10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2023; 19(6):1875-1893. doi:10.7150/ijbs.80605 This issue Cite
Research Paper
NLRP3 Inflammasome Mediates Silica-induced Lung Epithelial Injury and Aberrant Regeneration in Lung Stem/Progenitor Cell-derived Organotypic Models
Department of Pulmonary & Critical Care Medicine, The First Affiliated Hospital of Nanjing Medical University, Nanjing, Jiangsu 210029, P.R. China
†These authors contributed equally to this work.
Abstract
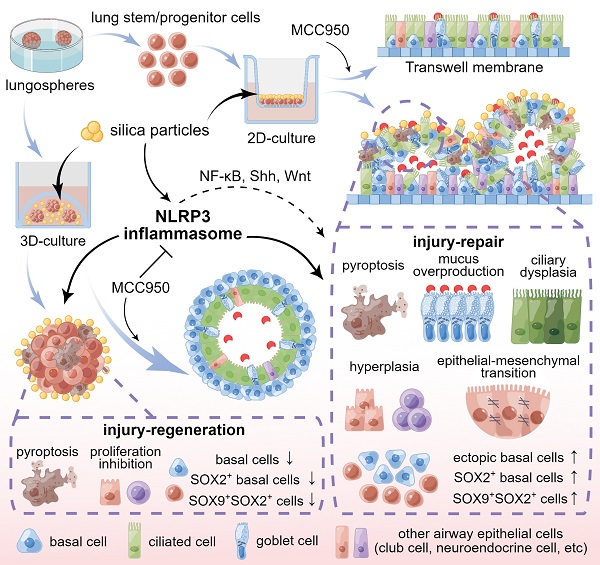
Silica-induced lung epithelial injury and fibrosis are vital pathogeneses of silicosis. Although the NOD-like receptor protein 3 (NLRP3) inflammasome contributes to silica-induced chronic lung inflammation, its role in epithelial injury and regeneration remains unclear. Here, using mouse lung stem/progenitor cell-derived organotypic systems, including 2D air-liquid interface and 3D organoid cultures, we investigated the effects of the NLRP3 inflammasome on airway epithelial phenotype and function, cellular injury and regeneration, and the potential mechanisms. Our data showed that silica-induced NLRP3 inflammasome activation disrupted the epithelial architecture, impaired mucociliary clearance, induced cellular hyperplasia and the epithelial-mesenchymal transition in 2D culture, and inhibited organoid development in 3D system. Moreover, abnormal expression of the stem/progenitor cell markers SOX2 and SOX9 was observed in the 2D and 3D organotypic models after sustained silica stimulation. Notably, these silica-induced structural and functional abnormalities were ameliorated by MCC950, a selective NLRP3 inflammasome inhibitor. Further studies indicated that the NF-κB, Shh-Gli and Wnt/β-catenin pathways were involved in NLRP3 inflammasome-mediated abnormal differentiation and dysfunction of the airway epithelium. Thus, prolonged NLRP3 inflammasome activation caused injury and aberrant lung epithelial regeneration, suggesting that the NLRP3 inflammasome is a pivotal target for regulating tissue repair in chronic inflammatory lung diseases.
Keywords: NLRP3 inflammasome, Lung stem/progenitor cells (LSPCs), Organoids, Repair, Regeneration

 Global reach, higher impact
Global reach, higher impact