10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2023; 19(5):1352-1368. doi:10.7150/ijbs.79022 This issue Cite
Research Paper
Neutrophils promote tumor invasion via FAM3C-mediated epithelial-to-mesenchymal transition in gastric cancer
1. Department of Surgical Oncology, Jiangsu Province Hospital of Chinese Medicine, Affiliated Hospital of Nanjing University of Chinese Medicine; Nanjing 210029, China.
2. Department of Pathology, Jiangsu Province Hospital of Chinese Medicine, Affiliated Hospital of Nanjing University of Chinese Medicine; Nanjing 210029, China.
3. Digestive Endoscopy Center, Jiangsu Province Hospital of Chinese Medicine, Affiliated Hospital of Nanjing University of Chinese Medicine; Nanjing 210029, China.
4. Department of Geriatrics, the Second Affiliated Hospital, Nanjing Medical University; Nanjing 210003, China.
5. Department of General Surgery, the First Affiliated Hospital, Nanjing Medical University; Nanjing 210029, China.
* These authors have contributed equally to this work.
Abstract
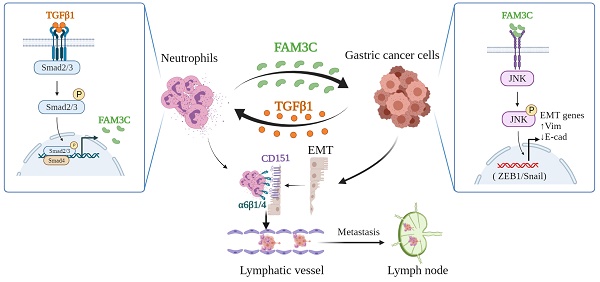
In gastric cancer, lymph node metastasis (LNM) is the major metastasis route, and lymphatic invasion is the precursor of LNM. Tumor-associated neutrophils (TANs) promote LNM. However, the molecular mechanisms underlying TANs-mediated lymphatic invasion and/or LNM remain unclear. Herein, we revealed that high level of TANs was the independent risk factor for lymphatic invasion and LNM respectively, and lymphatic tumor cell-neutrophil clusters were positively correlated with LNM. Crosstalk between neutrophils and tumor cells was required for enhanced tumor cell invasiveness, endowing neutrophils to boost epithelial-to-mesenchymal transition (EMT) of tumor cells and in turn promoting LNM. Mechanically, tumor cells educated neutrophils via TGFβ1 to produce more FAM3C through Smad2/3 signaling activation, and FAM3C promoted tumor cell EMT through JNK-ZEB1/Snail signaling pathway. The crosstalk enhanced the affinity of neutrophils with tumor cells through interaction of integrins α6β1 and α6β4 with CD151. Furthermore, studies using tumor-bearing mice demonstrated that neutrophils were the important driver for gastric cancer tumorigenesis and invasiveness. The study clearly identifies the functional roles of TANs in promoting tumor invasion, and facilitates a better understanding of novel mechanisms responsible for LNM of gastric cancer, which provides potential targets for developing new strategies to prevent or treat LNM in gastric cancer.
Keywords: Gastric cancer, Tumor-associated neutrophils, Lymph node metastasis, Epithelial-to-mesenchymal transformation, FAM3C.

 Global reach, higher impact
Global reach, higher impact