover, in cervical cancer cells, treatment with HK2 inhibitor 2-DG delayed the proliferation of radioresistant cells and sensitized them both in vitro and in vivo [32]. 3-bromopyruvate, a HK2 inhibitor, improved the sensitivity of pancreatic cancer cells to irradiation by reduce nucleotide biosynthesis and enhance DNA damage [33].
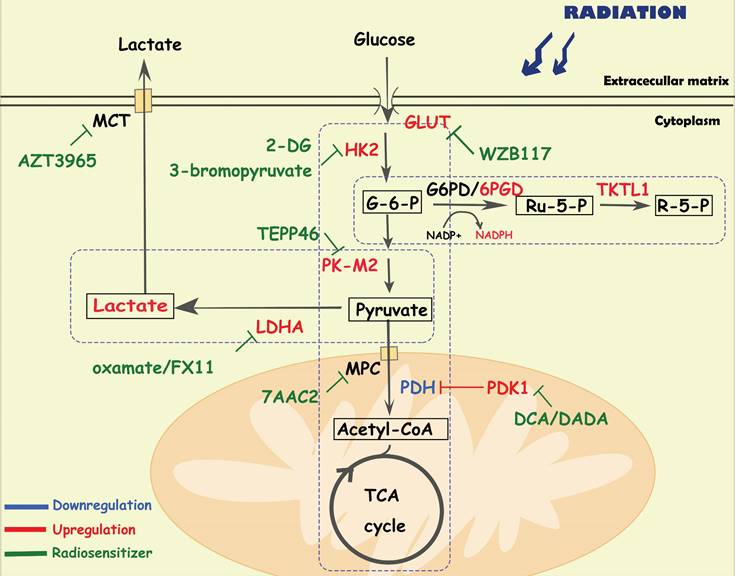
Diagram of glucose-related metabolic pathways involved in cancer radioresistance and its radiosensitizers. In response to radiation, cancer cells increase glucose flux via upregulating GLUT and HK2, facilitating the glucose metabolism including glycolysis, OXPHOS and PPP. In activated aerobic glycolysis, PK-M2/ LDHA/lactate are the major up-regulated metabolites and metabolic enzymes. In OXPHOS, metabolic enzymes (e g., PDH/MPC) and the integrity of mitochondrial function also play an important role in cancer radioresistance. In PPP, overexpression of 6PGD/ TKTL1 enhance the production of NAPDH and nucleotides.
PKM2, the main isoform expressed in tumor cells [34], is responsible for converting phosphoenolpyruvate into pyruvate. Wu S et al. found that the phosphorylation level of PKM2 s222 is related to the radioresistance and prognosis of glioblastoma [35]. Mechanistically, stimulated by radiation-induced DNA damage, PKM2 is phosphorylated at serine(s)222 and interacts with the FACT complex and histone chaperone, facilitating γH2AX-mediated chromatin loading, which promotes DNA damage repair and cell survival of tumor cells [35]. Silencing the expression of PKM2 inhibited the phosphorylation of AKT and PDK1, sensitizing non-small cell lung cancer cell lines and xenografts to radiotherapy by enhancing apoptosis and autophagy [36]. Combining PK-M2 activators (TEPP486) with radiation to enhance the effect of radiotherapy in resistant cancers, such as TNBC [37].
Elevated LDHA expression is also associated with poor prognosis of patients with multiple cancers (e g. head and neck squamous cell carcinoma, prostate cancer, non-small cell lung cancer) and has a positive correlation with radioresistance [38-41]. Indeed, LDHA5 overexpression suggests a hypoxic state in cancer cells, which is associated with cancer recurrence, metastasis and radioresistance [38, 39]. Treatment with LDHA inhibitors (e g, oxamate [41]) can overcome radioresistance by enhancing radiation-induced DNA damage and apoptosis. The long noncoding RNA LINC00518, an oncogene in cutaneous malignant melanoma, also induced radioresistance via a miR-33a-3p/ HIF-1α/LDHA negative feedback loop [42].
Lactic acid acts not only as a metabolic fuel for tumor tissues, but also a signaling molecule associated with the immunosuppressive microenvironment, which contributes to cancer proliferation, invasion, metastasis and radioresistance [43-45]. Rather than affecting tumor cells, lactate tends to influence the metabolic phenotype of nontumor cells in the TME, mediating immune escape [46]. Yang X et al. found that radiation-upregulated lactate enhanced the immunosuppressive activity of myeloid-derived suppressor cells (MDSCs), which inhibited antitumor T-cell activity and accumulation in the TME, accelerating the progression of pancreatic cancer [46]. Blocking lactate secretion or knocking out Hif-1α in MDSCs could reverse these responses and effectively inhibit cell progression after radiotherapy [46]. In addition, MCT and MPC, the major transporters of lactate, also play important roles in radioresistance. The combination of the MCT1 inhibitor AZD3965 and radiotherapy showed a greater therapeutic effect in small cell lung cancer xenografts than radiotherapy alone, suggesting that MCTs may be a potential target for radiosensitization [47]. Interfering with the expression of MPC1 also contributed to cancer epithelial-mesenchymal transition and radioresistance [48]. In cervical cancer xenograft models, 7AAC2, a novel MPC1 inhibitor, combined with radiation therapy significantly decreased tumor growth compared with 7AAC2 or radiation therapy alone [49].
Oxidative phosphorylation
Under physiological conditions, pyruvate in mitochondria is converted into acetyl-CoA and then enters the tricarboxylic acid cycle to produce ATP through OXPHOS, which satisfies more than 95% of cell energy demands. Indeed, tumor cells can adapt to altered metabolic environments by switching between glycolysis and OXPHOS. Increased OXPHOS and NADH/NAD+ metabolism mediated by mitochondrial membrane fusion contributes to tumor immortalization [50]. Age-related mitochondrial OXPHOS deficiency promotes intestinal cancer cell growth and survival by upregulating the serine de novo synthesis pathway (SSP) [51]. Inhibition of OXPHOS with gboxin restricts glioblastoma growth by inhibiting the activity of ATP synthase [52].
Pyruvate dehydrogenase (PDH) is responsible for the conversion of pyruvate to acetyl-CoA. Pyruvate dehydrogenase kinases (PDKs) inhibit the activity of PDH, which is a key enzyme regulating the switch between oxidative phosphorylation and glycolysis in cancer cells. Previous studies have indicated that PDK1 and phospho-pyruvate dehydrogenase (p-PDH) are positively associated with radioresistance [53-55]. In complex I-deficient mitochondrial cells, there was an increase in the expression of p-PDH, accompanied by radioresistance by reducing histone acetylation and promoting DNA damage repair [53]. Dichloroacetate (DCA), a specific inhibitor of PDK, can not only downregulate p-PDH levels, extracellular acidification rates and lactate production, but also significantly increase ROS levels in triple-negative breast cancer cells, leading to radiosensitization [54]. Moreover, diisopropylamine dichloroacetate (DADA), an inhibitor of PDK, enhanced radiosensitization in esophageal squamous cell carcinoma by increasing mitochondria-derived ROS levels [56].
Pentose phosphate pathway
In the pentose phosphate pathway (PPP), nicotinamide adenine dinucleotide phosphate (NADPH) and ribose 5-phosphate (R-5-P) are the main metabolites, the former can protect cells from ROS damage, and the latter is the essential material for nucleotide synthesis. The PPP can be divided into two parts: the oxidative portion is controlled by the key enzymes glucose-6-phosphate dehydrogenase (G6PD) and 6PGD, which are responsible for the production of NAPDH; and the non-redox portion is controlled by transketolase-like protein 1 (TKTL1), which is mainly responsible for the production of ribose. Studies have demonstrated upregulation of the PPP in several cancer types. Lin R et al. found that suppression of 6PGD decreased lipogenesis, and RNA biosynthesis and elevated ROS levels in cancer cells, which attenuated cell proliferation and tumor growth, suggesting that 6PGD could be an anticancer target [57]. Increased flux of the PPP was also confirmed in radioresistant head and neck squamous cell carcinoma cells [58]. The EGFR signaling pathway mediates the phosphorylation and activation of 6PGD, which promotes the metabolic flux of the PPP, and DNA synthesis and repair, facilitating tumor growth and radioresistance [59]. Moreover, suppression of TKTL1 increased the levels of ROS and sensitized tumor cells to ionizing radiation, indicating that TKTL2 may be a potential therapeutic target [60].
Amino acid metabolism and cancer radioresistance
Essential amino acids (histidine, lysine, methionine, phenylalanine, threonine, tryptophan, and branched-chain amino acids) cannot be produced by the de novo pathway in the human body. Cancer cells capture amino acids via transporters for biomacromolecules and energy production. Amino acid metabolism reprogramming significantly affects tumor cells and their immune microenvironment. In a leukemia stem cell (LSC) population, Jones CL et al. demonstrated the upregulation of the nutrient uptake rate, steady-state levels, and catabolism [61]. LSCs isolated from AML patients are uniquely reliant on amino acid metabolism for survival, which can be reversed by pharmacological inhibition of amino acid metabolism [61]. In addition, tumor cells are dependent on an exogenous supply of amino acids, suggesting that limiting the availability of these nutrients can selectively kill tumor cells.
Glutamine metabolism
Glutamine (Gln) is the most essential nutrient for intracellular energy production, redox homeostasis and biosynthesis in cells [62]. Gln is transported into cells by transporters (e.g. ASCT2/SLC1A5/SLC7A5/LAT1) and converted to glutamate (Glu) by mitochondrial glutaminase (GLS1/GLS2) [63]. Glu in mitochondria is then converted to α-ketoglutarate, an intermediate product of TCA, by glutamate dehydrogenase (GDH) or transaminase (TA). Additionally, in the cytoplasm, Glu can also be used to synthesize nucleotides, simultaneously producing glutathione (GSH) to maintain redox homeostasis and avoid oxidative stress [62]. Cells can also produce Gln from NH4 and Glu via the catalysis of glutamine synthetase (GS), participating in the transport of ammonium ions [63, 64]. Active glutamine metabolism provides sufficient carbon and nitrogen sources to promote tumor growth and proliferation [65]. Rather than glucose, cell-intrinsic programs drive the preferential acquisition of glutamine by cancer cells [66]. Additionally, multiple cancer cells (including breast cancer, pancreatic CSCs and GBM) received radioresistance in response to glutamine deprivation, suggesting a significant combination between glutamine metabolism and radioresistance [64, 67-70].
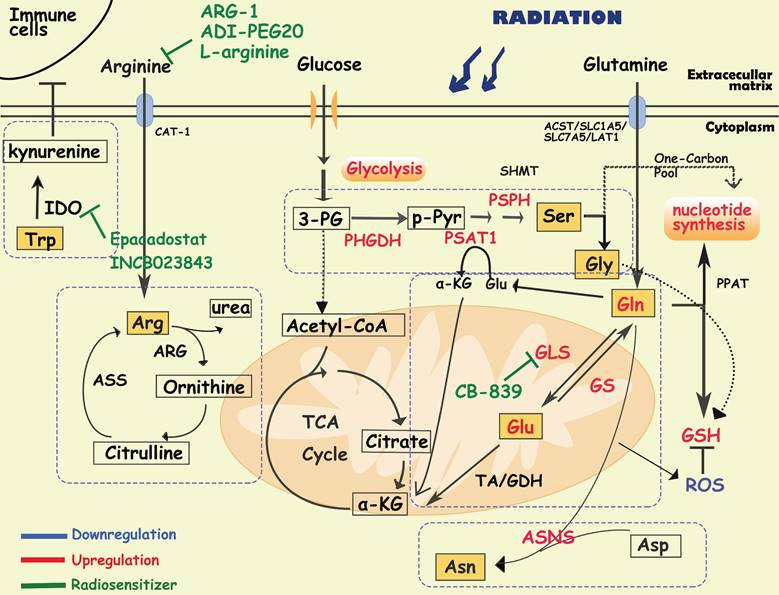
Diagram of amino acids metabolism involved in cancer radioresistance and its radiosensitizers. In response to radiotherapy, tumor cells enhance the metabolism of amino acids such as glutamate, serine, and asparagine, which provides the biomacromolecules and other materials required for nucleotides, GSH and energy production, prolonging the cancer cell survival. Tumor cells regulate glutamine metabolism by self-balancing the activities of GLS and GS, promoting cell resistance to radiation. Abnormally activated glycolysis also indirectly allows tumor cells to enhance serine synthesis, increasing one-carbon metabolic flux. In addition, amino acids, such as arginine, asparagine and tryptophan, also play important roles in the formatino of cancer radioresistance, which have been proved to be the effective targets for radiosensitization.
It has been reported that radiation increases the activity of GLS in multiple cancer cells such as head and neck squamous cell carcinoma, lung cancer, prostate cancer and cervical cancers, contributing to radioresistance [71-74]. In radioresistant cervical cancer tissues, GLS2 was overexpressed, and silencing GLS2 could reverse this radioresistant phenotype by decreasing GSH and NADH levels [73]. High requirements for glutamine are found in radioresistant prostate cancer (PCa) cells and CSCs [74]. Overexpression of GLS and MYC improved GSH production and maintained the redox state, which was significantly associated with a reduction in progression-free survival in PCa patients who received radiotherapy [74]. Glutamine catabolism regulates the epigenetic reset of chromatin-modifying dioxygenases by α-KG as well, contributing to the maintenance of CSCs [74]. Interestingly, activation of ATG5-mediated autophagy in the absence of glutamine is another tumor survival strategy to defend against radiation-mediated cellular damage [74]. In addition, Binkley MS et al. reported that GLS inhibition preferentially radiosensitizes KEAP1-mutant non-small cell lung cancer cells via depletion of GSH and enhanced DNA damage [75]. GLS inhibition also specifically sensitized IDH-mutant glioma cells to oxidative stress and radiation in vitro and in vivo [76]. Telaglenastat (CB-839), a specific GLS inhibitor, induced radiosensitization by enhancing oxidative stress in cervical cancer and head, neck squamous cell carcinoma and breast cancer [70, 71, 77]. Currently, the GLS1 inhibitor CB-839 is under phase 1 clinical evaluation in patients with advanced solid tumors (e.g., NCT02071862, NCT03875313, and NCT02861300), showing encouraging treatment effects, and further clinical trials are needed to establish its utility as a radiosensitizer [70, 76].
Several studies have indicated that high expression of GS contributes to radioresistance of cancer cells, such as lung cancer, pancreatic cancer and glioblastoma cells [64, 77-79]. High GS activity is associated with radioresistance in cancer cells through the regulation of nucleotide metabolism, G2/M recovery and DNA repair [79]. Interestingly, a recent study suggested that in addition to participating in glutamine metabolism, GS can directly interact with the nuclear pore protein NUP88, activating the anaphase-promoting complex (APC/CCDC20) to ensure the process of cell division and promote the proliferation of cancer cells, indicating a new function of GS [80]. It is worth mentioning that cancer cells can simultaneously overexpress GS and GLS sometimes, which may create an ineffective cycle. Therefore, how tumor cells dynamically regulate the expression of GS and GLS to maintain the growth of malignant tumor cells is an important issue in tumor metabolism.
Serine/glycine metabolism
Serine/glycine metabolism provides one-carbon units for the synthesis of proteins, lipids, and nucleic acids, which are essential for tumor growth and homeostasis. Cancer cells take up serine/glycine mainly from the extracellular microenvironment, as well as from de novo synthesis. In serine/glycine metabolism, tumor cells synthesize and utilize serine/glycine by a phosphoglycerate dehydrogenase (PHGDH)/phosphoserine aminotransferase 1 (PSAT1)/phosphoserine phosphatase (PSPH)/hydroxymethyltransferase (SHMT)-mediated continuous enzymatic reaction. To our knowledge, serine dependent-single carbon metabolism is closely related to nucleotide synthesis and is crucial to the proliferation of tumor cells [81]. Serine metabolism supports macrophage produce IL-1β, which contributes to the immunosuppression in breast cancer [82, 83]. Suppressing serine metabolism enhances the polarization of interferon-γ-activated macrophages (M(IFN-γ)) but suppresses that of interleukin-4-activated macrophages (M(IL-4)) by regulating the IGF1-p38 axis [84]. Recent evidence suggests that serine/glycine metabolism is not only an important driver of macrophage function [82-84] and revascularization [85, 86], but also contributes to the maintenance of CSCs in the TME. Silencing of PHGDH leads to downregulation of transcriptional regulators involved in self-renewal and pluripotency (e g. Oct4, Nanog, Sox2, Bmi1), reducing the formation of embryonic and breast CSCs [87].
Activation of serine/glycine metabolism could be a strategy for radioresistant cells to survive and recover during radiotherapy. After radiotherapy, the metabolome of B16 melanoma cells and the breast cancer cell line HCC1937 showed an increased level of glycine [88, 89]. Similarly, increased circulating levels of serine, leucine, and isoleucine in serum were observed in breast cancer patients who received local radiotherapy after tumor resection [90]. In resistant head and neck squamous cell carcinoma, activation of de novo serine/glycine biosynthesis has been observed [91]. Previous studies found that serine starvation combined with metformin and benzformin inhibited mitochondrial OXPHOS by targeting ETC complex I, facilitating radioresistance[92-94].
Hypoxia is one of the predisposing factors of radioresistance. It has been reported that under hypoxic conditions, the HIF-1/HIF-2 and PERK-eIF2α-ATF4 signaling pathways are activated, which induces upregulation of SHMT2/PHGDH/PSAT1/PSPH levels, increasing de novo serine/glycine biosynthesis [20, 95-97]. As glycine is one of the substrates for GSH synthesis, increased serine/glycine metabolism resulted in the accumulation of NAPDH/GSH, promoting the elimination of ROS. Serine/glycine metabolism is also associated with DNA stability. In neuroendocrine prostate tumors, loss of tumor suppressor protein kinase C (PKC) λ/ι enhanced serine metabolism through mTORC1/ATF4/PHGDH axis, which leads to aberrant DNA methylation by increased one-carbon metabolic flux, ultimately leading to treatment resistance and poor prognosis [98]. Wilson JL et al. found that PHGDH, as a metabolic checkpoint of M2 macrophages, induced the polarization of macrophages from M1 to M2, which are associated with radioresistance and relapse [99-101].
Arginine metabolism
Arginine is synthesized from citrulline by argininosuccinate synthase 1 (ASS1) and argininosuccinatelyase (ASL), and converted to ornithine and urea by arginase (ARG). ASS1 deficiency is common in many cancers, reported as arginine auxotrophic tumors, in which cancer cells are unable to synthesize endogenous arginine, resulting in an increased dependence on extracellular arginine [102, 103]. Arginine deiminase (ADI) and ARG are responsible for depleting extracellular arginine, resulting in antitumor activity. Therefore, enzymotherapeutic arginine deprivation therapy (ADT) has become a new metabolic strategy for cancers, and has already been proven to effectively inhibit the growth of cancers in both preclinical studies and clinical phase I-III trials [103-105].
Arginine metabolism is also associated with radiotherapy efficacy. In mice, overexpression of ARG1 in myeloid cells limits the control of residual disease after radiotherapy of tumors [106]. MDSCs have been reported to deplete L-arginine in the TME by overexpressing ARG1, which promotes the apoptosis of antitumor T cells and macrophages [107]. According to the metabolic characteristics of arginine in cancer cells, researchers invented D-arginine-loaded metal-organic framework nanoparticles, which can be used to sensitize osteosarcoma to radiotherapy [108]. Previous studies have shown that ADT can enhance radiosensitivity in certain tumors, even in ASS-positive colorectal cancer cells [109-111]. For example, in glioblastoma cells, ADT combined with radiotherapy improved the survival of tumor cells, especially in TP53-mutated and-knockdown cells [103]. Vynnytska-Myronovska B et al. found that arginine withdrawal alone or in combination with the arginine natural analog canavanine is a promising antitumor strategy with the potential to enhance cancer cure by radiotherapy [110]. Recently, another opposing strategy for radiosensitization in solid tumors was reported. Rossella Marullo et al. demonstrated that administration of oral L-arginine induced radiosensitization and local control in patients who underwent standard radiation therapy [112], which is associated with increased DNA damage via nitric oxide (NO)-mediated metabolic suppression [112]. It is worth mentioning that NO plays a dual role in tumor cells. An appropriate amount of NO can promote tumor growth; however, either too low or too high a level of NO will limit the proliferation of tumor cells. Since arginine is a precursor to NO [112, 113], it may explain the contradiction between ADT and oral L-arginine.
Other amino acid metabolism
Tryptophan metabolism
Tryptophan (Trp) is an essential amino acid ingested from the diet, and 95% of free Trp is catabolized through the kynurenine pathway and is involved in the regulation of immunity, neuronal function, and intestinal homeostasis. The imbalances of Trp metabolism in cancer have drawn great interest in targeting the kynurenine pathway for therapy, especially the rate-limiting enzymes indoleamine-2,3-dioxygenase 1 (IDO1), IDO2, tryptophan-2,3-dioxygenase (TDO) and kynurenine monooxygenase (KMO) [114, 115]. It has been found that IL4I1, a metabolic enzyme produced by tumors, breaks down tryptophan to activate aryl hydrocarbon receptors, enhancing tumor aggressiveness and suppressing antitumor immunity [116]. In addition, it has been reported that in UM-SCC-74A cells, a radiosensitive uveal melanoma cell line, the most pronounced metabolic change after irradiation is related to tryptophan metabolism [91]. IDO1 inhibitors, Epacadostat and INCB023843, are proved to be potential radiosensitivity agents [117, 118]. Recently, researchers have developed a nanomaterial delivery tool “coordination polymer-coated CaCO3” targeting IDO, which can reinforce radiotherapy by reprogramming the immunosuppressive metabolic microenvironment [119].
Asparagine metabolism
Mammalian cells synthesize asparagine from aspartate and glutamine by asparagine synthase (ASNS). Currently, the first antitumor drug that directly targets aspartate metabolism is L-asparaginase (L-ASPase) from Escherichia coli and Erwinia europaea, which has been approved by the FDA for the clinical treatment of acute lymphoblastic leukemia [120]. Asparagine can increase the synthesis of purine and pyrimidine by activating mTORC1 signaling when mitochondrial respiration is impaired, and promote tumor cell proliferation [121]. Elevated ASNS expression brought about antioxidative stress and rapid transformation between glycolysis and oxidative phosphorylation, leading to enhanced proliferation and radioresistance in tumor cells [122].
Lipid metabolism and cancer radioresistance
Lipids are not only important building blocks of biological membranes, but are also used for energy storage, biosynthesis and signaling pathways. Dysregulation of lipid metabolism, especially fatty acid (FA) metabolism, is an important metabolic phenotype alteration in cancer cells [123] and exhibits flexibility and vulnerability [124]. Lipid metabolism reprogramming influences cancer cell growth and migration, neovascularization, evasion of immune surveillance, and treatment resistance. For instance, Lim SA et al. demonstrated that inhibiting lipid synthesis in Treg cells unleashes effective antitumor immune responses without autoimmune toxicity, pointing to new mechanism of targeting lipid metabolism [125].
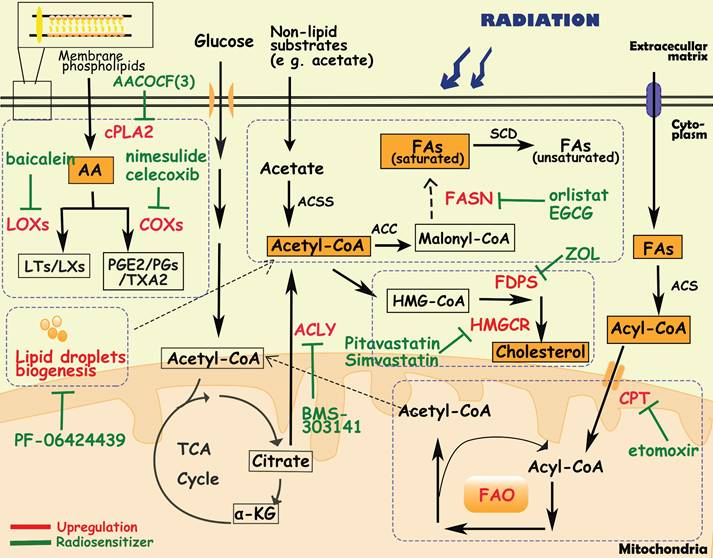
Diagram of lipids metabolism involved in cancer radioresistance and its radiosensitizers. Cancer cells develop radioresisitance via reprogramming lipids metabolism including fatty acids, cholesterol, arachidonic acids and lipid droplets. In lipids metabolism, acetyl-CoA is an important bridge connecting different metabolisms. In fatty acids metabolism, tumor cells upregulate the expression of FASN/CPT/ACLY to facilitate the synthesis and oxidation of FAs. In arachidonic acids metabolism, inhibition of metabolic enzymes (e g. cPLA2, LOXs, COXs) is proven to sensitize tumor cells to radiotherapy. Additionally, lipid droplets and cholesterol metabolism are positively correlated with radioresistance as well.
Fatty acid biosynthesis
Fatty acid synthesis in normal tissues is mainly restricted to hepatocytes and adipocytes. However, even in the presence of an exogenous lipid source, cancer cells activate lipogenesis in response to high metabolic demands, which is one of the characteristics of cancer cells and normal cells [124, 126]. The fatty acid metabolism of tumor cells involves glucose metabolism as well. The main substrate for FA synthesis is acetyl-CoA, which is synthesized from extracellular nonlipid substrates (e g. acetate) or citrate by ATP-citrate lyase (ACLY) and acetyl-CoA synthetase 2 (ACSS2). Afterward, acetyl-CoA is converted to FAs through a series of enzymatic reactions, in which acetyl-CoA carboxylase (ACC), fatty acid synthase (FASN) and stearoyl-CoA desaturase (SCD) are the main rate-limiting enzymes [127]. Previous studies have reported that overexpression of ACLY/ACSS2/ACC/FASN/SCD promotes tumor cell proliferation, invasion and metastasis in various types of cancer [128-131]. Moreover, in liver cancer cells and lung cancer cells with SCD suppression, cancer cells can secrete an uncommon unsaturated fatty acid, sapienate, through FADS2, which is mostly secreted by sebaceous glands in normal tissues [132].
In radioresistant tumor cells, FA synthesis is increased as well, producing more plasma membrane phospholipids and signaling molecules [128]. Göttgens EL et al. revealed that high expression of ACLY was associated with poor overall survival in HNSCC patients who received radiotherapy [127], and inhibition ACLY expression caused an impairment of DNA damage repair, leading to radiosensitization in HNSCC cell lines [127]. Moreover, elevated expression and activity of FASN have been observed in radioresistant nasopharyngeal and pancreatic carcinoma cells, and were positively correlated with poor prognosis of patients [133, 134]. In prostate cancer cells, overexpression of FASN upregulated androgen receptors via the Akt/NF-κB pathway, leading to cell cycle arrest and DNA repair enhancement, ultimately inducing radioresistance [135, 136]. Upregulation of FASN also induced radioresistance in nasopharyngeal carcinoma cells by frizzled class receptor 10[137]. Additionally, FASN inhibitors, such as EGCG (epigallocatechin gallate) and orlistat, were used as radiosensitizers to improve the efficacy of radiotherapy [135-137].
Fatty acid oxidation
In addition to providing essential phospholipids and signaling molecules for tumor growth, FAs also enter the mitochondria for β-oxidation, known as fatty acid oxidation (FAO). First, FAs are converted to acyl-CoA by the acyl-CoA synthase family (ACS). Then acyl-CoA is transported into mitochondria through carnitine palmitoyltransferase (CPT) on the mitochondrial membrane. Under the catalysis of the FAO system, acyl-CoA undergoes four consecutive reactions with dehydrogenation, water addition, re-dehydrogenation and thiolysis, and finally generates acetyl-CoA [138, 139]. Increased FAO activity has been observed in multiple cancers, such as lung cancer, triple negative breast cancer and glioma, to meet energy demands [140]. Enhanced FAO metabolism is required for the differentiation and activation of tumor-associated macrophages [141]. Duman C et al. demonstrated that acyl-CoA-binding protein drives glioblastoma tumorigenesis by sustaining fatty acid oxidation [139]. The nuclear receptor Nur77, which is essential for melanoma cells to adapt to the metabolic stress of glucose starvation, promotes FAO by protecting TPβ from oxidative inactivation, thereby maintaining ROS levels in cells to facilitate cell survival [142].
Active FAO metabolism and overexpression of FAO-related enzymes are also observed in radioresistant tumor cells [123]. In radioresistant breast cancer cells, CD47 expression is regulated by FAO metabolites, enabling tumor cells to escape phagocytosis by macrophages [143]. The combination of FAO inhibitors ((e.g., the CPT1 inhibitor etomoxir) and anti-CD47 antibodies improved tumor control in a mouse model of GBM that relapsed after radiotherapy [143]. Furthermore, during radiotherapy, lipids released by dead tumor cells can provide a survival advantage for CSCs, promoting the accumulation of CSCs in radioresistant tumors through FAO-mediated self-renewal and metastasis [144]. Long-chain acyl-CoA synthase (ACSL), a member of the ACS family, has been reported to mediate radioresistance by regulating FOXM1 to enhance DNA damage repair and inhibit apoptosis [145]. Tan Z et al. found that FAO was active in radiation-resistant nasopharyngeal carcinoma cells, and its rate-limiting enzyme CPT1A expression was also upregulated in these cells, which was significantly associated with overall survival after radiotherapy in nasopharyngeal carcinoma patients [146]. Mechanistically, CPT1A promotes fatty acid transportation by interacting with rab14, reducing radiation-induced lipid accumulation, and inducing radioresistance [146]. Another study also reported that the PGC1α/CEBPB/CPT1A axis promotes radiation resistance in nasopharyngeal carcinoma by activating FAO [147]. In radioresistant breast cancer cells and radioresistant breast CSCs, blocking FAO via an FAO inhibitor or by CRISPR-mediated CPT1A/CPT2 gene deficiency inhibited radiation-induced ERK activation and aggressive growth and radioresistance [148].
Arachidonic acid metabolism
Tumor cells utilize phospholipids to synthesize arachidonic acid (AA) through cytosolic phospholipase A2 (cPLA2), and then AA is converted to biologically active substances (e g. leukotrienes, prostacyclins, prostaglandins, thromboxanes) through lipoxygenases (LOXs) and cyclooxygenases (COXs), which contribute to the human cardiovascular system and immune system. In recent years, it has been found that arachidonic acid metabolic pathways also play an important role in tumors [149]. For instance, Liao P et al. found that CD8+ T-cell derived interferon (IFNγ) combined with AA induced tumor ferroptosis [150]. PIK3CA mutations drive multiple signaling networks involving mTORC2-PKCζ-mediated activation of cPLA2, and cPLA2 inhibition and dietary fat restriction restore tumor immune cell infiltration and inhibit breast tumor growth [149]. In addition, aspirin, a nonsteroidal anti-inflammatory drug targeting COXs, has been shown to anti-tumor effect [151, 152]. Professor John Burn's team at Newcastle University, UK, found that in high-risk groups of bowel cancer patients carrying hereditary mutations, taking aspirin for more than 2 years could reduce the incidence of bowel cancer by 50%, and this protective effect can last for up to 20 years [153].
By analysis of metabolic biomarkers from the multiomics classifier for radiation response, Joshua E. Lewis et al. found increased production of membrane phospholipids and arachidonic acid precursors, resulting in significant correlations between inflammation-mediating eicosanoids and radioresistance [21]. In GBM, prostaglandin E2 (PGE2), a major metabolite produced from AA metabolism, upregulated Id1 through the MAPK signaling pathway, inducing radioresistance [154]. Overexpression of cPLA2, LOXs and COXs also contributes to the radioresistance of tumors [154-156]. It has been reported that overexpression of 15-LOX-1 in colorectal cancer cells exposed to radiation induces radiation resistance through downregulation of MacroH2A2 [157]. Additionally, 12-LOX inhibitors (baicalein or bmd122) have been demonstrated to sensitize prostate cancer cells to radiation [158]. COX-2 inhibitors have shown radiosensitization effects in multiple cancers including renal cell carcinoma, breast cancer, cervical cancer, and non-small cell lung cancer [159-162]. A phase I clinical trial of patients with recurrent head and neck cancer demonstrated that the combination of COX-2 inhibitors (erlotinib, celecoxib) and radiotherapy can improve progression-free survival (37%) and overall survival (55%) [163].
Cholesterol biosynthesis
Cholesterol, an important component of the membrane structure, is also used as a precursor to synthesize other substances such as bile acids, steroid hormones, vitamins, and oxidized cholesterol. Cholesterol metabolism in tumor cells is characterized by upregulation of cholesterol synthesis and uptake, and enrichment of various cholesterol derivatives [162]. Microarray analysis revealed that many genes involved in cholesterol synthesis (e.g., FDPS, ACAT2, AG2, SLC12A2) were consistently associated with radioresistance in cell lines [164]. In cholesterol biosynthesis pathways, 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR) is the rate-limiting enzyme in in the conversion of HMG-CoA to mevalonate, whose expression is upregulated in gastric cancer, glioblastoma and prostate cancer. Pitavastatin (Livalo), an HMGCR inhibitor, when combined with radiotherapy, increased persistent DNA double-strand breaks (DSBs), induced senescence, and enhanced the acute effects of radiation on radioresistant melanoma tumors [165]. Seshacharyulu P et al. reported that farnesyl pyrophosphate synthase (FDPS), a branching enzyme of the cholesterol synthesis pathway, was overexpressed in pancreatic ductal adenocarcinoma tissues and was associated with poor radiotherapy response and survival [164, 166]. Inhibition of FDPS by zoledronic acid (Zol) exerted radiosensitization by affecting Rac1 and Rho prenylation, thereby modulating DNA damage and radiation response along with improved systemic immune cell activation [166].
Lipid droplet metabolism
Lipid droplets (LDs) are one of the major intracellular sites for the storage of triglycerides and cholesterol. When glucose is insufficient, cells use lipid hydrolases or the autophagy pathway to breakdown lipids in LDs and then transport FAs into mitochondria to provide energy through fatty acid oxidation [167]. Compared with normal cells, tumor cells continuously synthesize and take up large amounts of lipids, and store excess lipids in LDs to provide energy in a "starved" state [167-169]. According to some reports, large numbers of gene mutations and the unique microenvironment of tumors endow many metabolic enzymes (e g. phosphoenolpyruvate carboxykinase 1, and choline kinase α2) with protein kinase activity, and promote the formation and degradation of LDs. Additionally, the metabolic processes of LDs are also associated with radiosensitivity [167, 168]. It has been reported that inhibiting diacylglycerol acyltransferase 2 (DGAT2), an enzyme in the LD biogenesis process, by PF-06424439 reduced LD content, inhibited cell migration, and sensitized MCF7 breast cancer cells to radiation [170]. In addition, LD content is closely related to iron metabolism, especially ferritin heavy chain (FTH1). In breast and lung cancer cells, silencing the FTH1 gene downregulated the amounts of LDs and induced radioresistance, and FTH1 overexpression as well as iron-chelating treatment by dseferoxamine were able to restore these effects [171].
Nucleotide metabolism and cancer radioresistance
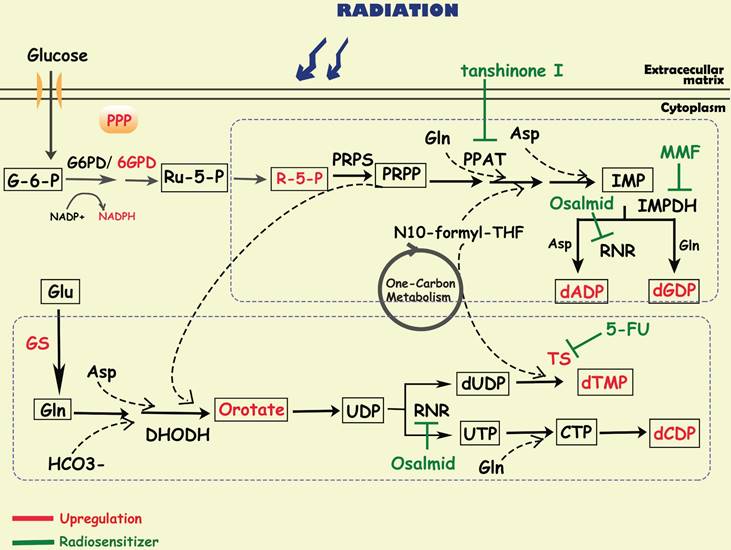
To satisfy the demands of rapid proliferation, tumor cells require large amounts of nucleotides to produce DNA and RNA. Nucleotides consist of purines and pyrimidines, both of which can be synthesized through two pathways: de novo synthesis and the salvage pathway. Although salvage pathways can recycle free purines and pyrimidines for nucleotide biosynthesis, most proliferating cells, especially cancer cells, still synthesize nucleotides via the de novo pathway. Substrates for de novo nucleotide synthesis come from ribose sugars produced by the PPP as well as amino acids and their derivatives, in which one-carbon metabolism is critical. In the de novo synthesis of purines, carbon and nitrogen atoms from glycine, glutamine, aspartic acid, and 10-CHO-THF are sequentially added to ribose 5-phosphate (R-5-P), of which phosphoribosyl pyrophosphate amidotransferase (PPAT), inosine monophosphate dehydrogenase (IMPDH), and ribonucleotide reductase (RNR) are the key rate-limiting enzymes [9]. The de novo pyrimidine pathway utilizes substrates such as glutamine, carbon dioxide, and aspartic acid to synthesize orotate, and then reacts with PPRP to generate UDP, which is further converted to CDP and dTMP by RNR and thymidylate synthase (TS) [9]. At present, most of the metabolism-related anticancer drugs in the clinic mainly focus on nucleotide metabolism, especially to interrupt DNA synthesis [172]. For example, methotrexate, a dihydrofolate reductase (DHFR) inhibitor, is widely used in tumor chemotherapy; and pemetrexed is used for the treatment of non-small cell lung cancer targeting TS and AICAR convertase.
Since radioresistant cancer cells require robust DNA repair capacity to compensate for radiation-induced DNA damage, enhanced nucleotide metabolism is needed in these cells. It has been reported that in glioma, efficient de novo nucleotide synthesis promotes the maintenance of tumor-initiating cells, which contributes to treatment resistance and tumor recurrence [173, 174]. Fu S et al. found that glutamine synthetase (GS), regulated by STAT5, was specifically overexpressed in radiation-resistant nasopharyngeal carcinoma and glioma cells, negatively correlating with treatment outcome [64]. The activation of GS increased the level of glutamine, which enhanced the metabolic flux of nucleotides and accelerated DNA repair via homologous recombination (HR), finally leading to radioresistance [64]. In addition, MUC1, an oncogene overexpressed in multiple solid tumors, was reported to reconnect the metabolic network to the pentose phosphate pathway, mediating the activation of nucleotide production and contributing to radiation resistance in pancreatic cancer cells [33]. After radiotherapy, these MUC1-expressing cells treated with 3-bromopyruvate, a glycolysis inhibitor, can abrogate the levels of metabolites in glycolysis, PPP and nucleotide biosynthesis pathways, resulting in radiosensitization by preventing DNA damage repair [33].
Diagram of nucleotide metabolism involved in cancer radioresistance and its radiosensitizers. DNA damage repair is one of the major mechanisms by which tumor cells develop radioresistance, requiring the accumulation of a large amount of nucleotides. To promote tumor cell survival during radiotherapy, activated glucose metabolism (e g. glycolysis, PPP) and amino acid metabolism (e g. glutamate, asparagine, serine) provide sufficient substrates and energy to accelerate purine and pyrimidine synthesis. Simultaneously, related enzymes in the de novo nucleotide synthesis pathway (e g. TS/PPAT/IMPDH) have been shown to be targets for radiosensitization.
Metabolism associated targets in radioresistance and the radiosensitization methods.
| Items | Targets | Radiosensitizer | Cancer cells | Function | Ref. |
|---|---|---|---|---|---|
| Glycose metabolism | GLUT1 | WZB117 | Breast cancer | - | [31] |
| HK2 | 2-DG | Cervical cancer | Increase ROS level | [32] | |
| 3-bromopyruvate | Pancreatic cancer | Reduce nucleotide biosynthesis and enhance DNA damage | [33] | ||
| PKM2 | TEPP46 | Triple negative breast cancers | Deplete breast cancer stem cells | [37] | |
| LDHA | Oxamate | Non-small cell lung cancer | Induced ROS accumulation and cellular ATP depletion, increase DNA injury | [41] | |
| MCT1 | AZD3965 | Small cell lung cancer | - | [47] | |
| MPC1 | 7AAC2 | Cervix cancer, Pharynx squamous cell carcinoma, Breast cancer | Reduce hypoxia | [49] | |
| PDK | Dichloroacetate (DCA) | Triple negative breast cancers | Increase ROS production | [54] | |
| Diisopropylamine dichloroacetate (DADA) | Esophageal squamous cell carcinoma | Increase intracellular levels of ROS | [56] | ||
| Amino acid metabolism | GLS | Terlaglenastat (CB-839) | Cervical cancers | Decreased total glutathione | [70] |
| Head and neck squamous cell carcinoma | Increased oxidative stress and DNA damage | [71] | |||
| Lung cancer | Reduce GSH | [72, 75] | |||
| Arginine | ARG-1 | Glioblastoma | - | [109] | |
| Epithelial cancer | - | [110] | |||
| ADI-PEG20 | Pancreatic cancer | Promote apoptosis | [111] | ||
| L-arginine | Brain metastases from solid tumors | Reduce glycolysis and impact ATP and NAD levels | [112] | ||
| IDO-1 | Epacadostat | Colorectal cancer | Reduce production of kynurenine; potentiated Th1 cytokines and myeloid cell-modulating factors in TME | [118] | |
| INCB023843 | Lung cancer | Reducing numbers of IDO1-expressing MDSCs in TME | [117] | ||
| Lipid metabolism | ACLY | BMS303141 | Head and neck squamous cell carcinoma | Inhibit DNA damage repair | [127] |
| FASN | Orlistat | prostate cancer | Decrease NF-κB activity | [135] | |
| Epigallocatechin gallate (EGCG) | Nasopharyngeal carcinoma | - | [137] | ||
| CPT-1 | Etomoxir | Glioblastoma multiforme | Boost macrophage phagocytosis | [143] | |
| Breast cancer | - | [148] | |||
| Nasopharyngeal carcinoma | - | [146] | |||
| cPLA2 | AACOCF(3) | Ovarian cancer | Inhibit activation of pro-survival Akt signaling and enhance cell death | [156] | |
| 12-LOX | Baicalein | Prostate cancer | - | [158] | |
| COX-2 | Nimesulide | Lung cancer | Increased apoptosis | [193] | |
| Celecoxib | Lung cancer | Regulate radiation-induced G2-M arrest | [194] | ||
| Esophageal squamous cell carcinoma | - | [161] | |||
| Celecoxib- Afatinib combination | Non-small cell lung cancer | Regulate cell sensitivity to apoptosis | [195] | ||
| HMG-CoA | Pitavastatin (Livalo) | Melanoma tumors | Delay DNA repair, enhance acute effects of radiation | [165] | |
| Simvastatin | Prostate cancer | Compromise DNA double-strand break repair | [196] | ||
| FDPS | Zoledronic acid (Zol) | Pancreatic ductal adenocarcinoma | Attenuate DNA damage repair and immunosuppressive signaling | [166] | |
| DGAT2 | PF-06424439 | Breast cancer | Reduce LD content | [170] | |
| Nucleotide metabolism | IMPDH1 | MMF | glioblastoma | Inhibit GTP synthesis | [175] |
| PPAT | Tanshinone I | lung cancer | Well-docked into the active pocket of the structure of PPAT | [177] | |
| TS | 5-FU | Colorectal cancer | - | [180] | |
| RNR | Osalmid | Esophageal cancer | Enhance DNA damage, apoptosis, and senescence | [184] | |
Purine metabolism
By analyzing the metabolomic information of GBM, researchers have discovered that purine metabolites, especially guanylate, are strongly associated with radioresistance [175]. Further study showed that purine metabolites induced radioresistance mainly by promoting the repair of radio-induced DNA double-stranded breaks (DSBs) [175]. Inhibiting GTP synthesis with MMF, an IMPDH1 inhibitor, could sensitize radioresistant GBM cells in vitro and in vivo [175, 176]. Moreover, it is reported that tanshinone I, a PPAT inhibitor, significantly improved the radiation sensitivity of radioresistant lung cancer cells [177]. These findings indicate that inhibiting de novo purine synthesis may be a promising strategy to overcome therapy resistance.
Pyrimidine metabolism
In glioblastoma, upregulation of proliferating cell nuclear antigen (PCNA)-associated factor (PAF) promoted the biosynthesis of pyrimidine metabolites, especially orotate, which maintained glioma stem cells self-renewal and is associated with radioresistance [178]. 5-Fluorouracil (5-FU), an analog of pyrimidine nucleoside, could enhance the radiosensitivity of colorectal cancer cells by inhibiting the activity of TS [179, 180]. And researchers developed multiple nanoparticles as carrier of 5-FU, which could enhance radio-sensitivity in cancer cells [181, 182]. Turnbull T et al. found that nanoparticle-induced impairment enhanced DNA damage by down-regulating repair proteins such as TS [183]. In addition, osalmid, a novel identified RNR inhibitor, have been proved to enhance radiosensitivity of esophageal cancer [184].
Others
In addition to glucose, lipid, amino acid and nucleotide metabolisms reprogramming, the metabolism of metal ions such as copper, iron and calcium are also involved in the regulation of malignant tumor progression and treatment resistance. Ferroptosis, a recently discovered form of cell death driven by iron-dependent lipid peroxidation, plays an important role in tumor radiotherapy. Recent evidence suggests that the synergistic effect of radiotherapy and immunotherapy is associated with increased susceptibility to ferroptosis. Combined radiotherapy and immunotherapy down-regulate SLC7A11, which is mediated by the DNA damage-activated kinases ATM and IFN-γ, resulting in decreased cystine uptake, increased ferroptosis, and limited tumor growth [182]. Cancer cells upregulate the expression of SLC7A11 and GPX4 to protect cells from radiation-induced ferroptosis, contributing to radioresistance [185].
Recently, the team of Todd R. Golub from the Broad Institute of Harvard and MIT discovered a new type of cell death, copper death, which has attracted great attention [186]. Further research revealed that FDX1 is a key regulator of copper death and an upstream regulator of protein fatty acylation [186]. Due to the abundance of FDX1 and fatty acylated proteins, which are highly correlated with a variety of human tumors, copper ionophores may be potential therapeutics against cancer cells with such metabolic signatures. Furthermore, Yang M et al. found that intracellular copper accumulation could promote radiotherapy resistance in multiple cancers including hepatic carcinoma, nasopharyngeal carcinoma and colorectal cancer [187]. Mechanistically, the accumulation of intracellular copper ions induced by the downregulation of COMMD10 can inhibit the ubiquitin-mediated degradation of HIF-1α, subsequently increasing the transcription and translation of ceruloplasmin, which forms a positive feedback regulation loop of HIF-1α/CP to inhibit ferroptosis, thereby promoting the radiotherapy resistance of liver cancer cells [187].
Discussion
It is worth noting that the development of radioresistance is not only associated with tumor cells, but also involves various cells in the tumor microenvironment (e g, immune cells, fibroblasts, endothelial cells, etc.). Rao E et al. reported that all-trans retinoic acid can induce inflammatory macrophages, which induce effector T-cell infiltration and enhance the effector T-cell to regulatory T-cell ratio, overcoming the radioresistance of solid tumors [188]. Compared with radiosensitive nasopharyngeal carcinoma tissues, radioresistant cancer cells had more infiltration of cancer-associated fibroblasts, which reduced radiation-induced DNA damage through the IL-8/NF-κB pathway and promoted tumor cells to acquire radiation resistance [189]. The protein kinase CK2 is involved in IR-induced cytokine production by endothelial cells, which ultimately leads to radiation resistance in non-small cell lung cancer cells [190]. Moreover, Yang X et al. found that radiation-induced lactate enhanced the immunosuppressive phenotype of pancreatic cancer myeloid-derived suppressor cells (MDSCs), and lactate blockade restored antitumor T-cell responses and effectively inhibited tumor progression after radiotherapy [46]. MDSCs with high expression of arginase-1 have been reported to deplete arginine to inhibit NO synthesis in macrophages, developing radioresistance [107]. These results suggest a significant connection between metabolic reprogramming and radioresistance in the TME. However, the specific molecular mechanism of tumor cell radioresistance of metabolites (e g. lactate, inflammatory factors, etc.) produced by cells in the TME remains unclear. Therefore, future studies on radioresistance can focus on the metabolic link between the tumor and TME.
With a deeper understanding of the complexity of tumor metabolism, it is not difficult to realize that different metabolic pathways weave into a large metabolic network, in which they are interconnected and regulated by each other, synergistically promoting cancer progression. For instance, Yuan Fang et.al. demonstrated that radioresistant liver cancer cells are highly addictive to glucose [191]. However, these glucose molecules did not enter the conventional glycolytic pathway but promoted the synthesis of cardiolipin, which inhibited the release of radiation-induced cytochrome C, blocked the initiation of apoptosis, and ultimately enhanced radiation resistance. This study reveals a new mechanism by which glucose links cardiolipin anabolism to mediate radiation resistance in liver cancer.
Flexible changes in cellular metabolism satisfy the demands of tumor tissue growth, and we have found that there is a large amount of metabolic heterogeneity in tumors. The metabolic heterogeneity of tumors can be mainly manifested in three aspects: one is between tumor cells and nontumor cells; the second is between different types of tumors; and the third is during the development process of tumor resistance. During tumor invasion, cancer cells activate glycolysis to release lactic acid and other organic acids to acidify the extracellular environment and promote the degradation of ECM, which is beneficial to tumor cell invasion [192]. However, when they become circulating tumor cells, the metabolic phenotype switches to PPP, and produces sufficient NADPH and GSH to counteract ROS [192]. Furthermore, it is interesting to note that different cell groups have different nutrient uptake propensities from the TME, with glutamine and fatty acids mainly distributed to cancer cells, while glucose is preferentially provided to immune cells [66]. Therefore, how tumors balance the metabolism of glucose and glutamine is a key issue. These studies have shown that there is metabolic heterogeneity in the radioresistance of tumor cells, and targeting only a single metabolic target is not enough to overcome this radioresistance. In the future, we hope to find common metabolic targets in different radiation-resistant tumors and implement pancancer therapy based on these findings.
In general, current studies on sensitizing cancer cells to radiation based on metabolic reprogramming are mainly focused on metabolic enzyme inhibitors and nutrient deprivation. Considering the complexity and heterogeneity of metabolism, future studies are needed to deeply explore the relationship between the mechanism of radioresistance and metabolism and distinguish tumor subtypes with unique metabolism alterations. To identify and sensitize radioresistant cancers early, it is necessary to explore new tracers and sensitizers targeting metabolism in preclinical and clinical studies.
Funding
This work was supported by Natural Science Foundation of China (NSFC 12275178, 82000939, 82203260), The Science and Technology Commission of Shanghai (20DZ2270800, 22Y31900700), Shanghai Pujiang Program22PJD036.
Competing Interests
The authors have declared that no competing interest exists.
References
1. de Martel C, Georges D, Bray F, Ferlay J, Clifford GM. Global burden of cancer attributable to infections in 2018: a worldwide incidence analysis. Lancet Glob Health. 2020;8:e180-e90
2. Allen C, Her S, Jaffray DA. Radiotherapy for Cancer: Present and Future. Adv Drug Deliv Rev. 2017;109:1-2
3. Suwa T, Kobayashi M, Nam JM, Harada H. Tumor microenvironment and radioresistance. Exp Mol Med. 2021;53:1029-35
4. Gogineni VR, Nalla AK, Gupta R, Dinh DH, Klopfenstein JD, Rao JS. Chk2-mediated G2/M cell cycle arrest maintains radiation resistance in malignant meningioma cells. Cancer Lett. 2011;313:64-75
5. Martínez-Reyes I, Chandel NS. Cancer metabolism: looking forward. Nat Rev Cancer. 2021;21:669-80
6. Warburg O, Wind F, Negelein E. THE METABOLISM OF TUMORS IN THE BODY. J Gen Physiol. 1927;8:519-30
7. Doherty JR, Cleveland JL. Targeting lactate metabolism for cancer therapeutics. J Clin Invest. 2013;123:3685-92
8. Farber S, Diamond LK. Temporary remissions in acute leukemia in children produced by folic acid antagonist, 4-aminopteroyl-glutamic acid. N Engl J Med. 1948;238:787-93
9. Stine ZE, Schug ZT, Salvino JM, Dang CV. Targeting cancer metabolism in the era of precision oncology. Nat Rev Drug Discov. 2022;21:141-62
10. Kantarjian H, Short NJ, DiNardo C, Stein EM, Daver N, Perl AE. et al. Harnessing the benefits of available targeted therapies in acute myeloid leukaemia. Lancet Haematol. 2021;8:e922-e33
11. Shim H, Dolde C, Lewis BC, Wu CS, Dang G, Jungmann RA. et al. c-Myc transactivation of LDH-A: implications for tumor metabolism and growth. Proc Natl Acad Sci U S A. 1997;94:6658-63
12. Sun L, Zhang H, Gao P. Metabolic reprogramming and epigenetic modifications on the path to cancer. Protein Cell. 2022;13:877-919
13. Ali ES, Sahu U, Villa E, O'Hara BP, Gao P, Beaudet C. et al. ERK2 Phosphorylates PFAS to Mediate Posttranslational Control of De Novo Purine Synthesis. Mol Cell. 2020;78:1178-91.e6
14. Priolo C, Pyne S, Rose J, Regan ER, Zadra G, Photopoulos C. et al. AKT1 and MYC induce distinctive metabolic fingerprints in human prostate cancer. Cancer Res. 2014;74:7198-204
15. Yu J, Chai P, Xie M, Ge S, Ruan J, Fan X. et al. Histone lactylation drives oncogenesis by facilitating m(6)A reader protein YTHDF2 expression in ocular melanoma. Genome Biol. 2021;22:85
16. Kumagai S, Koyama S, Itahashi K, Tanegashima T, Lin YT, Togashi Y. et al. Lactic acid promotes PD-1 expression in regulatory T cells in highly glycolytic tumor microenvironments. Cancer Cell. 2022;40:201-18.e9
17. Canale FP, Basso C, Antonini G, Perotti M, Li N, Sokolovska A. et al. Metabolic modulation of tumours with engineered bacteria for immunotherapy. Nature. 2021;598:662-6
18. Curtis M, Kenny HA, Ashcroft B, Mukherjee A, Johnson A, Zhang Y. et al. Fibroblasts Mobilize Tumor Cell Glycogen to Promote Proliferation and Metastasis. Cell Metab. 2019;29:141-55.e9
19. van Gastel N, Spinelli JB, Sharda A, Schajnovitz A, Baryawno N, Rhee C. et al. Induction of a Timed Metabolic Collapse to Overcome Cancer Chemoresistance. Cell Metab. 2020;32:391-403.e6
20. Engel AL, Lorenz NI, Klann K, Münch C, Depner C, Steinbach JP. et al. Serine-dependent redox homeostasis regulates glioblastoma cell survival. Br J Cancer. 2020;122:1391-8
21. Lewis JE, Kemp ML. Integration of machine learning and genome-scale metabolic modeling identifies multi-omics biomarkers for radiation resistance. Nat Commun. 2021;12:2700
22. Schmidt DR, Patel R, Kirsch DG, Lewis CA, Vander Heiden MG, Locasale JW. Metabolomics in cancer research and emerging applications in clinical oncology. CA Cancer J Clin. 2021;71:333-58
23. Schellenberg D, Quon A, Minn AY, Graves EE, Kunz P, Ford JM. et al. 18Fluorodeoxyglucose PET is prognostic of progression-free and overall survival in locally advanced pancreas cancer treated with stereotactic radiotherapy. Int J Radiat Oncol Biol Phys. 2010;77:1420-5
24. Ganapathy-Kanniappan S, Geschwind JF. Tumor glycolysis as a target for cancer therapy: progress and prospects. Mol Cancer. 2013;12:152
25. Shimura T, Noma N, Sano Y, Ochiai Y, Oikawa T, Fukumoto M. et al. AKT-mediated enhanced aerobic glycolysis causes acquired radioresistance by human tumor cells. Radiother Oncol. 2014;112:302-7
26. Liu Y, Liang G, Xu H, Dong W, Dong Z, Qiu Z. et al. Tumors exploit FTO-mediated regulation of glycolytic metabolism to evade immune surveillance. Cell Metab. 2021;33:1221-33.e11
27. Dholakia AS, Chaudhry M, Leal JP, Chang DT, Raman SP, Hacker-Prietz A. et al. Baseline metabolic tumor volume and total lesion glycolysis are associated with survival outcomes in patients with locally advanced pancreatic cancer receiving stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys. 2014;89:539-46
28. Kunkel M, Moergel M, Stockinger M, Jeong JH, Fritz G, Lehr HA. et al. Overexpression of GLUT-1 is associated with resistance to radiotherapy and adverse prognosis in squamous cell carcinoma of the oral cavity. Oral Oncol. 2007;43:796-803
29. Yan SX, Luo XM, Zhou SH, Bao YY, Fan J, Lu ZJ. et al. Effect of antisense oligodeoxynucleotides glucose transporter-1 on enhancement of radiosensitivity of laryngeal carcinoma. Int J Med Sci. 2013;10:1375-86
30. Jia C, Zhao Y, Huang H, Fan K, Xie T, Xie M. Apigenin sensitizes radiotherapy of mouse subcutaneous glioma through attenuations of cell stemness and DNA damage repair by inhibiting NF-κB/HIF-1α-mediated glycolysis. J Nutr Biochem. 2022;107:109038
31. Zhao F, Ming J, Zhou Y, Fan L. Inhibition of Glut1 by WZB117 sensitizes radioresistant breast cancer cells to irradiation. Cancer Chemother Pharmacol. 2016;77:963-72
32. Rashmi R, Huang X, Floberg JM, Elhammali AE, McCormick ML, Patti GJ. et al. Radioresistant Cervical Cancers Are Sensitive to Inhibition of Glycolysis and Redox Metabolism. Cancer Res. 2018;78:1392-403
33. Gunda V, Souchek J, Abrego J, Shukla SK, Goode GD, Vernucci E. et al. MUC1-Mediated Metabolic Alterations Regulate Response to Radiotherapy in Pancreatic Cancer. Clin Cancer Res. 2017;23:5881-91
34. Prakasam G, Bamezai RNK. Pyruvate Kinase. In: Boffetta P, Hainaut P, editors. Encyclopedia of Cancer (Third Edition). Oxford: Academic Press. 2019 p. 311-20
35. Wu S, Cao R, Tao B, Wu P, Peng C, Gao H. et al. Pyruvate Facilitates FACT-Mediated γH2AX Loading to Chromatin and Promotes the Radiation Resistance of Glioblastoma. Adv Sci (Weinh). 2022;9:e2104055
36. Meng MB, Wang HH, Guo WH, Wu ZQ, Zeng XL, Zaorsky NG. et al. Targeting pyruvate kinase M2 contributes to radiosensitivity of non-small cell lung cancer cells in vitro and in vivo. Cancer Lett. 2015;356:985-93
37. Zhang L, Bailleul J, Yazal T, Dong K, Sung D, Dao A. et al. PK-M2-mediated metabolic changes in breast cancer cells induced by ionizing radiation. Breast Cancer Res Treat. 2019;178:75-86
38. Koukourakis MI, Giatromanolaki A, Panteliadou M, Pouliliou SE, Chondrou PS, Mavropoulou S. et al. Lactate dehydrogenase 5 isoenzyme overexpression defines resistance of prostate cancer to radiotherapy. Br J Cancer. 2014;110:2217-23
39. Koukourakis MI, Kakouratos C, Kalamida D, Bampali Z, Mavropoulou S, Sivridis E. et al. Hypoxia-inducible proteins HIF1α and lactate dehydrogenase LDH5, key markers of anaerobic metabolism, relate with stem cell markers and poor post-radiotherapy outcome in bladder cancer. Int J Radiat Biol. 2016;92:353-63
40. Schwab M, Thunborg K, Azimzadeh O, von Toerne C, Werner C, Shevtsov M. et al. Targeting Cancer Metabolism Breaks Radioresistance by Impairing the Stress Response. Cancers (Basel). 2021 13
41. Yang Y, Chong Y, Chen M, Dai W, Zhou X, Ji Y. et al. Targeting lactate dehydrogenase a improves radiotherapy efficacy in non-small cell lung cancer: from bedside to bench. J Transl Med. 2021;19:170
42. Liu Y, He D, Xiao M, Zhu Y, Zhou J, Cao K. Long noncoding RNA LINC00518 induces radioresistance by regulating glycolysis through an miR-33a-3p/HIF-1α negative feedback loop in melanoma. Cell Death Dis. 2021;12:245
43. Sandulache VC, Chen Y, Skinner HD, Lu T, Feng L, Court LE. et al. Acute Tumor Lactate Perturbations as a Biomarker of Genotoxic Stress: Development of a Biochemical Model. Mol Cancer Ther. 2015;14:2901-8
44. Quennet V, Yaromina A, Zips D, Rosner A, Walenta S, Baumann M. et al. Tumor lactate content predicts for response to fractionated irradiation of human squamous cell carcinomas in nude mice. Radiother Oncol. 2006;81:130-5
45. Mair R, Wright AJ, Ros S, Hu DE, Booth T, Kreis F. et al. Metabolic Imaging Detects Low Levels of Glycolytic Activity That Vary with Levels of c-Myc Expression in Patient-Derived Xenograft Models of Glioblastoma. Cancer Res. 2018;78:5408-18
46. Yang X, Lu Y, Hang J, Zhang J, Zhang T, Huo Y. et al. Lactate-Modulated Immunosuppression of Myeloid-Derived Suppressor Cells Contributes to the Radioresistance of Pancreatic Cancer. Cancer Immunol Res. 2020;8:1440-51
47. Bola BM, Chadwick AL, Michopoulos F, Blount KG, Telfer BA, Williams KJ. et al. Inhibition of monocarboxylate transporter-1 (MCT1) by AZD3965 enhances radiosensitivity by reducing lactate transport. Mol Cancer Ther. 2014;13:2805-16
48. Takaoka Y, Konno M, Koseki J, Colvin H, Asai A, Tamari K. et al. Mitochondrial pyruvate carrier 1 expression controls cancer epithelial-mesenchymal transition and radioresistance. Cancer Sci. 2019;110:1331-9
49. Corbet C, Bastien E, Draoui N, Doix B, Mignion L, Jordan BF. et al. Interruption of lactate uptake by inhibiting mitochondrial pyruvate transport unravels direct antitumor and radiosensitizing effects. Nat Commun. 2018;9:1208
50. Bonnay F, Veloso A, Steinmann V, Köcher T, Abdusselamoglu MD, Bajaj S. et al. Oxidative Metabolism Drives Immortalization of Neural Stem Cells during Tumorigenesis. Cell. 2020;182:1490-507.e19
51. Smith AL, Whitehall JC, Bradshaw C, Gay D, Robertson F, Blain AP. et al. Age-associated mitochondrial DNA mutations cause metabolic remodelling that contributes to accelerated intestinal tumorigenesis. Nat Cancer. 2020;1:976-89
52. Shi Y, Lim SK, Liang Q, Iyer SV, Wang HY, Wang Z. et al. Gboxin is an oxidative phosphorylation inhibitor that targets glioblastoma. Nature. 2019;567:341-6
53. Shi Y, Wang Y, Jiang H, Sun X, Xu H, Wei X. et al. Mitochondrial dysfunction induces radioresistance in colorectal cancer by activating [Ca(2+)](m)-PDP1-PDH-histone acetylation retrograde signaling. Cell Death Dis. 2021;12:837
54. de Mey S, Dufait I, Jiang H, Corbet C, Wang H, Van De Gucht M. et al. Dichloroacetate Radiosensitizes Hypoxic Breast Cancer Cells. Int J Mol Sci. 2020 21
55. Cook KM, Shen H, McKelvey KJ, Gee HE, Hau E. Targeting Glucose Metabolism of Cancer Cells with Dichloroacetate to Radiosensitize High-Grade Gliomas. Int J Mol Sci. 2021 22
56. Dong G, Chen Q, Jiang F, Yu D, Mao Q, Xia W. et al. Diisopropylamine dichloroacetate enhances radiosensitization in esophageal squamous cell carcinoma by increasing mitochondria-derived reactive oxygen species levels. Oncotarget. 2016;7:68170-8
57. Lin R, Elf S, Shan C, Kang HB, Ji Q, Zhou L. et al. 6-Phosphogluconate dehydrogenase links oxidative PPP, lipogenesis and tumour growth by inhibiting LKB1-AMPK signalling. Nat Cell Biol. 2015;17:1484-96
58. Mims J, Bansal N, Bharadwaj MS, Chen X, Molina AJ, Tsang AW. et al. Energy metabolism in a matched model of radiation resistance for head and neck squamous cell cancer. Radiat Res. 2015;183:291-304
59. Liu R, Li W, Tao B, Wang X, Yang Z, Zhang Y. et al. Tyrosine phosphorylation activates 6-phosphogluconate dehydrogenase and promotes tumor growth and radiation resistance. Nat Commun. 2019;10:991
60. Heller S, Maurer GD, Wanka C, Hofmann U, Luger AL, Bruns I. et al. Gene Suppression of Transketolase-Like Protein 1 (TKTL1) Sensitizes Glioma Cells to Hypoxia and Ionizing Radiation. Int J Mol Sci. 2018 19
61. Jones CL, Stevens BM, D'Alessandro A, Reisz JA, Culp-Hill R, Nemkov T. et al. Inhibition of Amino Acid Metabolism Selectively Targets Human Leukemia Stem Cells. Cancer Cell. 2019;35:333-5
62. Hensley CT, Wasti AT, DeBerardinis RJ. Glutamine and cancer: cell biology, physiology, and clinical opportunities. J Clin Invest. 2013;123:3678-84
63. Altman BJ, Stine ZE, Dang CV. From Krebs to clinic: glutamine metabolism to cancer therapy. Nat Rev Cancer. 2016;16:619-34
64. Fu S, Li Z, Xiao L, Hu W, Zhang L, Xie B. et al. Glutamine Synthetase Promotes Radiation Resistance via Facilitating Nucleotide Metabolism and Subsequent DNA Damage Repair. Cell Rep. 2019;28:1136-43.e4
65. Cluntun AA, Lukey MJ, Cerione RA, Locasale JW. Glutamine Metabolism in Cancer: Understanding the Heterogeneity. Trends Cancer. 2017;3:169-80
66. Reinfeld BI, Madden MZ, Wolf MM, Chytil A, Bader JE, Patterson AR. et al. Cell-programmed nutrient partitioning in the tumour microenvironment. Nature. 2021;593:282-8
67. Dornier E, Rabas N, Mitchell L, Novo D, Dhayade S, Marco S. et al. Glutaminolysis drives membrane trafficking to promote invasiveness of breast cancer cells. Nat Commun. 2017;8:2255
68. Zacharias NM, McCullough C, Shanmugavelandy S, Lee J, Lee Y, Dutta P. et al. Metabolic Differences in Glutamine Utilization Lead to Metabolic Vulnerabilities in Prostate Cancer. Sci Rep. 2017;7:16159
69. Le Grand M, Mukha A, Püschel J, Valli E, Kamili A, Vittorio O. et al. Interplay between MycN and c-Myc regulates radioresistance and cancer stem cell phenotype in neuroblastoma upon glutamine deprivation. Theranostics. 2020;10:6411-29
70. Rashmi R, Jayachandran K, Zhang J, Menon V, Muhammad N, Zahner M. et al. Glutaminase Inhibitors Induce Thiol-Mediated Oxidative Stress and Radiosensitization in Treatment-Resistant Cervical Cancers. Mol Cancer Ther. 2020;19:2465-75
71. Wicker CA, Hunt BG, Krishnan S, Aziz K, Parajuli S, Palackdharry S. et al. Glutaminase inhibition with telaglenastat (CB-839) improves treatment response in combination with ionizing radiation in head and neck squamous cell carcinoma models. Cancer Lett. 2021;502:180-8
72. Boysen G, Jamshidi-Parsian A, Davis MA, Siegel ER, Simecka CM, Kore RA. et al. Glutaminase inhibitor CB-839 increases radiation sensitivity of lung tumor cells and human lung tumor xenografts in mice. Int J Radiat Biol. 2019;95:436-42
73. Xiang L, Xie G, Liu C, Zhou J, Chen J, Yu S. et al. Knock-down of glutaminase 2 expression decreases glutathione, NADH, and sensitizes cervical cancer to ionizing radiation. Biochim Biophys Acta. 2013;1833:2996-3005
74. Mukha A, Kahya U, Linge A, Chen O, Löck S, Lukiyanchuk V. et al. GLS-driven glutamine catabolism contributes to prostate cancer radiosensitivity by regulating the redox state, stemness and ATG5-mediated autophagy. Theranostics. 2021;11:7844-68
75. Binkley MS, Jeon YJ, Nesselbush M, Moding EJ, Nabet BY, Almanza D. et al. KEAP1/NFE2L2 Mutations Predict Lung Cancer Radiation Resistance That Can Be Targeted by Glutaminase Inhibition. Cancer Discov. 2020;10:1826-41
76. McBrayer SK, Mayers JR, DiNatale GJ, Shi DD, Khanal J, Chakraborty AA. et al. Transaminase Inhibition by 2-Hydroxyglutarate Impairs Glutamate Biosynthesis and Redox Homeostasis in Glioma. Cell. 2018;175:101-16.e25
77. Gross MI, Demo SD, Dennison JB, Chen L, Chernov-Rogan T, Goyal B. et al. Antitumor activity of the glutaminase inhibitor CB-839 in triple-negative breast cancer. Mol Cancer Ther. 2014;13:890-901
78. Marin-Valencia I, Yang C, Mashimo T, Cho S, Baek H, Yang XL. et al. Analysis of tumor metabolism reveals mitochondrial glucose oxidation in genetically diverse human glioblastomas in the mouse brain in vivo. Cell Metab. 2012;15:827-37
79. Peng Y, Fu S, Hu W, Qiu Y, Zhang L, Tan R. et al. Glutamine synthetase facilitates cancer cells to recover from irradiation-induced G2/M arrest. Cancer Biol Ther. 2020;21:43-51
80. Zhao JS, Shi S, Qu HY, Keckesova Z, Cao ZJ, Yang LX. et al. Glutamine synthetase licenses APC/C-mediated mitotic progression to drive cell growth. Nat Metab. 2022;4:239-53
81. Yang M, Vousden KH. Serine and one-carbon metabolism in cancer. Nat Rev Cancer. 2016;16:650-62
82. Rodriguez AE, Ducker GS, Billingham LK, Martinez CA, Mainolfi N, Suri V. et al. Serine Metabolism Supports Macrophage IL-1β Production. Cell Metab. 2019;29:1003-11.e4
83. Kaplanov I, Carmi Y, Kornetsky R, Shemesh A, Shurin GV, Shurin MR. et al. Blocking IL-1β reverses the immunosuppression in mouse breast cancer and synergizes with anti-PD-1 for tumor abrogation. Proc Natl Acad Sci U S A. 2019;116:1361-9
84. Shan X, Hu P, Ni L, Shen L, Zhang Y, Ji Z. et al. Serine metabolism orchestrates macrophage polarization by regulating the IGF1-p38 axis. Cell Mol Immunol. 2022;19:1263-78
85. Fuks Z, Kolesnick R. Engaging the vascular component of the tumor response. Cancer Cell. 2005;8:89-91
86. Vandekeere S, Dubois C, Kalucka J, Sullivan MR, García-Caballero M, Goveia J. et al. Serine Synthesis via PHGDH Is Essential for Heme Production in Endothelial Cells. Cell Metab. 2018;28:573-87.e13
87. Sharif T, Martell E, Dai C, Ghassemi-Rad MS, Lee K, Singh SK. et al. Phosphoglycerate dehydrogenase inhibition induces p-mTOR-independent autophagy and promotes multilineage differentiation in embryonal carcinoma stem-like cells. Cell Death Dis. 2018;9:990
88. Wu L, Hu Z, Huang Y, Yu Y, Liang W, Zheng Q. et al. Radiation Changes the Metabolic Profiling of Melanoma Cell Line B16. PLoS One. 2016;11:e0162917
89. Bhute VJ, Ma Y, Bao X, Palecek SP. The Poly (ADP-Ribose) Polymerase Inhibitor Veliparib and Radiation Cause Significant Cell Line Dependent Metabolic Changes in Breast Cancer Cells. Sci Rep. 2016;6:36061
90. Arenas M, Rodríguez E, García-Heredia A, Fernández-Arroyo S, Sabater S, Robaina R. et al. Metabolite normalization with local radiotherapy following breast tumor resection. PLoS One. 2018;13:e0207474
91. Lindell Jonsson E, Erngren I, Engskog M, Haglöf J, Arvidsson T, Hedeland M. et al. Exploring Radiation Response in Two Head and Neck Squamous Carcinoma Cell Lines Through Metabolic Profiling. Front Oncol. 2019;9:825
92. Gravel SP, Hulea L, Toban N, Birman E, Blouin MJ, Zakikhani M. et al. Serine deprivation enhances antineoplastic activity of biguanides. Cancer Res. 2014;74:7521-33
93. McCann E, O'Sullivan J, Marcone S. Targeting cancer-cell mitochondria and metabolism to improve radiotherapy response. Transl Oncol. 2021;14:100905
94. Sánchez-Castillo A, Vooijs M, Kampen KR. Linking Serine/Glycine Metabolism to Radiotherapy Resistance. Cancers (Basel). 2021 13
95. Samanta D, Park Y, Andrabi SA, Shelton LM, Gilkes DM, Semenza GL. PHGDH Expression Is Required for Mitochondrial Redox Homeostasis, Breast Cancer Stem Cell Maintenance, and Lung Metastasis. Cancer Res. 2016;76:4430-42
96. Kim D, Fiske BP, Birsoy K, Freinkman E, Kami K, Possemato RL. et al. SHMT2 drives glioma cell survival in ischaemia but imposes a dependence on glycine clearance. Nature. 2015;520:363-7
97. Rouschop KM, Dubois LJ, Keulers TG, van den Beucken T, Lambin P, Bussink J. et al. PERK/eIF2α signaling protects therapy resistant hypoxic cells through induction of glutathione synthesis and protection against ROS. Proc Natl Acad Sci U S A. 2013;110:4622-7
98. Reina-Campos M, Linares JF, Duran A, Cordes T, L'Hermitte A, Badur MG. et al. Increased Serine and One-Carbon Pathway Metabolism by PKCλ/ι Deficiency Promotes Neuroendocrine Prostate Cancer. Cancer Cell. 2019;35:385-400.e9
99. Shi X, Shiao SL. The role of macrophage phenotype in regulating the response to radiation therapy. Transl Res. 2018;191:64-80
100. Okubo M, Kioi M, Nakashima H, Sugiura K, Mitsudo K, Aoki I. et al. M2-polarized macrophages contribute to neovasculogenesis, leading to relapse of oral cancer following radiation. Sci Rep. 2016;6:27548
101. Wilson JL, Nägele T, Linke M, Demel F, Fritsch SD, Mayr HK. et al. Inverse Data-Driven Modeling and Multiomics Analysis Reveals Phgdh as a Metabolic Checkpoint of Macrophage Polarization and Proliferation. Cell Rep. 2020;30:1542-52.e7
102. Li F, Simon MC. Cancer Cells Don't Live Alone: Metabolic Communication within Tumor Microenvironments. Dev Cell. 2020;54:183-95
103. Delage B, Fennell DA, Nicholson L, McNeish I, Lemoine NR, Crook T. et al. Arginine deprivation and argininosuccinate synthetase expression in the treatment of cancer. Int J Cancer. 2010;126:2762-72
104. Feun LG, Kuo MT, Savaraj N. Arginine deprivation in cancer therapy. Curr Opin Clin Nutr Metab Care. 2015;18:78-82
105. Butler M, van der Meer LT, van Leeuwen FN. Amino Acid Depletion Therapies: Starving Cancer Cells to Death. Trends Endocrinol Metab. 2021;32:367-81
106. Crittenden MR, Savage T, Cottam B, Baird J, Rodriguez PC, Newell P. et al. Expression of arginase I in myeloid cells limits control of residual disease after radiation therapy of tumors in mice. Radiat Res. 2014;182:182-90
107. Leonard W, Dufait I, Schwarze JK, Law K, Engels B, Jiang H. et al. Myeloid-derived suppressor cells reveal radioprotective properties through arginase-induced l-arginine depletion. Radiother Oncol. 2016;119:291-9
108. Du C, Zhou M, Jia F, Ruan L, Lu H, Zhang J. et al. D-arginine-loaded metal-organic frameworks nanoparticles sensitize osteosarcoma to radiotherapy. Biomaterials. 2021;269:120642
109. Hinrichs CN, Ingargiola M, Käubler T, Löck S, Temme A, Köhn-Luque A. et al. Arginine Deprivation Therapy: Putative Strategy to Eradicate Glioblastoma Cells by Radiosensitization. Mol Cancer Ther. 2018;17:393-406
110. Vynnytska-Myronovska B, Bobak Y, Garbe Y, Dittfeld C, Stasyk O, Kunz-Schughart LA. Single amino acid arginine starvation efficiently sensitizes cancer cells to canavanine treatment and irradiation. Int J Cancer. 2012;130:2164-75
111. Singh PK, Deorukhkar AA, Venkatesulu BP, Li X, Tailor R, Bomalaski JS. et al. Exploiting Arginine Auxotrophy with Pegylated Arginine Deiminase (ADI-PEG20) to Sensitize Pancreatic Cancer to Radiotherapy via Metabolic Dysregulation. Mol Cancer Ther. 2019;18:2381-93
112. Marullo R, Castro M, Yomtoubian S, Calvo-Vidal MN, Revuelta MV, Krumsiek J. et al. The metabolic adaptation evoked by arginine enhances the effect of radiation in brain metastases. Sci Adv. 2021;7:eabg1964
113. Koga Y, Akita Y, Nishioka J, Yatsuga S, Povalko N, Tanabe Y. et al. L-arginine improves the symptoms of strokelike episodes in MELAS. Neurology. 2005;64:710-2
114. Platten M, Nollen EAA, Röhrig UF, Fallarino F, Opitz CA. Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nat Rev Drug Discov. 2019;18:379-401
115. Prendergast GC, Smith C, Thomas S, Mandik-Nayak L, Laury-Kleintop L, Metz R. et al. Indoleamine 2,3-dioxygenase pathways of pathogenic inflammation and immune escape in cancer. Cancer Immunol Immunother. 2014;63:721-35
116. Sadik A, Somarribas Patterson LF, Öztürk S, Mohapatra SR, Panitz V, Secker PF. et al. IL4I1 Is a Metabolic Immune Checkpoint that Activates the AHR and Promotes Tumor Progression. Cell. 2020;182:1252-70.e34
117. Li A, Barsoumian HB, Schoenhals JE, Caetano MS, Wang X, Menon H. et al. IDO1 Inhibition Overcomes Radiation-Induced "Rebound Immune Suppression" by Reducing Numbers of IDO1-Expressing Myeloid-Derived Suppressor Cells in the Tumor Microenvironment. Int J Radiat Oncol Biol Phys. 2019;104:903-12
118. Chen B, Alvarado DM, Iticovici M, Kau NS, Park H, Parikh PJ. et al. Interferon-Induced IDO1 Mediates Radiation Resistance and Is a Therapeutic Target in Colorectal Cancer. Cancer Immunol Res. 2020;8:451-64
119. Wang C, Dong Z, Hao Y, Zhu Y, Ni J, Li Q. et al. Coordination Polymer-Coated CaCO(3) Reinforces Radiotherapy by Reprogramming the Immunosuppressive Metabolic Microenvironment. Adv Mater. 2022;34:e2106520
120. Vrooman LM, Stevenson KE, Supko JG, O'Brien J, Dahlberg SE, Asselin BL. et al. Postinduction dexamethasone and individualized dosing of Escherichia Coli L-asparaginase each improve outcome of children and adolescents with newly diagnosed acute lymphoblastic leukemia: results from a randomized study-Dana-Farber Cancer Institute ALL Consortium Protocol 00-01. J Clin Oncol. 2013;31:1202-10
121. Krall AS, Mullen PJ, Surjono F, Momcilovic M, Schmid EW, Halbrook CJ. et al. Asparagine couples mitochondrial respiration to ATF4 activity and tumor growth. Cell Metab. 2021;33:1013-26.e6
122. Thomas TM, Miyaguchi K, Edwards LA, Wang H, Wollebo H, Aiguo L. et al. Elevated Asparagine Biosynthesis Drives Brain Tumor Stem Cell Metabolic Plasticity and Resistance to Oxidative Stress. Mol Cancer Res. 2021;19:1375-88
123. Broadfield LA, Pane AA, Talebi A, Swinnen JV, Fendt SM. Lipid metabolism in cancer: New perspectives and emerging mechanisms. Dev Cell. 2021;56:1363-93
124. Röhrig F, Schulze A. The multifaceted roles of fatty acid synthesis in cancer. Nat Rev Cancer. 2016;16:732-49
125. Lim SA, Wei J, Nguyen TM, Shi H, Su W, Palacios G. et al. Lipid signalling enforces functional specialization of T(reg) cells in tumours. Nature. 2021;591:306-11
126. Hoy AJ, Nagarajan SR, Butler LM. Tumour fatty acid metabolism in the context of therapy resistance and obesity. Nat Rev Cancer. 2021;21:753-66
127. Göttgens EL, van den Heuvel CN, de Jong MC, Kaanders JH, Leenders WP, Ansems M. et al. ACLY (ATP Citrate Lyase) Mediates Radioresistance in Head and Neck Squamous Cell Carcinomas and is a Novel Predictive Radiotherapy Biomarker. Cancers (Basel). 2019 11
128. Khwairakpam AD, Banik K, Girisa S, Shabnam B, Shakibaei M, Fan L. et al. The vital role of ATP citrate lyase in chronic diseases. J Mol Med (Berl). 2020;98:71-95
129. Comerford SA, Huang Z, Du X, Wang Y, Cai L, Witkiewicz AK. et al. Acetate dependence of tumors. Cell. 2014;159:1591-602
130. German NJ, Yoon H, Yusuf RZ, Murphy JP, Finley LW, Laurent G. et al. PHD3 Loss in Cancer Enables Metabolic Reliance on Fatty Acid Oxidation via Deactivation of ACC2. Mol Cell. 2016;63:1006-20
131. Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer. 2007;7:763-77
132. Vriens K, Christen S, Parik S, Broekaert D, Yoshinaga K, Talebi A. et al. Evidence for an alternative fatty acid desaturation pathway increasing cancer plasticity. Nature. 2019;566:403-6
133. Kao YC, Lee SW, Lin LC, Chen LT, Hsing CH, Hsu HP. et al. Fatty acid synthase overexpression confers an independent prognosticator and associates with radiation resistance in nasopharyngeal carcinoma. Tumour Biol. 2013;34:759-68
134. Yang Y, Liu H, Li Z, Zhao Z, Yip-Schneider M, Fan Q. et al. Role of fatty acid synthase in gemcitabine and radiation resistance of pancreatic cancers. Int J Biochem Mol Biol. 2011;2:89-98
135. Chuang HY, Lee YP, Lin WC, Lin YH, Hwang JJ. Fatty Acid Inhibition Sensitizes Androgen-Dependent and -Independent Prostate Cancer to Radiotherapy via FASN/NF-κB Pathway. Sci Rep. 2019;9:13284
136. Dolcet X, Llobet D, Pallares J, Matias-Guiu X. NF-kB in development and progression of human cancer. Virchows Arch. 2005;446:475-82
137. Chen J, Zhang F, Ren X, Wang Y, Huang W, Zhang J. et al. Targeting fatty acid synthase sensitizes human nasopharyngeal carcinoma cells to radiation via downregulating frizzled class receptor 10. Cancer Biol Med. 2020;17:740-52
138. Carracedo A, Cantley LC, Pandolfi PP. Cancer metabolism: fatty acid oxidation in the limelight. Nat Rev Cancer. 2013;13:227-32
139. Duman C, Yaqubi K, Hoffmann A, Acikgöz AA, Korshunov A, Bendszus M. et al. Acyl-CoA-Binding Protein Drives Glioblastoma Tumorigenesis by Sustaining Fatty Acid Oxidation. Cell Metab. 2019;30:274-89.e5
140. Koundouros N, Poulogiannis G. Reprogramming of fatty acid metabolism in cancer. Br J Cancer. 2020;122:4-22
141. Su P, Wang Q, Bi E, Ma X, Liu L, Yang M. et al. Correction: Enhanced Lipid Accumulation and Metabolism Are Required for the Differentiation and Activation of Tumor-Associated Macrophages. Cancer Res. 2022;82:945
142. Li XX, Wang ZJ, Zheng Y, Guan YF, Yang PB, Chen X. et al. Nuclear Receptor Nur77 Facilitates Melanoma Cell Survival under Metabolic Stress by Protecting Fatty Acid Oxidation. Mol Cell. 2018;69:480-92.e7
143. Jiang N, Xie B, Xiao W, Fan M, Xu S, Duan Y. et al. Fatty acid oxidation fuels glioblastoma radioresistance with CD47-mediated immune evasion. Nat Commun. 2022;13:1511
144. Wang T, Fahrmann JF, Lee H, Li YJ, Tripathi SC, Yue C. et al. JAK/STAT3-Regulated Fatty Acid β-Oxidation Is Critical for Breast Cancer Stem Cell Self-Renewal and Chemoresistance. Cell Metab. 2018;27:136-50.e5
145. Kwon YS, Lee MG, Baek J, Kim NY, Jang H, Kim S. Acyl-CoA synthetase-4 mediates radioresistance of breast cancer cells by regulating FOXM1. Biochem Pharmacol. 2021;192:114718
146. Tan Z, Xiao L, Tang M, Bai F, Li J, Li L. et al. Targeting CPT1A-mediated fatty acid oxidation sensitizes nasopharyngeal carcinoma to radiation therapy. Theranostics. 2018;8:2329-47
147. Du Q, Tan Z, Shi F, Tang M, Xie L, Zhao L. et al. PGC1α/CEBPB/CPT1A axis promotes radiation resistance of nasopharyngeal carcinoma through activating fatty acid oxidation. Cancer Sci. 2019;110:2050-62
148. Han S, Wei R, Zhang X, Jiang N, Fan M, Huang JH. et al. CPT1A/2-Mediated FAO Enhancement-A Metabolic Target in Radioresistant Breast Cancer. Front Oncol. 2019;9:1201
149. Koundouros N, Karali E, Tripp A, Valle A, Inglese P, Perry NJS. et al. Metabolic Fingerprinting Links Oncogenic PIK3CA with Enhanced Arachidonic Acid-Derived Eicosanoids. Cell. 2020;181:1596-611.e27
150. Liao P, Wang W, Wang W, Kryczek I, Li X, Bian Y. et al. CD8(+) T cells and fatty acids orchestrate tumor ferroptosis and immunity via ACSL4. Cancer Cell. 2022;40:365-78.e6
151. Merritt MA, Rice MS, Barnard ME, Hankinson SE, Matulonis UA, Poole EM. et al. Pre-diagnosis and post-diagnosis use of common analgesics and ovarian cancer prognosis (NHS/NHSII): a cohort study. Lancet Oncol. 2018;19:1107-16
152. Bosetti C, Santucci C, Gallus S, Martinetti M, La Vecchia C. Aspirin and the risk of colorectal and other digestive tract cancers: an updated meta-analysis through 2019. Ann Oncol. 2020;31:558-68
153. Burn J, Sheth H, Elliott F, Reed L, Macrae F, Mecklin JP. et al. Cancer prevention with aspirin in hereditary colorectal cancer (Lynch syndrome), 10-year follow-up and registry-based 20-year data in the CAPP2 study: a double-blind, randomised, placebo-controlled trial. Lancet. 2020;395:1855-63
154. Cook PJ, Thomas R, Kingsley PJ, Shimizu F, Montrose DC, Marnett LJ. et al. Cox-2-derived PGE2 induces Id1-dependent radiation resistance and self-renewal in experimental glioblastoma. Neuro Oncol. 2016;18:1379-89
155. Nix P, Lind M, Greenman J, Stafford N, Cawkwell L. Expression of Cox-2 protein in radioresistant laryngeal cancer. Ann Oncol. 2004;15:797-801
156. Schulte RR, Linkous AG, Hallahan DE, Yazlovitskaya EM. Cytosolic phospholipase A2 as a molecular target for the radiosensitization of ovarian cancer. Cancer Lett. 2011;304:137-43
157. Na YJ, Kim BR, Kim JL, Kang S, Jeong YA, Park SH. et al. Deficiency of 15-LOX-1 Induces Radioresistance through Downregulation of MacroH2A2 in Colorectal Cancer. Cancers (Basel). 2019 11
158. Lövey J, Nie D, Tóvári J, Kenessey I, Tímár J, Kandouz M. et al. Radiosensitivity of human prostate cancer cells can be modulated by inhibition of 12-lipoxygenase. Cancer Lett. 2013;335:495-501
159. Zhang P, He D, Song E, Jiang M, Song Y. Celecoxib enhances the sensitivity of non-small-cell lung cancer cells to radiation-induced apoptosis through downregulation of the Akt/mTOR signaling pathway and COX-2 expression. PLoS One. 2019;14:e0223760
160. Xia S, Zhao Y, Yu S, Zhang M. Activated PI3K/Akt/COX-2 pathway induces resistance to radiation in human cervical cancer HeLa cells. Cancer Biother Radiopharm. 2010;25:317-23
161. Yusup G, Akutsu Y, Mutallip M, Qin W, Hu X, Komatsu-Akimoto A. et al. A COX-2 inhibitor enhances the antitumor effects of chemotherapy and radiotherapy for esophageal squamous cell carcinoma. Int J Oncol. 2014;44:1146-52
162. Huang B, Song BL, Xu C. Cholesterol metabolism in cancer: mechanisms and therapeutic opportunities. Nat Metab. 2020;2:132-41
163. Kao J, Genden EM, Chen CT, Rivera M, Tong CC, Misiukiewicz K. et al. Phase 1 trial of concurrent erlotinib, celecoxib, and reirradiation for recurrent head and neck cancer. Cancer. 2011;117:3173-81
164. Souchek JJ, Baine MJ, Lin C, Rachagani S, Gupta S, Kaur S. et al. Unbiased analysis of pancreatic cancer radiation resistance reveals cholesterol biosynthesis as a novel target for radiosensitisation. Br J Cancer. 2014;111:1139-49
165. Efimova EV, Ricco N, Labay E, Mauceri HJ, Flor AC, Ramamurthy A. et al. HMG-CoA Reductase Inhibition Delays DNA Repair and Promotes Senescence After Tumor Irradiation. Mol Cancer Ther. 2018;17:407-18
166. Seshacharyulu P, Halder S, Nimmakayala R, Rachagani S, Chaudhary S, Atri P. et al. Disruption of FDPS/Rac1 axis radiosensitizes pancreatic ductal adenocarcinoma by attenuating DNA damage response and immunosuppressive signalling. EBioMedicine. 2022;75:103772
167. Xu D, Wang Z, Xia Y, Shao F, Xia W, Wei Y. et al. The gluconeogenic enzyme PCK1 phosphorylates INSIG1/2 for lipogenesis. Nature. 2020;580:530-5
168. Liu R, Lee JH, Li J, Yu R, Tan L, Xia Y. et al. Choline kinase alpha 2 acts as a protein kinase to promote lipolysis of lipid droplets. Mol Cell. 2021;81:2722-35.e9
169. Yang K, Rich JN. A delicate initiation: Lipolysis of lipid droplets fuels glioblastoma. Mol Cell. 2021;81:2686-7
170. Nisticò C, Pagliari F, Chiarella E, Fernandes Guerreiro J, Marafioti MG, Aversa I. et al. Lipid Droplet Biosynthesis Impairment through DGAT2 Inhibition Sensitizes MCF7 Breast Cancer Cells to Radiation. Int J Mol Sci. 2021 22
171. Tirinato L, Marafioti MG, Pagliari F, Jansen J, Aversa I, Hanley R. et al. Lipid droplets and ferritin heavy chain: a devilish liaison in human cancer cell radioresistance. Elife. 2021 10
172. Wu HL, Gong Y, Ji P, Xie YF, Jiang YZ, Liu GY. Targeting nucleotide metabolism: a promising approach to enhance cancer immunotherapy. J Hematol Oncol. 2022;15:45
173. Wang X, Yang K, Xie Q, Wu Q, Mack SC, Shi Y. et al. Purine synthesis promotes maintenance of brain tumor initiating cells in glioma. Nat Neurosci. 2017;20:661-73
174. Wang X, Yang K, Wu Q, Kim LJY, Morton AR, Gimple RC. et al. Targeting pyrimidine synthesis accentuates molecular therapy response in glioblastoma stem cells. Sci Transl Med. 2019 11
175. Zhou W, Yao Y, Scott AJ, Wilder-Romans K, Dresser JJ, Werner CK. et al. Purine metabolism regulates DNA repair and therapy resistance in glioblastoma. Nat Commun. 2020;11:3811
176. Zhou W, Wahl DR. Purine metabolism promotes radioresistance and is a therapeutic target in glioblastoma. Mol Cell Oncol. 2020;7:1834902
177. Yan Y, Su W, Zeng S, Qian L, Chen X, Wei J. et al. Effect and Mechanism of Tanshinone I on the Radiosensitivity of Lung Cancer Cells. Mol Pharm. 2018;15:4843-53
178. Ong DST, Hu B, Ho YW, Sauvé CG, Bristow CA, Wang Q. et al. PAF promotes stemness and radioresistance of glioma stem cells. Proc Natl Acad Sci U S A. 2017;114:E9086-e95
179. Wang W, Cui J, Ma H, Lu W, Huang J. Targeting Pyrimidine Metabolism in the Era of Precision Cancer Medicine. Front Oncol. 2021;11:684961
180. Tang M, Lu X, Zhang C, Du C, Cao L, Hou T. et al. Downregulation of SIRT7 by 5-fluorouracil induces radiosensitivity in human colorectal cancer. Theranostics. 2017;7:1346-59
181. Lee KJ, Ko EJ, Park YY, Park SS, Ju EJ, Park J. et al. A novel nanoparticle-based theranostic agent targeting LRP-1 enhances the efficacy of neoadjuvant radiotherapy in colorectal cancer. Biomaterials. 2020;255:120151
182. Lang X, Green MD, Wang W, Yu J, Choi JE, Jiang L. et al. Radiotherapy and Immunotherapy Promote Tumoral Lipid Oxidation and Ferroptosis via Synergistic Repression of SLC7A11. Cancer Discov. 2019;9:1673-85
183. Turnbull T, Douglass M, Williamson NH, Howard D, Bhardwaj R, Lawrence M. et al. Cross-Correlative Single-Cell Analysis Reveals Biological Mechanisms of Nanoparticle Radiosensitization. ACS Nano. 2019;13:5077-90
184. Tang Q, Wu L, Xu M, Yan D, Shao J, Yan S. Osalmid, a Novel Identified RRM2 Inhibitor, Enhances Radiosensitivity of Esophageal Cancer. Int J Radiat Oncol Biol Phys. 2020;108:1368-79
185. Lei G, Zhang Y, Koppula P, Liu X, Zhang J, Lin SH. et al. The role of ferroptosis in ionizing radiation-induced cell death and tumor suppression. Cell Res. 2020;30:146-62
186. Tsvetkov P, Coy S, Petrova B, Dreishpoon M, Verma A, Abdusamad M. et al. Copper induces cell death by targeting lipoylated TCA cycle proteins. Science. 2022;375:1254-61
187. Yang M, Wu X, Hu J, Wang Y, Wang Y, Zhang L. et al. COMMD10 inhibits HIF1α/CP loop to enhance ferroptosis and radiosensitivity by disrupting Cu-Fe balance in hepatocellular carcinoma. J Hepatol. 2022;76:1138-50
188. Rao E, Hou Y, Huang X, Wang L, Wang J, Zheng W. et al. All-trans retinoic acid overcomes solid tumor radioresistance by inducing inflammatory macrophages. Sci Immunol. 2021 6
189. Huang W, Zhang L, Yang M, Wu X, Wang X, Huang W. et al. Cancer-associated fibroblasts promote the survival of irradiated nasopharyngeal carcinoma cells via the NF-κB pathway. J Exp Clin Cancer Res. 2021;40:87
190. Li Q, Zong Y, Li K, Jie X, Hong J, Zhou X. et al. Involvement of endothelial CK2 in the radiation induced perivascular resistant niche (PVRN) and the induction of radioresistance for non-small cell lung cancer (NSCLC) cells. Biol Res. 2019;52:22
191. Fang Y, Zhan Y, Xie Y, Du S, Chen Y, Zeng Z. et al. Integration of glucose and cardiolipin anabolism confers radiation resistance of HCC. Hepatology. 2022;75:1386-401
192. Faubert B, Solmonson A, DeBerardinis RJ. Metabolic reprogramming and cancer progression. Science. 2020 368
193. Kim BM, Won J, Maeng KA, Han YS, Yun YS, Hong SH. Nimesulide, a selective COX-2 inhibitor, acts synergistically with ionizing radiation against A549 human lung cancer cells through the activation of caspase-8 and caspase-3. Int J Oncol. 2009;34:1467-73
194. Shin YK, Park JS, Kim HS, Jun HJ, Kim GE, Suh CO. et al. Radiosensitivity enhancement by celecoxib, a cyclooxygenase (COX)-2 selective inhibitor, via COX-2-dependent cell cycle regulation on human cancer cells expressing differential COX-2 levels. Cancer Res. 2005;65:9501-9
195. Zhang P, Song E, Jiang M, Song Y. Celecoxib and Afatinib synergistic enhance radiotherapy sensitivity on human non-small cell lung cancer A549 cells. Int J Radiat Biol. 2021;97:170-8
196. Chen YA, Shih HW, Lin YC, Hsu HY, Wu TF, Tsai CH. et al. Simvastatin Sensitizes Radioresistant Prostate Cancer Cells by Compromising DNA Double-Strand Break Repair. Front Pharmacol. 2018;9:600
Author contact
![]() Corresponding authors: Shengfang Ge, M.D., Ph.D., Shanghai Ninth People's Hospital, Shanghai JiaoTong University School of Medicine, Shanghai, 200025, P.R. China, E-mail: geshengfangedu.cn. Yun Su, M.D., Ph.D., Shanghai Ninth People's Hospital, Shanghai JiaoTong University School of Medicine, Shanghai, 200025, P.R. China, E-mail: suyun_sycom. Xianqun Fan, M.D., Ph.D., Shanghai Ninth People's Hospital, Shanghai JiaoTong University School of Medicine, Shanghai, 200025, P.R. China, E-mail: fanxqedu.cn
Corresponding authors: Shengfang Ge, M.D., Ph.D., Shanghai Ninth People's Hospital, Shanghai JiaoTong University School of Medicine, Shanghai, 200025, P.R. China, E-mail: geshengfangedu.cn. Yun Su, M.D., Ph.D., Shanghai Ninth People's Hospital, Shanghai JiaoTong University School of Medicine, Shanghai, 200025, P.R. China, E-mail: suyun_sycom. Xianqun Fan, M.D., Ph.D., Shanghai Ninth People's Hospital, Shanghai JiaoTong University School of Medicine, Shanghai, 200025, P.R. China, E-mail: fanxqedu.cn

 Global reach, higher impact
Global reach, higher impact