10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2023; 19(6):1894-1909. doi:10.7150/ijbs.76756 This issue Cite
Research Paper
PYGL-mediated glucose metabolism reprogramming promotes EMT phenotype and metastasis of pancreatic cancer
1. Department of Radiation Oncology, Institute of Oncology, Affiliated Hospital of Jiangsu University, Zhenjiang 212001, China.
2. Department of Pancreatic surgery, Huashan Hospital, Fudan University, Shanghai 200040, China.
3. Department of General Surgery, Hepato-biliary-pancreatic Center, Huadong Hospital, Fudan University, Shanghai 200040, China.
4. Department of Burn and Plastic Surgery, Affiliated Hospital of Jiangsu University, Zhenjiang 212001, China.
5. Department of Oncology, Shanghai East Hospital, School of Medicine, Tongji University, Shanghai 200123, China.
6. Jiangsu Key Laboratory of Medical Science and Laboratory Medicine, School of Medicine, Jiangsu University, Zhenjiang 212013, China.
*These authors contributed equally to this work.
Abstract
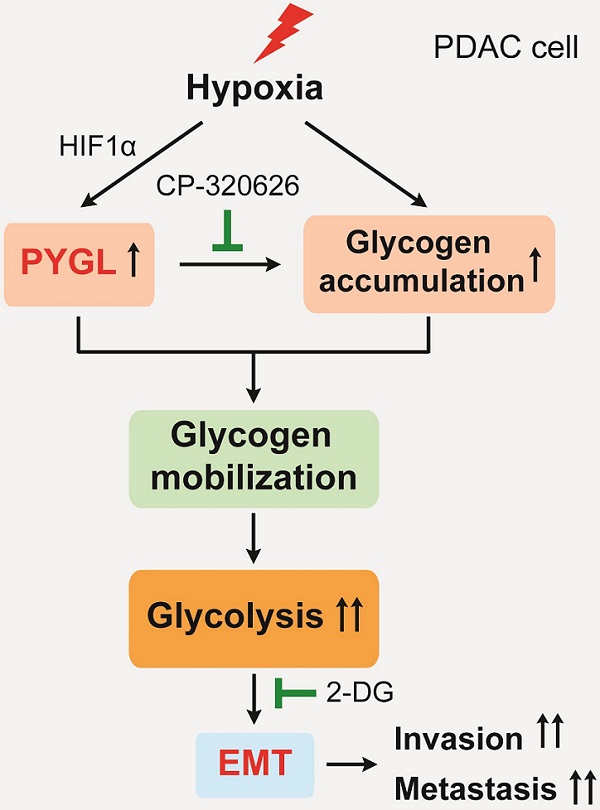
Epithelial-mesenchymal transition (EMT) is closely associated with tumor invasion and metastasis. However, key regulators of EMT in pancreatic ductal adenocarcinoma (PDAC) need to be further studied. Bioinformatics analyses of pancreatic cancer public datasets showed that glycogen phosphorylase L (PYGL) expression is elevated in quasimesenchymal PDAC (QM-PDAC) and positively associated with EMT. In vitro cellular experiments further confirm PYGL as a crucial EMT regulator in PDAC cells. Functionally, PYGL overexpression promotes cell migration and invasion in vitro and facilitates liver metastasis in vivo, while PYGL knockdown has opposite effects. Mechanically, hypoxia induces PYGL expression in a hypoxia inducible factor 1α (HIF1α)-dependent manner and promotes glycogen accumulation. Elevated PYGL mobilizes accumulated glycogen to fuel glycolysis via its activity as a glycogen phosphorylase, thus inducing the EMT process, which could be suppressed by the glycolysis inhibitor 2-deoxy-D-glucose (2-DG). Clinically, PYGL expression is upregulated in PDAC and correlates with its malignant features and poor prognosis. Collectively, the data from our study reveal that the hypoxia/PYGL/glycolysis-induced EMT promotes PDAC metastasis, which establishes the rational for targeting hypoxia/PYGL/glycolysis/EMT signaling pathway against PDAC.
Keywords: PDAC, Hypoxia, PYGL, Glucose metabolism reprogramming, EMT, Metastasis

 Global reach, higher impact
Global reach, higher impact