10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2023; 19(8):2428-2442. doi:10.7150/ijbs.82776 This issue Cite
Review
Current Status in Rechallenge of Immunotherapy
1. Department of Medical Oncology, Sichuan Clinical Research Center for Cancer, Sichuan Cancer Hospital & Institute, Sichuan Cancer Center, Affliated Cancer Hospital of University of Electronic Science and Technology of China, Chengdu, China.
2. Division of Nutritional Medicine, Sichuan Clinical Research Center for Cancer, Sichuan Cancer Hospital & Institute, Sichuan Cancer Center, Affliated Cancer Hospital of University of Electronic Science and Technology of China, Chengdu, China.
3. Department of Radiotherapy, Sichuan Clinical Research Center for Cancer, Sichuan Cancer Hospital & Institute, Sichuan Cancer Center, Affliated Cancer Hospital of University of Electronic Science and Technology of China, Chengdu, China.
Received 2023-4-14; Accepted 2023-4-24; Published 2023-5-7
Abstract
The treatment of malignant tumors has entered the era of immunotherapy, and immune checkpoint inhibitors (ICIs) have brought significant benefits to patients. However, some patients are required to discontinue treatment with ICIs owing to factors such as disease progression and intolerable side effects. Faced with limited subsequent treatment options and complex medical needs, we searched PubMed, Embase, Cochrane library, and the NIH clinical trials database and found that ICI rechallenge could be a relevant clinical strategy. The factors that could affect the rechallenge efficacy include the patients' characteristics, therapeutic strategy selection, and the timing of treatment. Multiple factors are used to identify target population, of which clinical features and PD-L1 expression are more potential. Both single ICI rechallenge and combination therapy may have survival benefits. Patients who have tolerated initial immunotherapy well could undergo ICI rechallenge, while patients who have experienced grade 3 or higher immune-related adverse events should be carefully assessed prior to rechallenge. Interventions and the interval between two courses of ICI will clearly have an impact on the efficacy of subsequent treatment. Preliminary data evaluation supports further investigation on ICI rechallenge to identify the factors that could contribute to its efficacy.
Keywords: cancers, immune checkpoint inhibitors, rechallenge, target population, strategy, safety
Introduction
The incidence and mortality of patients with malignant tumors is increasing, and the public health burden of cancer treatment is increasing accordingly [1-4]. There were 18.1 million new cancer cases and 9.6 million cancer deaths worldwide in 2018 [5], and the numbers increased to 19.3 million and 9.96 million, respectively, in 2020 [6]. Cancer is considered one of the major health threats to humans. However, cancer therapy has advanced significantly in the last few decades, including the recent development of immunotherapy, which has long-term benefits for some patients with cancer [7-9].
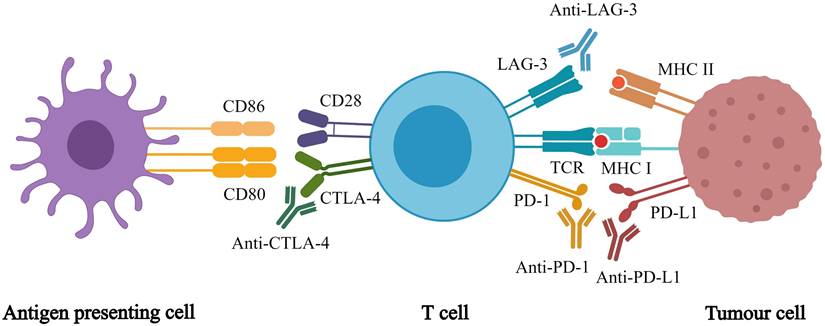
Immunotherapy enhances the immune system's tumor recognition, blocks the immunosuppressive signals from the tumor cell, and weakens the immunosuppressive nature of the tumor microenvironment [10]. Compared with traditional anti-tumor therapy, immunotherapies have incomparable advantages, which can prolong progression free survival (PFS) and overall survival (OS) [11]. While many studies are being conducted to discover new immunotherapies, immune checkpoint inhibitors (ICIs) are currently the most effective cancer immunotherapy [12]. ICIs are monoclonal antibodies that target inhibitory immune checkpoints, which are exploited by cancer cells to escape the immune system. Approved ICIs include inhibitors of [13] programmed cell death 1 (PD-1), programmed cell death ligand 1 (PD-L1), cytotoxic T lymphocyte antigen 4 (CTLA-4), and lymphocyte activation gene 3 (Figure 1). ICI monotherapy or in combination with other anti-cancer treatments improves anti-tumor efficacy for many cancer types, which is effective in the treatment of malignant tumors [14-17]. Meanwhile, immunotherapy introduces new problems and challenges. For example, ICIs only work for a few patients, and there is a lack of biomarkers to identify them. Currently, the level of PD-L1 expression is used but it has several limitations [18-19]. In addition, clinicians have many unanswered questions, such as how to determine the treatment regimen and whether to use monotherapy or combination therapy. Moreover, if combination therapy is chosen, there are still questions regarding which drugs should be combined. In addition, from a clinical standpoint, how can immunotoxicity be predicted? To maximize the application of ICIs, many studies must be conducted to answer these questions.
Different immune checkpoint inhibitors and their respective targets. Immune checkpoint inhibitors act on different targets to enhance T cell responses, which in turn promote anti-tumor effects of whole-body.
When employing ICI therapy for tumors, we found that some patients have to discontinue ICI treatment owing to immune-related adverse events (irAEs) or progressive disease (PD). If the subsequent therapy option is limited for those patients, should or can the clinicians order another course of ICI treatment (rechallenge)? We considered this a viable option because of the long-term benefits of immunotherapy. Some studies have revealed that ICI rechallenge can be beneficial to some patients [20-27]. In this review, we summarized literature data on ICI rechallenge, and hope to contribute to standardizing ICI rechallenge clinical protocols.
Immunotherapy rechallenge/retreatment
Definition
There are two protocols for restarting immunotherapy: retreatment and rechallenge. ICI retreatment refers to the clinical strategy whereby patients restart ICI treatment without any other cancer treatments in between [28-31], whereas the ICI rechallenge strategy involves other treatments between the two ICI courses. This is an important distinction because the additional treatments can influence the homeostasis of the patients' immune system, resulting in the need for a second course of immunotherapy. We focused on ICI rechallenge in this review because it is more broadly used [32-33].
Significance
Cancer treatment has entered the era of immunotherapy. Patients with advanced cancers can particularly benefit from it. Nevertheless, treatment resistance and tumor progression can still happen over time. When these happen, clinical research indicates that the reuse of immunotherapy could still benefit patients, and it may be a better option than conventional cancer therapies, such as chemotherapy and radiation therapy. Immunotherapy has additional advantages such as low side effects, which allows patients to maintain a higher quality of life. Furthermore, ICIs are affordable in China, making it a viable option for many patients. On the other hand, whilst a profound number of patients are being treated with ICIs, more knowledge is needed to maximize their benefits.
Current guidance
Current studies on ICI rechallenge are sparse. The National Comprehensive Cancer Network, the European Society for Medical Oncology, and the Society for Immunotherapy of Cancer recommend immunotherapy rechallenge for melanoma treatment; however, they have no consensus for the timing [34-37]. A few guidelines recommend immunotherapy rechallenge for renal cancer and head and neck squamous cell carcinoma, but the data were insufficient to support them. Lung cancer is the second most common cancer and the leading cause of cancer death [38-39]. ICI treatment significantly improved the prognosis of lung cancer in patients. However, no guidelines have been published for lung cancer ICI rechallenge. We investigated the lung cancer subgroups of recent publications on ICI rechallenge, and our findings suggest that some patients with lung cancer benefited from ICI rechallenge. Notably, the sample sizes from these subgroups were not large enough to statistically conclude the benefit of ICI rechallenge in patients with lung cancer. Based on the available data, we believe that it is worthwhile to study ICI rechallenge regarding lung cancer.
We list some of the ongoing clinical trials of ICI rechallenge in Tables 1 and 2. Most of them are Phase II clinical trials using ICI rechallenge to treat lung cancer, malignant melanoma, and urinary system tumors. The ICI rechallenge strategy includes using a single ICI, two ICIs, and an ICI combined with other anti-tumor medications. We want to point out that most studies do not focus on ICI rechallenge; rather, ICI rechallenge was used as one of the treatment options, and it was used independently or in combination therapies. Moreover, the decision whether to use other anti-tumor treatments between two courses of ICIs varied, and the primary endpoints were efficacy or safety. In other words, the information solely on ICI rechallenge is limited. In this review, we highlighted the data in an attempt to spark interest in the study of ICI rechallenge.
Ongoing ICI rechallenge clinical trials related to lung cancer
| Cancer type | Prior ICI | Rechallenge regimen | Endpoints | Phase | Trial |
|---|---|---|---|---|---|
| NSCLC | Nivolumab+Ipilimumab | Nivolumab+Ipilimumab | PFS | III | NCT03469960 |
| NSCLC | ICI | Nivolumab+Anlotinib | ORR | Ib/IIa | NCT04507906 |
| NSCLC | Anti-PD-1 | Atezolizumab+platinum doublet chemotherapy | ORR | II | NCT03977467 |
| NSCLC | ICI | Atezolizumab+Tocilizumab | ORR | Ib/II | NCT04691817 |
| NSCLC | ICI | Atezolizumab+Ramucirumab | ORR | II | NCT03689855 |
| NSCLC | Anti-PD-(L)1 | Camrelizumab+Apatinib | PFS | II | NCT04670913 |
| NSCLC | Anti-PD-(L)1 | Camrelizumab+famitinib | OS | III | NCT05106335 |
| NSCLC | Anti-PD-(L)1 | Pembrolizumab | ORR | II | NCT03526887 |
| NSCLC | Anti-PD-(L)1 | Pembrolizumab+Docetaxel/Pemetrexed/Gemcitabine | PFS | II | NCT03083808 |
| NSCLC | Anti-PD-(L)1 | Durvalumab | ORR | II | NCT03334617 |
| SCLC | Anti-PD-(L)1 | Durvalumab+Topotecan hydrochloride | OS | II | NCT04607954 |
Ongoing ICI rechallenge clinical trials related to other cancers
| Cancer type | Prior ICI | Rechallenge regimen | Endpoints | Phase | Trial |
|---|---|---|---|---|---|
| Melanoma | Anti-PD-(L)1 | Pembrolizumab+Ipilimumab | ORR | II | NCT02743819 |
| Melanoma | Anti-PD-1±Ipilimumab | Pembrolizumab+4SC-202 | safety | Ib/II | NCT03278665 |
| HCC | ICI | Camrelizumab+Apatinib | ORR | II | NCT04826406 |
| HCC | ICI | Sintilimab+Lenvatinib | ORR | II | NCT05010681 |
| HCC | Anti-PD-(L)1 | Pembrolizumab+Regorafenib | ORR | II | NCT04696055 |
| GC/CRC | Anti-PD-(L)1 | Tislelizumab+Anlotinib | ORR | II | NCT04777162 |
| UC | ICI | Same ICI | Efficiency | II | NCT04322643 |
| UC | Anti-PD-(L)1 | Atezolizumab+Carboplatin+Gemcitabine | PFS | II | NCT03737123 |
| TCC | ICI | Pembrolizumab+Ramucirumab | ORR | II | NCT04179110 |
| NPC | Anti-PD-(L)1 | Sintilimab+IBI310 | ORR | Ib/II | NCT04945421 |
| RCC | Nivolumab | Nivolumab+Ipilimumab | ORR | II | NCT03177239 |
| RCC | Nivolumab+Ipilimumab | Nivolumab+Ipilimumab | ORR | II | NCT03126331 |
| RCC | Nivolumab+Ipilimumab | Nivolumab+Ipilimumab | DCR | II | NCT04088500 |
| RCC | Anti-PD-(L)1 | Atezolizumab+Cabozantinib | PFS/OS | III | NCT04338269 |
| SCCHN | Anti-PD-1 | Pembrolizumab+Radiation | ORR | II | NCT03085719 |
| Solid tumor | Durvalumab | Durvalumab | safety | II | NCT03847649 |
| Solid tumor | Anti-PD-(L)1 | Pembrolizumab+BI 1206 | safety | I/IIa | NCT04219254 |
Table 1 and Table 2 NSCLC, non-small-cell lung cancer; SCLC, small-cell lung cancer; HCC, hepatocellular carcinoma; GC, gastric cancer; CRC, colorectal cancer; UC, urothelial carcinoma; TCC, transitional cell carcinoma; NPC, nasopharyngeal carcinoma; RCC, renal cell carcinoma; SCCHN, squamous cell carcinoma of the head and neck; ICI, immune checkpoint inhibitor; ORR, objective response rate; DCR, disease control rate; PFS, progression free survival; OS, overall survival.
The current research status
ICI rechallenge target population
Currently, ICIs can only benefit small patient populations, and how to identify them has received increasing interest. PD-L1 expression level is the primary choice [40-41]. The same applies to ICI rechallenge; however, clinicians are also aware of its limitations. No conclusion has been reached to identify patients who can benefit from ICI rechallenge, and we believe this should be a priority for ICI rechallenge clinical studies. Meanwhile, we summarize the main inclusion and exclusion criteria from the ongoing studies in Tables 3 and 4.
Clinical features
Patient's general condition
ICI treatment outcomes vary greatly among patients. The main contributing factors include Eastern Cooperative Oncology Group performance status (ECOG/PS) and nutritional status. In a retrospective study [42], 35 patients with advanced non-small cell lung cancer (NSCLC) were retreated with ICIs after pausing the initial ICI treatment, owing to disease progression. The median PFS and OS were 81 days (95% confidence interval [CI] 41-112 days) and 225 days (95% CI 106-361 days), respectively. Multivariate analysis showed that an ECOG/PS score ≥2 (hazard ratio [HR] 2.38; 95% CI 1.03-5.52; p=0.043) was negatively associated with PFS, while body mass index (BMI) >20 (HR 0.43; 95% CI 0.19-0.95; p=0.036) was positively associated with PFS. Therefore, the researchers proposed that ECOG/PS score and BMI could be evaluation factors to decide if patients should receive ICI rechallenge. In another study, Gobbini et al. [43] analyzed the outcome of ICI rechallenge in 144 patients with advanced NSCLC and found that patients with ECOG/PS score of 0, 1, and ≥2 have a median OS of NR (95% CI 2.1-not reached), 1.4 years (95% CI 0.2-2.1), and 1.1 years (95% CI 0.7-1.6), respectively. These studies suggest that patients with NSCLC who had better ECOG/PS scores (ECOG/PS ≤1) could benefit from ICI rechallenge. We suspect that this correlation is linked to treatment tolerance. Patients with better physical health can tolerate ICI rechallenge better, and the ICI efficacy is correlated with the clinical treatment duration. We need to point out that physical fitness is subjective; we would like to see clinical studies to list both patient fitness data and ICI rechallenge tolerance data.
The main inclusion and exclusion criteria from the ongoing clinical trials on lung cancer
| Trial | Main inclusion criteria | Main exclusion criteria | ||||||
|---|---|---|---|---|---|---|---|---|
| Cancer type | ECOG/KPS | PD-L1 | Prior treatment | irAE | Metastases | Driver mutation | Other | |
| NCT03469960 | stage IV NSCLC | 0-1 | ≥1% | PD on/after prior ICI | Grade ≥3 | active untreated brain | EGFR/ALK/ROS1/HER | - |
| NCT04507906 | stage IIIB/IIIC/IV NSCLC | 0-1 | - | PD on/after prior ICI | Grade ≥3 | active untreated brain | EGFR/ALK/ROS1/unknown | obvious hemorrhage symptom |
| NCT03977467 | advanced NSCLC | 0-2 | ≥1% | PD after initial disease control of prior ICI | Grade ≥2 | active untreated brain | EGFR/ALK/ROS1 | uncontrolled tumor-related pain |
| NCT04691817 | stage IV or recurrent NSCLC | 0-2 | - | PD on/after prior ICI | Grade ≥3 | active untreated brain | susceptible to targeted therapy | uncontrolled tumor-related pain |
| NCT03689855 | NSCLC | 0-1 | - | PD on/after prior ICI | Grade ≥3 | active untreated brain | EGFR/ALK but not treated | - |
| NCT04670913 | stage IV or recurrent NSCLC | 0-1 | - | SD ≥3 months with the first immunotherapy | Grade ≥2 | active untreated brain | EGFR/ALK | squamous cell NSCLC |
| NCT05106335 | metastatic or recurrent NSCLC | 0-1 | - | PD on/after prior anti-PD-(L)1 | - | - | - | uncontrolled pleural effusion or ascites |
| NCT03526887 | recurrent NSCLC | 0-1 | ≥1% | PD >12 weeks after the last dose of prior ICI | Grade ≥3 | active untreated brain | EGFR/ALK | - |
| NCT03083808 | stage IV NSCLC | 0-1 | - | PFS ≥3 months with the first immunotherapy | Grade ≥2 | active untreated brain | EGFR/ALK/ROS1 but not treated | - |
| NCT03334617 | metastatic or recurrent NSCLC | 0-1 | - | PD on prior anti-PD-(L)1 | - | symptomatic brain | EGFR/ALK/ROS1/BRAF/MET/RET | - |
| NCT04607954 | SCLC | 0-1 | - | PD after prior anti-PD-(L)1 | Grade ≥3 | active untreated brain | - | uncontrolled intercurrent illness |
The main inclusion and exclusion criteria from the ongoing clinical trials on other cancers
| Trial | Main inclusion criteria | Main exclusion criteria | ||||||
|---|---|---|---|---|---|---|---|---|
| Cancer type | ECOG/KPS | PD-L1 | Prior treatment | irAE | Metastases | Targeted therapy | Other | |
| NCT02743819 | advanced melanoma | 0-1 | - | SD ≥24 weeks with first-line anti-PD-(L)1 | - | active untreated brain | - | active pneumonitis or active infection |
| NCT03278665 | unresectable stage III/IV melanoma | - | - | non-responding to prior anti-PD-1 | - | symptomatic brain | - | CR or PR on/after prior ICI |
| NCT04826406 | HCC | 0-1 | - | PD after 2 cycles of prior ICI | intolerable | central nervous system | Apatinib | hepatic encephalopathy or symptomatic ascites |
| NCT05010681 | unresectable or metastatic HCC | 0-2 | - | PD on/after prior anti-PD-(L)1 | - | central nervous system | Lenvatinib | gastrointestinal bleeding |
| NCT04696055 | unresectable advanced HCC | 0-1 | - | SD ≥8 weeks with first-line anti-PD-(L)1 | Grade ≥2 | active central nervous system | Regorafenib | pleural effusion or ascites |
| NCT04777162 | unresectable or metastatic GC/CRC | 0-1 | ≥1% | CR or PR with first-line anti-PD-(L)1 | severe | brain | Anlotinib | uncontrolled pleural effusion |
| NCT04322643 | advanced or metastatic UC | ≥70 | - | SD ≥24 weeks with the first immunotherapy | - | - | - | serious medical condition |
| NCT03737123 | metastatic or unresectable UC | 0-2 | - | PD after prior anti-PD-(L)1 | Grade ≥2 | active central nervous system | - | - |
| NCT04179110 | metastatic or unresectable TCC | 0-1 | - | PD on/after prior ICI | - | brain | VEGF/VEGFR targeting drug | uncontrolled pleural effusion or ascites |
| NCT04945421 | metastatic or recurrent NPC | 0-1 | - | Failed to prior Anti-PD-1 resistance | - | - | - | uncontrolled life-threatening illness |
| NCT03177239 | unresectable or metastatic RCC | 0-1 | - | PD on/after prior ICI | - | untreated brain | - | - |
| NCT03126331 | advanced or metastatic RCC | ≥70 | - | CR, PR or SD after 24 weeks of nivolumab | - | active untreated brain | - | serious medical condition |
| NCT04088500 | advanced RCC | - | - | PD of maintenance treatment of nivolumab | - | active central nervous system | - | - |
| NCT04338269 | advanced or metastatic RCC | ≥70 | - | PD on/after prior ICI | - | active untreated brain | Cabozantinib | uncontrolled pleural effusion or ascites |
| NCT03085719 | metastatic SCCHN | 0-1 | - | CR, PR or SD after 6 cycles of anti-PD-1 | intolerable | active untreated brain | - | uncontrolled intercurrent illness |
| NCT03847649 | advanced solid tumor | 0-1 | - | SD ≥8 weeks with first-line durvalumab | Grade ≥3 | symptomatic brain | - | - |
| NCT04219254 | advanced solid tumor | 0-1 | - | PD <12 weeks after prior anti-PD-(L)1 | Grade ≥3 | active central nervous system | - | serious medical condition |
Table 3 and Table 4 NSCLC, non-small-cell lung cancer; SCLC, small-cell lung cancer; HCC, hepatocellular carcinoma; GC, gastric cancer; CRC, colorectal cancer; UC, urothelial carcinoma; TCC, transitional cell carcinoma; NPC, nasopharyngeal carcinoma; RCC, renal cell carcinoma; SCCHN, squamous cell carcinoma of the head and neck; ECOG, Eastern Cooperative Oncology Group; KPS, Karnofsky Performance Status; PD-1, programmed cell death 1; PD-L1, programmed cell death ligand 1; CR, complete remission; PR, partial remission; SD, stable disease; PD, progressive disease; ICI, immune checkpoint inhibitor; PFS, progression free survival; irAE, immune-related adverse event.
Initial immunotherapy
Researchers have investigated the relationship between ICI rechallenge clinical outcomes and initial immunotherapy. Levra et al. [44] evaluated 1,517 patients who received ICI rechallenge in the French national hospital database of 10,452 patients with locally advanced or metastatic NSCLC. Between the initial ICI treatments and rechallenge, 1,127 patients did not receive any therapy, whilst 390 patients received chemotherapy. Among the 1,127 patients, Levra found that the median OS increased for patients who received the initial ICI treatment for ≥6 months (HR 0.19; 95% CI 0.14-0.25; p<0.0001) and 3-6 months (HR 0.56; 95% CI 0.46-0.70; p<0.0001) compared with patients who received the initial treatment for <3 months. Therefore, patients' ICI rechallenge outcome is positively correlated with the length of their initial treatment. One hypothesis states that longer initial immunotherapy strengthens the immune memory. Another hypothesis states that patients with longer initial treatment are the dominant population for immunotherapy. Vauchier et al. [45] analyzed the data of 45 patients with metastatic renal cell carcinoma in a multicenter study. Patients with a PFS of 6-12 months (HR 0.55; 95% CI 0.17-1.78; p=0.07) and >12 months (HR 0.25; 95% CI 0.08-0.84; p=0.07) responded better to their initial ICI treatment than patients with a PFS ≤6 months. A melanoma study with a small sample size also revealed similar results [46]. In addition, Niki et al. [47] conducted a retrospective study and found that four out of five patients with advanced NSCLC who responded to ICI rechallenge also responded to their initial ICI therapy. In contrast, a retrospective study by Santini et al. [20] showed that of 482 patients with NSCLC who received ICI treatment, 20 achieved a partial response (PR) for the initial ICI therapy; however, the treatment was stopped owing to irAEs, PFS (HR 0.68; 95% CI 0.19-2.24; p=0.56) and OS (HR 0.37; 95% CI 0.06-2.21; p=0.28), which were similar between the rechallenge (N=12) and control (N=8) groups. Another study revealed a similar outcome for patients with advanced melanoma treated with nivolumab and ipilimumab [48]. This suggests that the initial ICI treatment outcome cannot be used as the sole indicator to predict ICI rechallenge outcome, particularly when severe irAEs occur during treatment. Summarily, initial ICI therapy efficacy can be a good indicator for ICI rechallenge; however, it should not be the sole consideration.
Interruptive reasons for immunotherapy
Most initial immunotherapy was discontinued abruptly for two reasons: irAEs or PD. Reschke et al. [49] analyzed the data of 570 patients with advanced melanoma from different studies who paused their initial ICI treatments and found that the most common cause as PD (381/570, 67%), followed by severe irAEs (189/570, 33%). Patients (N=85) who received anti-PD-1 antibody rechallenge following PD during their initial anti-PD-1 therapy had a mean disease control rate (DCR) of 45.8%, mean objective response rate (ORR) of 15.5%, and mean PFS of >8.2 months. Patients (N=114) who received anti-PD-1 and anti-CTLA-4 antibody rechallenge had a mean DCR of 40.6% and a mean ORR of 20%. In addition, patients (N=182) rechallenged with anti-CTLA-4 antibody following PD during their initial anti-CTLA-4 therapy had a mean DCR of 50.9% and a mean ORR of 20.4%. Meanwhile, patients (N=189) who received anti-PD-1 antibody rechallenge following toxicity-related treatment discontinuation had a mean DCR of 89.5%, a mean ORR of 70.2%, and a mean PFS of >7.4 months. This indicates that ICI rechallenge could benefit patients who paused their initial immunotherapy regardless of the cause. Two separate cohorts have a significantly different DCR and ORR but a similar PFS. The reason behind this phenomenon is worth exploring. The meta-analysis by Inno et al. [50] found similar outcomes. The overall response rate of ICI rechallenge was 21.8% regardless of the reason for pausing the initial treatments. This is important because it shows that ICI rechallenge could translate into survival benefits for patients who did not finish their initial course of ICI treatment. From the data, we hypothesize that ICI rechallenge might restore some treatment benefits that were lost owing to the incompletion of the initial treatment. Sheth et al. [51] provided partial data for patients (N=168) with advanced solid tumors who completed 1 year of initial durvalumab treatment and then restarted (N=70) after tumor relapse. More than half of the 70 patients achieved disease control (eight patients had PR, 42 had stable disease [SD]), and four had ≥ grade 3 irAEs. The retrospective study by Gobbini et al. [43] agrees with these outcomes. The PFS (irAE: HR 0.54, 95% CI 0.33-0.86; clinical decision: HR 0.63, 95% CI 0.37-1.07) and OS (irAE: HR 0.44, 95% CI 0.23-0.82; clinical decision: HR 0.61, 95% CI 0.23-0.82) of rechallenged patients who were suspended prior to ICI rechallenge owing to irAEs or clinical decisions were prolonged compared with those with PD. Taken together, the reasons for pausing the initial ICI treatment might affect the rechallenge outcome; however, there are different tendencies. If patients discontinued ICI therapy owing to irAEs, clinicians should try to design treatment regimens to avoid adverse reactions and allow patients to receive therapy for as long as possible. If patients discontinued owing to PD, different ICIs for rechallenge should also be considered, although choosing depends on more clinical trials.
Biomarkers
Immunotherapy still lacks effective biomarkers to predict efficacy. Based on clinical studies, including KEYNOTE-010, KEYNOTE-024, and CheckMate026, PD-L1 expression level and tumor mutation burden (TMB) are the most commonly used biomarkers [52-54], and the former is frequently used [55]. ICI rechallenge also lacks biomarkers, and clinicians try to use PD-L1 and TMB as substitutes. Regrettably, retesting for PD-L1 before rechallenge is still rarely achieved in the real-world. A retrospective study examined 12 patients with NSCLC who were administered pembrolizumab rechallenge after pausing initial nivolumab treatment; the authors found that all patients who showed responses (PR and SD) had high PD-L1 expression (tumor proportion score ≥80%) [56]. However, a different study analyzed 35 patients with NSCLC from six Japanese institutions who received ICI rechallenge and did not find the rechallenge efficacy to be correlated with PD-L1 expression level [42]. Therefore, it is unclear whether the PD-L1 expression level could reliably predict the outcome of ICI rechallenge. The PD-L1 expression level in many retrospective studies was only determined during the initial ICI treatment, and we do not know if the PD-L1 expression level changes following the initial immunotherapy. We believe it is important to measure before the rechallenge.
The anti-tumor immune responses are heavily influenced by the microenvironment. Therefore, researchers studied whether inflammatory indicators, such as neutrophil to lymphocyte ratio (NLR), lymphocyte to monocyte ratio (LMR), and platelet to lymphocyte ratio (PLR), could predict immunotherapy outcomes [57-59]. The aforementioned study of patients with NSCLC [42] found that PFS from ICI rechallenge correlated with a NLR ≥5 and a LMR <1.7, and the OS correlated with a PLR >262. Kan et al. [60] found that patients with advanced melanoma who achieved a PR showed an increase in the NLR during initial immunotherapy but a decrease after sequential non-ICI treatment, whereas the NLR remained unchanged in patients who did not respond. A change in the NLR might result from a fluctuating tumor microenvironment. Some inflammatory indicators may be used as reference elements for the ICI rechallenge efficacy; however, the mechanisms are unclear.
Rechallenge strategies
Single ICI rechallenge
No conclusion has been reached on whether ICI rechallenge should use the initial regimen or switch to other ICIs. Chiarion et al [61] examined 855 patients with advanced melanoma treated with ipilimumab, an anti-CTLA-4 antibody, and 51 patients underwent ipilimumab rechallenge after disease progression. Overall, 55% of the rechallenged patients achieved disease control (two cases of complete response, four cases of PR, and 22 cases of SD), and the median OS of the rechallenged group was significantly prolonged compared with the control group (21 months vs. 13 months, p<0.0001). Yang et al. [62] analyzed 22 prospective studies using ipilimumab and identified three studies (204 cases) that have ipilimumab monotherapy rechallenge data. The ORR was 12-23%, DCR was 48.4-67.7%, and median OS was 12 months. In a similar analysis of 13 studies using PD-1/PD-L1 inhibitors, six of them applied the initial drug to rechallenge, and the ORR and DCR were 11.4-53% and 47.1-83%, respectively. Several other independent studies found similar outcomes [63-64]. These data indicate that ICI rechallenge using the original regimens of CTLA-4 or PD-1/PD-L1 inhibitors can benefit patients. Furthermore, other studies found that it was safe and effective to switch to different PD-1/PD-L1 inhibitors [65-67]; however, their outcomes were inconsistent. Kitagawa et al. [22] retrospectively examined 17 patients with advanced NSCLC who were rechallenged with different PD-1/PD-L1 inhibitors, and 10 (58.8%) achieved a PR or SD. Watanabe et al. [68] reported that switching to different PD-1/PD-L1 inhibitors did not achieve clinical benefits. Martini et al.'s [69] data for two patients also revealed similar outcomes. Rechallenge using different ICIs could in theory achieve efficacy while reducing irAEs, and the inconsistency might be from the small sample size, which introduced large variations.
The current dominant types of ICIs are anti-CTLA-4 and anti-PD-1/PD-L1 antibodies, and they work through different biological pathways [70]. The CTLA-4 pathway restricts immune responses in the early stages of T cell activation, while the PD-1/PD-L1 pathway mainly limits T cell activities in the tumor microenvironment. Switching between the two for ICI rechallenge has drawn some interest [71]. Larkin et al. [72] compared patients with ipilimumab-refractory melanoma who received nivolumab or chemotherapy. They found that the former had a higher overall response rate (27% vs. 10%), longer median duration of response (31.9 vs. 12.8 months), and fewer grade 3 and 4 treatment-related adverse events (14% vs. 34%). However, the survival rates showed no difference. In the aforementioned meta-analysis by Yang et al. [62], patients with PD during the initial anti-CTLA-4 treatment had higher ORR when rechallenged with anti-PD-1 antibodies (22-36%) compared with anti-CTLA-4 antibodies (12-23%). Only two studies in this meta-analysis evaluated the efficacy of anti-CTLA-4 antibodies after PD from the initial anti-PD-1 antibody treatment. One did not observe an objective response after a median follow-up of 21.2 months; the other study reported an ORR of 22.4%. This meta-analysis revealed that anti-CTLA-4 antibody monotherapy for rechallenge has limited efficacy; therefore, anti-PD-1/PD-L1 antibodies should be used (either as monotherapy or in combination). We speculate that the systematical stimulation of T cells (through anti-CTLA-4 inhibitors) is insufficient; therefore, resolving the immunosuppression of the tumor microenvironment is also needed. In addition, anti-CTLA-4 and anti-PD-1 antibodies have different irAE profiles [73]. Clinicians could consider switching provided that the treatment efficacy is not sacrificed.
Combination therapy
Combination of ICIs
The approved ICIs activate the immune system through different mechanisms, and the US Food and Drug Administration has already approved several ICI combination treatments [74-78]. Glutsch et al. [79] described five patients with Merkel cell carcinoma refractory to avelumab that benefited from nivolumab and ipilimumab combination rechallenge after PD. Other studies reported similar outcomes for different cancers [80-81], although they were based on individual case studies. Silva et al. [82] examined 355 patients with PD-1/PD-L1-resistant advanced melanoma at 15 institutions for a median follow-up period of 22.1 months. Patients rechallenged with an anti-PD-1 antibody in combination with ipilimumab had longer OS (HR 0.50; 95% CI 0.38-0.66; p<0.0001), longer PFS (HR 0.69; 95% CI 0.55-0.87; p=0.0019), and higher ORR (31% vs. 13%, p<0.0001) than those rechallenged with ipilimumab monotherapy. In this study, patients rechallenged with a single or multiple ICIs had a similar ratio of grade 3 and 5 adverse events (31% vs. 33%). These data suggest that ICI rechallenge involving the combination of different types of ICIs was superior to using a single ICI. Studies on other cancers showed similar outcomes [83-85]; however, they lack a control group using a single ICI. Moreover, Reschke et al. [49] found that anti-PD-1 plus anti-CTLA-4 antibody dual rechallenge regimen (mean DCR: 40.6%, mean ORR: 20%) was not superior to anti-PD-1/PD-L1 monotherapy for patients who relapsed after the initial anti-PD-1 therapy. Zimmer et al. [86] found a similar outcome where 1-year OS rates were 54% and 55% for dual and mono ICI rechallenge, respectively. In addition, Ravi et al. [87] found that more patients achieved a PR or SD with mono-ICI rechallenge (N=26) than with dual-ICIs rechallenge (N=22). Summarily, dual-ICIs rechallenge which activates the anti-tumor immune response from different angles remains a fascinating concept; however, the outcome was mixed. Further large, multi-center sample studies may help to explain the findings.
Combining with non-ICI anti-cancer therapies
Chemoradiotherapy and targeted therapy are still the dominant anti-cancer treatments [88-89]. Several clinical studies are being conducted to combine these treatments with immunotherapies. This could potentially expand the patient population that can benefit from immunotherapy, which remains low. Several studies [90-91] have reported that adding chemotherapy or anti-angiogenic drugs to ICI rechallenge showed objective and durable tumor responses. A phase II study [92] assessed patients with metastatic renal clear cell carcinoma who had PD during the initial ICI treatment and were rechallenged with the combination of pembrolizumab and levatinib (an anti-VEGF inhibitor). Half of the patients had an objective response (ORR: 51%). Moreover, ICIs combined with mitogen-activated protein kinase kinase inhibitors, gonadotropin-releasing agents, and inhibitors of apoptosis protein antagonists are also being actively explored in clinical trials as second-, third- or fourth-line treatments [93-97]. Lemaire et al. [98] used the quantitative scoring methodology to help screen drugs and further discovered combinations with potential for success. This provides a novel and promising idea for future studies and utilization of ICI rechallenge. Selected ongoing clinical trials for such combination are presented in Table 5. One more issue to take into account is that the differences in drug dosages between initial immunotherapy and ICI retreatment/rechallenge lack an integrative systematic analysis. And there are also few related studies on the dose-dependent actions of subsequent ICI. But it might be worth a word to mention that the dose of subsequent ICI was essentially the same as the initial treatment from a clinical perspective. The optimal dose still requires further exploration.
Safety management
Immunotherapy is a new field compared with chemo- and radiation therapies in which the tolerance profile is largely under-explored. The safety concerns are particularly important for patients who paused the initial ICI treatments owing to irAEs [99-100]. Fujisaki et al. [101] showed that patients who paused their initial ICI treatment owing to irAEs could tolerate ICI rechallenge and also achieved improved OS (p=0.025). Bhatlapenumarthi et al. [102] identified 27 patients who received ICI rechallenge in their retrospective analysis of 465 patients with advanced solid tumors, of which 18 patients showed good tolerance (18/27). A cohort study conducted by Dolladille et al. [21] observed the recurrence rate of irAEs to be 28.8% (95% CI 24.8-33.1) for ICI rechallenge. Simonaggio et al. [103] examined rechallenged patients who experienced ≥ grade 2 irAEs during the initial ICI treatment. They found that 22 patients (55%) experienced the recurrence of irAEs; however, none of them had irAEs that were more severe than the initial ones, and the irAE onset time was delayed compared with the initial treatment (9.15 weeks vs. 15 weeks, p=0.04). Several other studies have shown that ICI rechallenge was better tolerated, even for patients who paused the initial treatments owing to irAEs [68, 104-107]. However, not all studies revealed this trend. Zhao et al. [108] performed a meta-analysis that included 789 cases from 18 cohort studies and five case series. Their findings revealed that ICI rechallenge produced equal incidences of ≥ grade 3 irAEs (p>0.05) but higher incidence in other categories (OR=3.81; 95% CI 2.15-6.74; p<0.0001). Pollack et al. [109] examined patients with advanced melanoma who paused the initial anti-PD-1 plus anti-CTLA-4 antibody regiment owing to irAEs and were then rechallenged with anti-PD-1 antibodies. Approximately 50% of them had irAEs; however, whilst most were low-grade, life-threatening cases were also observed. They also discovered that the severity of irAEs from the initial treatments could not predict the outcome of the rechallenge (p=0.9). Particularly, clinicians should pay attention to the cardiac toxicities, neurological toxicities, and any grade 4 irAEs during the rechallenge regardless of the initial adverse effects [110-112]. Some scholars believe clinicians should be cautious about applying ICI rechallenge to patients who could benefit from rechallenge but suffer from grade 3 or higher irAEs. The cost-benefit should be carefully balanced [113-114]. Currently, we cannot predict either the efficacy or irAEs of ICI rechallenge. Managing immunotoxicities should, therefore, be a high priority for clinicians, especially for patients who experienced ≥ grade 3 irAEs [115-116]. We listed partial safety data from the ICI rechallenge in Table 6.
Ongoing clinical trials of combination therapy
| Combination therapy | Cancer type | Rechallenge regimen | Endpoints | Phase | Trial |
|---|---|---|---|---|---|
| ICI+ICI | NSCLC | Nivolumab+Ipilimumab | PFS | III | NCT03469960 |
| RCC | Nivolumab+Ipilimumab | ORR | II | NCT03177239 | |
| RCC | Nivolumab+Ipilimumab | DCR | II | NCT04088500 | |
| RCC | Nivolumab+Ipilimumab | ORR | II | NCT03126331 | |
| Melanoma | Pembrolizumab+Ipilimumab | ORR | II | NCT02743819 | |
| NPC | Sintilimab+IBI310 (Anti-CTLA-4) | ORR | Ib/II | NCT04945421 | |
| ICI+Targeted therapy | NSCLC | Atezolizumab+Ramucirumab | ORR | II | NCT03689855 |
| NSCLC | Nivolumab+Anlotinib | ORR | Ib/IIa | NCT04507906 | |
| NSCLC | Camrelizumab+famitinib | OS | III | NCT05106335 | |
| NSCLC | Camrelizumab+Apatinib | PFS | II | NCT04670913 | |
| HCC | Camrelizumab+Apatinib | ORR | II | NCT04826406 | |
| HCC | Sintilimab+Lenvatinib | ORR | II | NCT05010681 | |
| HCC | Pembrolizumab+Regorafenib | ORR | II | NCT04696055 | |
| ICI+Targeted therapy | GC/CRC | Tislelizumab+Anlotinib | ORR | II | NCT04777162 |
| TCC | Pembrolizumab+Ramucirumab | ORR | II | NCT04179110 | |
| RCC | Atezolizumab+Cabozantinib | PFS/OS | III | NCT04338269 | |
| ICI+Chemotherapy | NSCLC | Atezolizumab+platinum doublet chemotherapy | ORR | II | NCT03977467 |
| NSCLC | Pembrolizumab+Docetaxel/Pemetrexed/Gemcitabine | PFS | II | NCT03083808 | |
| SCLC | Durvalumab+Topotecan hydrochloride | OS | II | NCT04607954 | |
| UC | Atezolizumab+Carboplatin+Gemcitabine | PFS | II | NCT03737123 | |
| ICI+Radiotherapy | SCCHN | Pembrolizumab+Radiation | ORR | II | NCT03085719 |
| ICI+Other | NSCLC | Atezolizumab+Tocilizumab | ORR | Ib/II | NCT04691817 |
| Melanoma | Pembrolizumab+4SC-202 | safety | Ib/II | NCT03278665 | |
| Solid tumor | Pembrolizumab+BI 1206 | safety | I/IIa | NCT04219254 |
Table 5 NSCLC, non-small-cell lung cancer; SCLC, small-cell lung cancer; HCC, hepatocellular carcinoma; GC, gastric cancer; CRC, colorectal cancer; UC, urothelial carcinoma; TCC, transitional cell carcinoma; NPC, nasopharyngeal carcinoma; RCC, renal cell carcinoma; SCCHN, squamous cell carcinoma of the head and neck; ICI, immune checkpoint inhibitor; ORR, objective response rate; DCR, disease control rate; PFS, progression free survival; OS, overall survival.
The clinical data of safety from ICI rechallenge
| Cancer type | Rechallenge regimen | ≥G3 irAEs No.(%) | IrAEs during ICI rechallenge No.(%) | ORR | DCR | Author | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Prior ICI | Rechallenge ICI | All irAEs | Recurrence of irAEs | Death related to irAE | Cause of death | |||||
| NSCLC | Nivolumab | 7 (33.3) | 1 (4.7) | 15 (71.4) | - | - | - | 14.3 | 85.7 | Mouri et al[117] |
| NSCLC | Anti-PD-1±Anti-CTLA-4 | 13 (34.2) | 8 (21) | 20 (52.6) | 10 (26.3) | 2 | Pneumonitis/ hepatic failure | 47.3 | 81.5 | Santini et al[20] |
| NSCLC | Anti-PD-(L)1 | 3 (20) | 2 (22.2) | 9 (100) | 4 (23.5) | 1 | pneumonitis | 5.9 | 58.8 | Kitagawa et al[22] |
| NSCLC | Anti-PD-(L)1 | 5 (20.8) | 3 (12.5) | 4 (16.6) | 1 (4.2) | - | - | 8.3 | 45.8 | Takahara et al[118] |
| Melanoma | Anti-PD-1 | 55 (68.7) | 14 (17.5) | 40 (50) | 14 (17.5) | 1 | TEN | 70 | 88.7 | Pollack et al[109] |
| Melanoma | Anti-PD-1 | 58 (86.5) | 14 (20.9) | 67 (100) | 2 (3) | - | - | 40 | - | Menzies et al[119] |
| Melanoma | Anti-PD-(L)1 | 10 (25.6) | 6 (15.4) | 19 (48.7) | - | - | - | 15.4 | 25.6 | Amode et al[122] |
| Melanoma | Ipilimumab | 3 (8) | 14 (35) | - | - | 1 | pneumonitis | 10 | 18 | Bowyer et al[120] |
| Melanoma | Ipilimumab | 45 (38.7) | 31 (26.7) | 54 (46.5) | - | 1 | colitis | 7.7 | 41.4 | Cybulska-Stopa et al[126] |
| RCC | ICI | 18 (26) | 11 (15.9) | - | - | - | - | 23.4 | 64 | Ravi et al[87] |
| RCC | Nivolumab+Ipilimumab | 3 (6.7) | 6 (13.3) | 29 (64.4) | - | - | - | 20 | 35.6 | Gul et al[83] |
| Genitourinary cancer | ICI | 17 (16) | 14 (30) | 46 (100) | 16 (26.2) | - | - | 11 | - | Siddiqui et al[107] |
| Various | Anti-PD-1±Anti-CTLA-4 | 62 (37.1) | 6 (3.6) | 57 (34.1) | - | - | - | - | - | Abu-Sbeih et al[121] |
| Various | ICI | 7 (25.9) | 5 (18.5) | 9 (33.3) | 7 (25.9) | - | - | - | - | Bhatlapenumarthi et al[102] |
| Various | ICI | 22 (52.5) | 15 (68) | 22 (55) | 17 (42.5) | - | - | - | - | Simonaggio et al[103] |
| Various | ICI | 7 (17.5) | 7 (17.5) | 31 (77.5) | 19 (47.5) | - | - | - | - | Kartolo et al[110] |
Table 6 NSCLC, non-small-cell lung cancer; RCC, renal cell carcinoma; ICI, immune checkpoint inhibitor; irAE, immune-related adverse events; ORR, objective response rate; DCR, disease control rate; TEN, toxic epidermal necrolysis.
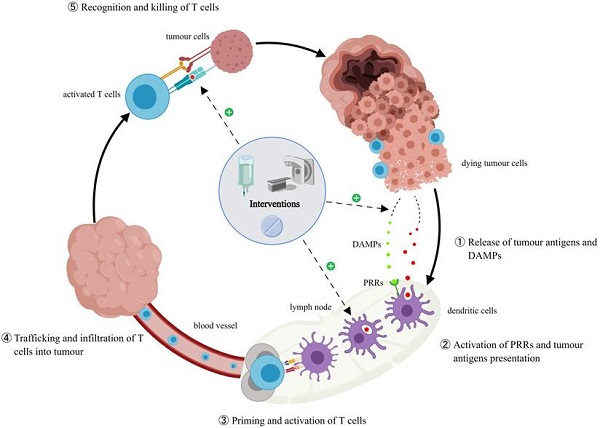
The cancer immunity cycle and the effects of interventions. Additional interventions promote the release of tumor antigens and DAMPs in the host, increase the number of antigen-presenting cells and their antigen presentation ability, induce the expression of major histocompatibility complex class I antigen, and change tumor immunosuppressive microenvironment and PD-L1 expression, thus having an impact on ICI rechallenge.
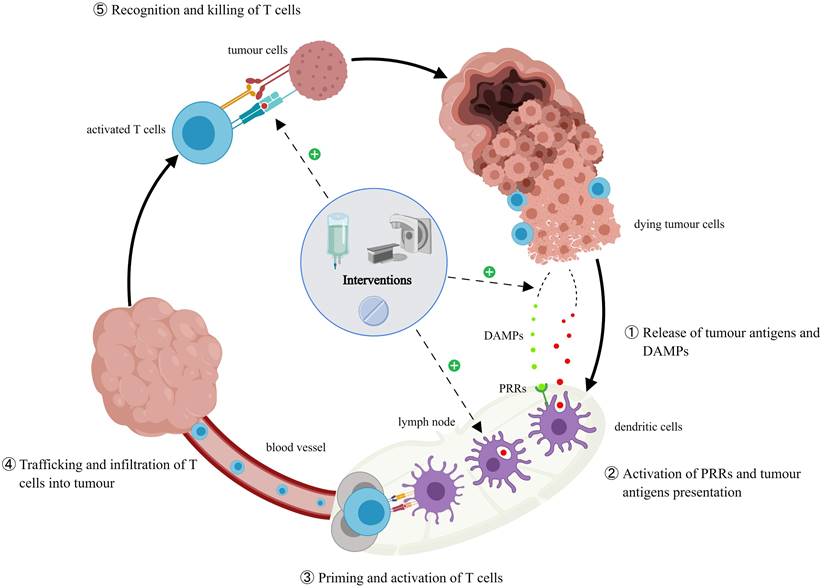
Rechallenge timing
Interval between two ICI courses
In the real-world clinical setting, a treatment interval should exist between the initial treatment and ICI rechallenge. If the rechallenge is performed too soon, the ICIs from the initial treatment could still exist in the patients' blood circulation because some ICIs have long half-lives [123-125]. A sustained drug effect could keep tumor cells in a dormant state, and tumor progression was inhibited by immune mechanisms. Additionally, the time from immune induction to tumor death varies between individuals; therefore, the length of the intervals may also affect ICI rechallenge outcome. Cybulska et al. [126] retrospectively examined 116 patients with advanced melanoma who were rechallenged with ipilimumab after the initial anti-PD-1 antibody treatment. The interval varied and the medium length was 4 weeks. They did not find a correlation between the length of the intervals with median PFS (p=0.12), median OS (p=0.938), or irAEs from the rechallenge (p=0.7403). Fujisawa et al. [127] discovered a similar outcome (p=0.32), and the incidence of irAEs was similar between the patients with intervals ≤28 days and >28 days. Patients with longer intervals had a significantly reduced incidence of ≥3 types of irAEs (3% vs. 25%, p=0.013). The mechanism of action of the subsequent ICI could differ from the initial ICI, which could persist in the patients' blood circulation; thus producing combinational therapy-like effects [128]. When such patients could not be distinguished from rechallenged patients after long intervals, the findings of such investigations should be scrutinized. NiKi et al. [47] retrospectively examined 11 patients with advanced NSCLC who received ICI rechallenge using the initial ICI. They found that patients who responded to rechallenge had a shorter treatment interval than those who did not (1.6 vs. 4.7 months), suggesting that the immunological memory from the initial treatments could last after the treatment ends. Additionally, when the immune response from the initial treatment is weakened, the originally established tumor dormancy may be broken which in turn loses the control of tumor growth, and rebuilding it might take substantial effort [129]. Therefore, the researchers recommended rechallenging within 3 months. In conclusion, the interval between two courses of ICI may affect the rechallenge efficacy. The maintenance of tumor dormancy during immune balance is indispensable, and drug half-lives and immunological memory are believed to be contributing factors.
Interventions during immunotherapies
Immunotherapies stimulate immune responses through tumor-specific neoantigens, which can be influenced by other antineoplastic therapies, such as chemotherapy, radiotherapy, targeted therapy, and anti-vascular therapy [130]. An active endogenous anti-tumor immune response induces and maintains tumor dormancy under continued action, and traditional therapies can reinvigorate debilitating endogenous immune response pathways by enhancing tumor immunogenicity [131-132]. Consequently, interventions before ICI rechallenge could impact its efficacy. Some potential pathways include [133-138]: increasing the expression of major histocompatibility complex class I antigen on tumor cells, recruiting more antigen-presenting cells, changing the tumor immunosuppressive microenvironment or up-regulating PD-L1 expression on tumor cells, and inducing cytotoxic effects to promote the release of tumor antigens and damage-associated molecular patterns (DAMPs) therefore reactivating the cancer-immune cycle. ICI rechallenge after interventions revealed an increased immune response in several small sample studies on patients with advanced melanoma and NSCLC [139-140]. Watanabe et al. [68] included 14 patients who received ICI rechallenge in their retrospective study of 434 patients with advanced NSCLC. Of the three cases achieving disease control (PR=1, SD=2), two received radiotherapy before rechallenge. Reinhorn et al. [141] reported 45 patients with advanced NSCLC who received ICI rechallenge; nine underwent local radiotherapy for oligometastases, while another had active adrenal metastases and underwent surgery before the rechallenge. Patients who received interventions (92% vs. 58%, p=0.17) achieved better disease control compared with the ones who did not (31% vs. 58%, p=0.008). Some researchers also believe that ablation therapy could stimulate the local immune system [142]. Wei et al. [143] described two patients with NSCLC who discontinued combination therapy of camrelizumab with ablation therapy owing to severe immune-related pneumonia (G2-G3). They achieved a PR after retreatment. In another study, four patients with advanced melanoma [60] were administered dacarbazine between the initial nivolumab treatment (paused owing to PD) and pembrolizumab rechallenge, and two patients achieved a PR. Notably, dacarbazine may have other advantageous effects on the immune system. Tedbirt et al. [144] had similar findings. Cabezas et al. [145] described one patient with squamous cell carcinoma of the head and neck who paused the initial PD-L1 treatment owing to PD and achieved a PR after receiving paclitaxel followed by nivolumab rechallenge. Levra et al. [44] found that patients who underwent chemotherapy between two lines of ICI treatments had a median OS of 18.1 months (95% CI 14.6-21.6), while those who did not had a median OS of 14.8 months (95% CI 13.4-16.5). Unfortunately, not all studies reached the same conclusion. Gobbini et al. [43] found that patients who underwent chemotherapy before ICI rechallenge had a shorter PFS (HR 1.81; 95% CI 1.21-2.72; p=0.004) and OS (HR 1.52; 95% CI 0.90-2.60; p=0.1). Similar outcomes (PFS: 6.6 vs. 2.1 months, p=0.001) were reported by another retrospective analysis [146]. Vauchier et al. [45] reported 26 patients who received therapies (nine of whom received more than one) before the ICI rechallenge and their PFS was shorter. In conclusion, interventions between two lines of ICI treatments have two contrary effects. It could stimulate exhausted tumor-specific T cells in the immune system to maintain tumor dormancy and also weaken the patients' body condition thereby dampening the immune system [147]. Not all therapies may be useful before the rechallenge. A prospective study to identify the drugs or treatments that can improve ICI rechallenge efficacy may be warranted.
Conclusion and Perspectives
With the growing advent of new ICIs, many patients could benefit from them. The potential of ICIs in the field of tumor treatment has attracted increased attention, and new therapeutic strategies are continually being explored to gain better oncotherapy efficiency. In this review, we assess the current status of ICI rechallenge and recognize that patients could benefit from this treatment paradigm. Many factors can increase the complexity of the treatment and influence the outcomes of ICI rechallenge, such as patients' clinical and pathological features, different rechallenge strategies, length of treatment pause interval, and additional treatments before the rechallenge. However, most clinical studies identified were based on a retrospective analysis of a subset of other immunotherapy clinical studies; hence, some aforementioned factors were not included in the data. In addition, the number of patients in some studies were low and produced contradictory outcomes among different publications.
Based on the effective survival benefit, ICI rechallenge is one promising way to release the underexplored potential of immunotherapy. Future studies are needed to address the following questions: How can patients who are most likely to benefit from ICI rechallenge be identified? How should the optimal rechallenge treatment regimen for the target population be selected? How can the therapeutic efficacy and safety of ICI rechallenge be maximized while minimizing adverse effects? These findings will help to establish a standardized treatment regimen, which can be applied in routine clinical practice.
Abbreviations
ICI: Immune checkpoint inhibitor; irAE: Immune-related adverse events; NSCLC: Non-small-cell lung cancer; PD-1: Programmed cell death 1; PD-L1: Programmed cell death ligand 1; CTLA-4: Cytotoxic T lymphocyte antigen 4; TMB: Tumor mutation burden; NLR: Neutrophil to lymphocyte ratio; LMR: Lymphocyte to monocyte ratio; PLR: Platelet to lymphocyte ratio; PR: Partial response; SD: Stable disease; PD: Progressive disease; OS: Overall survival; PFS: Progression free survival; DCR: Disease control rate; ORR: Objective response rate; OR: Odds ratio; HR: Hazard ratio; ECOG/PS: Eastern Cooperative Oncology Group performance status; BMI: Body mass index; DAMPs: damage associated molecular patterns; PRRs: pattern recognition receptors.
Acknowledgements
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author Contributions
HH designed the study, performed the literature search and wrote the original manuscript. KW and RJ conceived the project and prepared the figures and tables. HH, ZXZ, and MZ drafted the manuscript. YLD, ZJX, JNT, HX, YW, PZ, and JD critically revised the manuscript. JZ revised the manuscript draft and gave final approval of the version to be submitted. All authors read and approved the final manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Cao W, Chen HD, Yu YW, Li N, Chen WQ. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chin Med J (Engl). 2021;134:783-91
2. Xia C, Dong X, Li H, Cao M, Sun D, He S. et al. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J (Engl). 2022;135:584-90
3. Torre LA, Siegel RL, Ward EM, Jemal A. Global Cancer Incidence and Mortality Rates and Trends-An Update. Cancer Epidemiol Biomarkers Prev. 2016;25:16-27
4. Soerjomataram I, Bray F. Planning for tomorrow: global cancer incidence and the role of prevention 2020-2070. Nat Rev Clin Oncol. 2021;18:663-72
5. Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Pineros M. et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144:1941-53
6. Ferlay J, Colombet M, Soerjomataram I, Parkin DM, Pineros M, Znaor A. et al. Cancer statistics for the year 2020: An overview. Int J Cancer. 2021;149:778-89
7. Rehman FU, Al-Waeel M, Naz SS, Shah KU. Anticancer therapeutics: a brief account on wide refinements. Am J Cancer Res. 2020;10:3599-3621
8. Malik D, Mahendiratta S, Kaur H, Medhi B. Futuristic approach to cancer treatment. Gene. 2021;805:145906
9. Pennock GK, Chow LQ. The Evolving Role of Immune Checkpoint Inhibitors in Cancer Treatment. Oncologist. 2015;20:812-22
10. Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39:1-10
11. Tan S, Li D, Zhu X. Cancer immunotherapy: Pros, cons and beyond. Biomed Pharmacother. 2020;124:109821
12. Kakimi K, Karasaki T, Matsushita H, Sugie T. Advances in personalized cancer immunotherapy. Breast Cancer. 2017;24:16-24
13. Qin S, Xu L, Yi M, Yu S, Wu K, Luo S. Novel immune checkpoint targets: moving beyond PD-1 and CTLA-4. Mol Cancer. 2019;18:155-69
14. Yi M, Zheng X, Niu M, Zhu S, Ge H, Wu K. Combination strategies with PD-1/PD-L1 blockade: current advances and future directions. Mol Cancer. 2022;21:28
15. Wu M, Huang Q, Xie Y, Wu X, Ma H, Zhang Y. et al. Improvement of the anticancer efficacy of PD-1/PD-L1 blockade via combination therapy and PD-L1 regulation. J Hematol Oncol. 2022;15:24
16. Yap TA, Parkes EE, Peng W, Moyers JT, Curran MA, Tawbi HA. Development of Immunotherapy Combination Strategies in Cancer. Cancer Discov. 2021;11:1368-97
17. Meric-Bernstam F, Larkin J, Tabernero J, Bonini C. Enhancing anti-tumour efficacy with immunotherapy combinations. Lancet. 2021;397:1010-22
18. Yarchoan M, Albacker LA, Hopkins AC, Montesion M, Murugesan K, Vithayathil TT. et al. PD-L1 expression and tumor mutational burden are independent biomarkers in most cancers. JCI Insight. 2019;4:e126908
19. Davis AA, Patel VG. The role of PD-L1 expression as a predictive biomarker: an analysis of all US Food and Drug Administration (FDA) approvals of immune checkpoint inhibitors. J Immunother Cancer. 2019;7:278
20. Santini FC, Rizvi H, Plodkowski AJ, Ni A, Lacouture ME, Gambarin-Gelwan M. et al. Safety and efficacy of re-treating with immunotherapy after immune-related adverse events in patients with NSCLC. Cancer Immunol Res. 2018;6:1093-9
21. Dolladille C, Ederhy S, Sassier M, Cautela J, Thuny F, Cohen AA. et al. Immune checkpoint inhibitor rechallenge after immune-related adverse events in patients with cancer. JAMA Oncol. 2020;6:865-71
22. Kitagawa S, Hakozaki T, Kitadai R, Hosomi Y. Switching administration of anti-PD-1 and anti-PD-L1 antibodies as immune checkpoint inhibitor rechallenge in individuals with advanced non-small cell lung cancer: Case series and literature review. Thorac Cancer. 2020;11:1927-33
23. Allouchery M, Lombard T, Martin M, Rouby F, Sassier M, Bertin C. et al. Safety of immune checkpoint inhibitor rechallenge after discontinuation for grade≥2 immune-related adverse events in patients with cancer. J Immunother Cancer. 2020;8:e001622
24. Alaiwi SA, Xie W, Nassar AH, Dudani S, Martini D, Bakouny Z. et al. Safety and efficacy of restarting immune checkpoint inhibitors after clinically significant immune-related adverse events in metastatic renal cell carcinoma. J Immunother Cancer. 2020;8:e000144
25. Park R, Lopes L, Saeed A. Outcomes following immunotherapy re-challenge after immune-related adverse event: systematic review and meta-analysis. Immunotherapy. 2020;12:1183-93
26. Sasaki T, Tabata T, Yoshimura N. For which lung cancer patients is re-administration of immune checkpoint inhibitors effective? J Rural Med. 2021;16:256-62
27. Plazy C, Hannani D, Gobbini E. Immune checkpoint inhibitor rechallenge and resumption: a systematic review. Curr Oncol Rep. 2022;24:1095-106
28. Levra MG, Cotte FE, Corre Romain, Calvet C, Gaudin AF, Penrod JR. et al. Immunotherapy rechallenge after nivolumab treatment in advanced non-small cell lung cancer in the real-world setting: A national data base analysis. Lung Cancer. 2020;140:99-106
29. Borea R, Damassi A, Rebuzzi SE, Banna GL, Murianni V, Catalano F. et al. Immunotherapy retreatment: case report, review of the literature and proposal for the definition of different scenarios. Immunotherapy. 2021;13:645-52
30. Zaremba A, Eggermont AMM, Robert C, Dummer R, Ugurel S, Livingstone E. et al. The concepts of rechallenge and retreatment with immune checkpoint blockade in melanoma patients. Eur J Cancer. 2021;155:268-80
31. Gebhardt C, Ascierto P, Atkinson V, Corrie P, Dummer R, Schadendorf D. The concepts of rechallenge and retreatment in melanoma: A proposal for consensus definitions. Eur J Cancer. 2020;138:68-76
32. Xu S, Shukuya T, Tamura J, Shimamura S, Kurokawa K, Miura K. et al. Heterogeneous Outcomes of Immune Checkpoint Inhibitor Rechallenge in Patients With NSCLC: A Systematic Review and Meta-Analysis. JTO Clin Res Rep. 2022;3:100309
33. Hanovich E, Asmis T, Ong M, Stewart D. Rechallenge strategy in cancer therapy. Oncology. 2020;98:669-79
34. Michielin O, van Akkooi ACJ, Ascierto PA, Dummer R, Keilholz U e Committee, ESMO Guidelines. Cutaneous melanoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30:1884-1901
35. Swetter SM, Thompson JA, Albertini MR, Braker CA, Baumgartner J, Boland G. et al. NCCN Guidelines Insights: Melanoma: Cutaneous, Version 2.2021. J Natl Compr Canc Netw. 2021;19:364-76
36. Sullivan RJ, Atkins MB, Kirkwood JM, Agarwala SS, Clark JI, Ernstoff MS. et al. An update on the society for immunotherapy of cancer consensus statement on tumor immunotherapy for the treatment of cutaneous melanoma: version 2.0. J Immunother C. 2018;6:44
37. Bimbatti D, Maruzzo M, Pierantoni F, Diminutto A, Dionese M, Deppieri FM. et al. Immune checkpoint inhibitors rechallenge in urological tumors: An extensive review of the literature. Crit Rev Oncol Hematol. 2022;170:103579
38. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F. et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-32
39. Krist AH, Davidson KW, Mangione CM, Barry MJ, Cabana M, Caughey AB. et al. Screening for Lung Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;325:962-70
40. Patel SP, Kurzrock R. PD-L1 Expression as a Predictive Biomarker in Cancer Immunotherapy. Mol Cancer Ther. 2015;14:847-56
41. Gibney GT, Weiner LM, Atkins MB. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol. 2016;17:e542-51
42. Katayama Y, Shimamoto T, Yamada T, Takeda T, Yamada T, Shiotsu S. et al. Retrospective efficacy analysis of immune checkpoint inhibitor rechallenge in patients with non-small cell lung cancer. J Clin Med. 2019;9:102-12
43. Gobbini E, Toffart AC, Perol M, Assie JB, Duruisseaux M, Coupez D. et al. Immune checkpoint inhibitors rechallenge efficacy in non-small-cell lung cancer patients. Clin Lung Cancer. 2020;21:e497-e510
44. Levra MG, Cotte FE, Corre R, Calvet C, Gaudin AF, Penrod JR. et al. Immunotherapy rechallenge after nivolumab treatment in advanced non-small cell lung cancer in the real-world setting: A national data base analysis. Lung Cancer. 2020;140:99-106
45. Vauchier C, Auclin E, Barthelemy P, Carril-Ajuria L, Ryckewaert T, Borchiellini D. et al. REchallenge of NIVOlumab (RENIVO) or Nivolumab-Ipilimumab in metastatic renal cell carcinoma: An ambispective multicenter study. J Oncol. 2022;2022:3449660
46. Nomura M, Otsuka A, Kondo T, Nagai H, Nonomura Y, Kaku Y. et al. Efficacy and safety of retreatment with nivolumab in metastatic melanoma patients previously treated with nivolumab. Cancer Chemother Pharmacol. 2017;80:999-1004
47. Niki M, Nakaya A, Kurata T, Yoshioka H, Kaneda T, Kibata K. et al. Immune checkpoint inhibitor re-challenge in patients with advanced non-small cell lung cancer. Oncotarget. 2018;9:32298-304
48. Schadendorf D, Wolchok JD, Hodi FS, Chiarion-Sileni V, Gonzalez R, Rutkowski P. et al. Efficacy and safety outcomes in patients with advanced melanoma who discontinued treatment with nivolumab and ipilimumab because of adverse events: A pooled analysis of randomized phase II and III trials. J Clin Oncol. 2017;35:3807-14
49. Reschke R, Ziemer M. Rechallenge with checkpoint inhibitors in metastatic melanoma. J Dtsch Dermatol Ges. 2020;18:429-36
50. Inno A, Roviello G, Ghidini A, Luciani A, Catalano M, Gori S. et al. Rechallenge of immune checkpoint inhibitors: A systematic review and meta-analysis. Crit Rev Oncol Hematol. 2021;165:103434
51. Sheth S, Gao C, Mueller N, Angra N, Gupta A, Germa C. et al. Durvalumab activity in previously treated patients who stopped durvalumab without disease progression. J Immunother Cancer. 2020;8:e000650
52. Herbst RS, Baas P, Kim DW, Felip E, Pérez-Gracia JL, Han JY. et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540-50
53. Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A. et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823-33
54. Chan TA, Yarchoan M, Jaffee E, Swanton C, Quezada SA, Stenzinger A. et al. Development of tumor mutation burden as an immunotherapy biomarker: utility for the oncology clinic. Ann Oncol. 2019;30:44-56
55. Tumor Pathology Committee of Chinese Anti-Cancer Association, Expert Committee on Pathology of Chinese Society of Clinical Oncology, Expert Committee on Non-Small Cell Lung Cancer of Chinese Society of Clinical Oncology. Expert consensus on PD-L1 expression testing in non-small-cell lung cancer in China. Zhonghua Zhong Liu Za Zhi. 2020;42:513-21
56. Fujita K, Uchida N, Kanai O, Okamura M, Nakatani K, Mio T. Retreatment with pembrolizumab in advanced non-small cell lung cancer patients previously treated with nivolumab: emerging reports of 12 cases. Cancer Chemother Pharmacol. 2018;81:1105-9
57. Bagley SJ, Kothari S, Aggarwal C, Bauml JM, Alley EW, Evans TL. et al. Pretreatment neutrophil-to-lymphocyte ratio as a marker of outcomes in nivolumab-treated patients with advanced non-small-cell lung cancer. Lung Cancer. 2017;106:1-7
58. Diem S, Schmid S, Krapf M, Flatz L, Born D, Jochum W. et al. Neutrophil-to-Lymphocyte ratio (NLR) and Platelet-to-Lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer. 2017;111:176-81
59. Failing JJ, Yan Y, Porrata LF, Markovic SN. Lymphocyte-to-monocyte ratio is associated with survival in pembrolizumab-treated metastatic melanoma patients. Melanoma Res. 2017;27:596-600
60. Kan T, Takahagi S, Kawai M, Matsubara D, Tanaka A, Hide M. Rechallenge of programmed cell death 1 inhibitor after an interval with dacarbazine treatment may be effective for advanced malignant melanoma. J Dermatol. 2020;47:907-10
61. Chiarion-Sileni V, Pigozzo J, Ascierto PA, Simeone E, Maio M, Calabrò L. et al. Ipilimumab retreatment in patients with pretreated advanced melanoma: the expanded access programme in Italy. Br J Cancer. 2014;110:1721-6
62. Yang K, Li J, Sun Z, Zhao L, Bai C. Retreatment with immune checkpoint inhibitors in solid tumors: a systematic review. Ther Adv Med Oncol. 2020;12:1-18
63. Cai Z, Zhan P, Song Y, Liu H, Lv T. Safety and efficacy of retreatment with immune checkpoint inhibitors in non-small cell lung cancer: a systematic review and meta-analysis. Transl Lung Cancer Res. 2022;11:1555-66
64. Yao Y, Liu Z, Li Q, Cao B, Wang M. Successful immune checkpoint inhibitor-based rechallenge in a patient with advanced esophageal squamous cell cancer: A case report. Thorac Oncol. 2022;13:497-501
65. Hakozaki T, Okuma Y, Kashima J. Re-challenging immune checkpoint inhibitor in a patient with advanced non-small cell lung cancer: a case report. BMC Cancer. 2018;18:302
66. Aya F, Gonzalez EA, Martinez C, Carcelero E, Arance A. Safe anti-programmed cell death-1 rechallenge with antibody switching after immune-related adverse events: brief communication. Immunotherapy. 2021;13:745-52
67. Guaitoli G, Barbieri F, Barbolini M, Molinaro E, Emidio KD, Borghi V. et al. Pembrolizumab rechallenge in squamous non-small-cell lung cancer and HIV-positivity: a case report. Immunotherapy. 2021;13:277-81
68. Watanabe H, Kubo T, Ninomiya K, Kudo K, Minami D, Murakami E. et al. The effect and safety of immune checkpoint inhibitor rechallenge in non-small cell lung cancer. Jpn J Clin Oncol. 2019;49:762-5
69. Martini DJ, Lalani AA, Bosse D, Steinharter JA, Harshman LC, Hodi FS. et al. Response to single agent PD-1 inhibitor after progression on previous PD-1/PD-L1 inhibitors: a case series. J Immunother Cancer. 2017;5:66
70. Wei SC, Duffy CR, Allison JP. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov. 2018;8:1069-86
71. Schoenfeld AJ, Antonia SJ, Awad MM, Felip E, Gainor J, Gettinger SN. et al. Clinical definition of acquired resistance to immunotherapy in patients with metastatic non-small cell lung cancer. Ann Oncol. 2021;32:1597-1607
72. Larkin J, Minor D, D'Angelo S, Neyns B, Smylie M Jr WHM. et al. Overall survival in patients with advanced melanoma who received nivolumab versus investigator's choice chemotherapy in CheckMate 037: A randomized, controlled, open-label phase III trial. J Clin Oncol. 2018;36:383-90
73. Khoja L, Day D, Chen TWW, Siu LL, Hansen AR. Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: a systematic review. Ann Oncol. 2017;28:2377-85
74. Hellmann MD, Paz-Ares L, Bernabe Caro R, Zurawski B, Kim SW, Carcereny Costa E. et al. Nivolumab plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer. N Engl J Med. 2019;381:2020-31
75. Brahmer JR, Lee JS, Ciuleanu TE, Caro RB, Nishio M, Urban L. et al. Five-Year Survival Outcomes With Nivolumab Plus Ipilimumab Versus Chemotherapy as First-Line Treatment for Metastatic Non-Small Cell Lung Cancer in CheckMate 227. J Clin Oncol. 2023;41:1200-12
76. Johnson ML, Cho BC, Luft A, Alatorre-Alexander J, Geater SL, Laktionov K. et al. Durvalumab With or Without Tremelimumab in Combination With Chemotherapy as First-Line Therapy for Metastatic Non-Small-Cell Lung Cancer: The Phase III POSEIDON Study. J Clin Oncol. 2023;41:1213-27
77. Tawbi HA, Schadendorf D, Lipson EJ, Ascierto PA, Matamala L, Castillo Gutiérrez E. et al. Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. N Engl J Med. 2022;386:24-34
78. Chocarro L, Bocanegra A, Blanco E, Fernández-Rubio L, Arasanz H, Echaide M. et al. Cutting-Edge: Preclinical and Clinical Development of the First Approved Lag-3 Inhibitor. Cells. 2022;11:2351
79. Glutsch V, Kneitz H, Gesierich A, Goebeler M, Haferkamp S, Becker JC. et al. Activity of ipilimumab plus nivolumab in avelumab-refractory Merkel cell carcinoma. Cancer Immunol Immunother. 2021;70:2087-93
80. Gim G, Kim Y, Park Y, Kim MJ, Nam M, Yang W. et al. Response to Nivolumab and Ipilimumab in Microsatellite Instability-High (MSI-H) Cervical Carcinoma with Acquired Resistance to Pembrolizumab: A Case Report and Literature Review. Oncologist. 2022;27:525-31
81. Afzal MZ, Mabaera R, Shirai K. Metastatic uveal melanoma showing durable response to anti-CTLA-4 and anti-PD-1 combination therapy after experiencing progression on anti-PD-1 therapy alone. J Immunother Cancer. 2018;6:13
82. Silva IP, Ahmed T, Reijers ILM, Weppler AM, Warner AB, Patrinely JR. et al. Ipilimumab alone or ipilimumab plus anti-PD-1 therapy in patients with metastatic melanoma resistant to anti-PD-(L)1 monotherapy: a multicentre, retrospective, cohort study. Lancet Oncol. 2021;22:836-47
83. Gul A, Stewart TF, Mantia CM, Shah NJ, Gatof ES, Long Y. et al. Salvage ipilimumab and nivolumab in patients with metastatic renal cell carcinoma after prior immune checkpoint inhibitors. J Clin Oncol. 2020;38:3088-94
84. Carril-Ajuria L, Lora D, Carretero-Gonzalez A, Martín-Soberón M, Rioja-Viera P, Castellano D. et al. Systemic analysis and review of nivolumab-ipilimumab combination as a rescue strategy for renal cell carcinoma after treatment with anti-PD-1/PD-L1 therapy. Clin Genitourin Cancer. 2021;19:95-102
85. Wong JSL, Kwok GGW, Tang V, Li BCW, Leung R, Chiu J. et al. Ipilimumab and nivolumab/pembrolizumab in advanced hepatocellular carcinoma refractory to prior immune checkpoint inhibitors. J Immunother Cancer. 2021;9:e001945
86. Zimmer L, Apuri S, Eroglu Z, Kottschade LA, Forschner A, Gutzmer R. et al. Ipilimumab alone or in combination with nivolumab after progression on anti-PD-1 therapy in advanced melanoma. Eur J Cancer. 2017;75:47-55
87. Ravi P, Mantia C, Su C, Sorenson K, Elhag D, Rathi N. et al. Evaluation of the safety and efficacy of immunotherapy rechallenge in patients with renal cell carcinoma. JAMA Oncol. 2020;6:1606-10
88. Rallis KS, Lai Yau TH, Sideris M. Chemoradiotherapy in Cancer Treatment: Rationale and Clinical Applications. Anticancer Res. 2021;41:1-7
89. Lee YT, Tan YJ, Oon CE. Molecular targeted therapy: Treating cancer with specificity. Eur J Pharmacol. 2018;834:188-96
90. Xu Z, Hao X, Yang K, Wang Q, Wang J, Lin L. et al. Immune checkpoint inhibitor rechallenge in advanced or metastatic non-small cell lung cancer: a retrospective cohort study. J Cancer Res Clin Oncol. 2022;148:3081-9
91. Zhang Z, Cheng S, Qi C, Zhang X, Peng Z, Shen L. Response to the rechallenge of combination immunotherapy in a patient with late-stage gastric cancer: case report. Ann Palliat Med. 2022;11:818-26
92. Lee CH, Shah AY, Rasco D, Rao A, Taylor MH, Di Simone C. et al. Lenvatinib plus pembrolizumab in patients with either treatment-naive or previously treated metastatic renal cell carcinoma (Study 111/KEYNOTE-146): a phase 1b/2 study. Lancet Oncol. 2021;22:946-58
93. Sandhu S, Atkinson V, Cao MG, Medina T, Rivas AS, Menzies AM. et al. Phase 1b study of cobimetinib plus atezolizumab in patients with advanced BRAFV600 wild-type melanoma progressing on prior anti-programmed death-1 therapy. Eur J Cancer. 2023;178:180-90
94. Robert C, Lebbé C, Lesimple T, Lundström E, Nicolas V, Gavillet B. et al. Phase I study of Androgen deprivation therapy in combination with anti-PD-1 in melanoma patients pretreated with anti-PD-1. Clin Cancer Res. 2023;29:858-65
95. Niu J, Maurice-Dror C, Lee DH, Kim DW, Nagrial A, Voskoboynik M. et al. First-in-human phase 1 study of the anti-TIGIT antibody vibostolimab as monotherapy or with pembrolizumab for advanced solid tumors, including non-small-cell lung cancer. Ann Oncol. 2022;33:169-80
96. Márquez-Rodas I, Longo F, Rodriguez-Ruiz ME, Calles A, Ponce S, Jove M. et al. Intratumoral nanoplexed poly I:C BO-112 in combination with systemic anti-PD-1 for patients with anti-PD-1-refractory tumors. Sci Transl Med. 2020;12:eabb0391
97. Beug ST, Conrad DP, Alain T, Korneluk RG, Lacasse EC. Combinatorial cancer immunotherapy strategies with proapoptotic small-molecule IAP antagonists. Int J Dev Biol. 2015;59:141-7
98. Lemaire V, Shemesh CS, Rotte A. Pharmacology-based ranking of anti-cancer drugs to guide clinical development of cancer immunotherapy combinations. J Exp Clin Cancer Res. 2021;40:311
99. Cheng M, Zhang S, Liu J, Jiang S, Dong M. Evaluating the efficacy and safety of immune checkpoint inhibitors by detecting the exposure-response: An inductive review. Int Immunopharmacol. 2021;97:107703
100. Sullivan RJ, Weber JS. Immune-related toxicities of checkpoint inhibitors: mechanisms and mitigation strategies. Nat Rev Drug Discov. 2022;21:495-508
101. Fujisaki T, Watanabe S, Ota T, Kushiro K, Sato Y, Takahashi M. et al. The prognostic significance of the continuous administration of anti-PD-1 antibody via continuation or rechallenge after the occurrence of immune-related adverse events. Front Oncol. 2021;11:704475
102. Bhatlapenumarthi V, Patwari A, Harb AJ. Immune-related adverse events and immune checkpoint inhibitor tolerance on rechallenge in patients with irAEs: a single-center experience. J Cancer Res Clin Oncol. 2021;147:2789-2800
103. Simonaggio A, Michot JM, Voisin AL, Pavec JL, Collins M, Lallart A. et al. Evaluation of readministration of immune checkpoint inhibitors after immune-related adverse events in patients with cancer. JAMA Oncol. 2019;5:1310-7
104. Li M, Sack JS, Rahma OE, Hodi FS, Zucker SD, Grover S. Outcomes after resumption of immune checkpoint inhibitor therapy after high-grade immune-mediated hepatitis. Cancer. 2020;126:5088-97
105. Shah P, Punekar SR, Pavlick AC. Response to immune checkpoint inhibitor rechallenge after high-grade immune related adverse events in patients with advanced melanoma. Melanoma Res. 2021;31:242-8
106. Nakajima EC, Lipson EJ, Brahmer JR. Challenge of rechallenge: When to resume immunotherapy following an immune-related adverse event. J Clin Oncol. 2019;37:2714-8
107. Siddiqui BA, Gheeya J, Goswamy R, Bathala TK, Surasi DS, Gao J. et al. Durable responses in patients with genitourinary cancers following immune checkpoint therapy rechallenge after moderate-to-severe immune-related adverse events. J Immunother Cancer. 2021;9:e002850
108. Zhao Q, Zhang J, Xu L, Yang H, Liang N, Zhang L. et al. Safety and efficacy of the rechallenge of immune checkpoint inhibitors after immune-related adverse events in patients with cancer: a systemic review and meta-analysis. Front Immunol. 2021;12:730320
109. Pollack MH, Betof A, Dearden H, Rapazzo K, Valentine I, Brohl AS. et al. Safety of resuming anti-PD-1 in patients with immune-related adverse events (irAEs) during combined anti-CTLA-4 and anti-PD1 in metastatic melanoma. Ann Oncol. 2018;29:250-5
110. Kartolo A, Holstead R, Khalid S, Emack J, Hopman W, Baetz T. Safety of immunotherapy rechallenge after immune-related adverse events in patients with advanced cancer. J Immunother. 2021;44:41-8
111. Villagran-Garcia M, Velasco R. Neurotoxicity and safety of the rechallenge of immune checkpoint inhibitors: a growing issue in neuro-oncology practice. Neurol Sci. 2022;43:2339-61
112. Tsui A, Edmondson L, Julius J. An evaluation of the use of corticosteroids for the management of immune-mediated adverse events in cancer patients treated with immune checkpoint inhibitors. J Adv Pract Oncol. 2021;12:137-45
113. Haanen John, Ernstoff M, Wang Y, Menzies A, Puzanov I, Grivas P. et al. Rechallenge patients with immune checkpoint inhibitors following severe immune-related adverse events: review of the literature and suggested prophylactic strategy. J Immunother Cancer. 2020;8:e000604
114. Johnson DB, Jackubovic BD, Sibaud V, Sise ME. Balancing cancer immunotherapy efficacy and toxicity. J Allergy Clin Immunol Pract. 2020;8:2898-2906
115. Espana S, Guasch I, Carcereny E. Immunotherapy rechallenge in patients with non-small-cell lung cancer. Pulmonology. 2020;26:252-4
116. Gobbini E, Charles J, Toffart AC, Leccia MT, Moro-Sibilot D, Levra MG. Current opinions in immune checkpoint inhibitors rechallenge in solid cancers. Crit Rev Oncol Hematol. 2019;144:102816
117. Mouri A, Kaira K, Yamaguchi O, Shiono A, Miura Y, Hashimoto K. et al. Clinical difference between discontinuation and retreatment with nivolumab after immune-related adverse events in patients with lung cancer. Cancer Chemother Pharmacol. 2019;84:873-80
118. Takahara Y, Tanaka T, Ishige Y, Shionoya I, Yamamura K, Sakuma T. et al. Efficacy and predictors of rechallenge with immune checkpoint inhibitors in non-small cell lung cancer. Thorac Cancer. 2022;13:624-30
119. Menzies AM, Johnson DB, Ramanujam S, Atkinson VG, Wong ANM, Park JJ. et al. Anti-PD-1 therapy in patients with advanced melanoma and preexisting autoimmune disorders or major toxicity with ipilimumab. Ann Oncol. 2017;28:368-76
120. Bowyer S, Prithviraj P, Lorigan p, Larkin J, McArthur G, Atkinson V. et al. Efficacy and toxicity of treatment with the anti-CTLA-4 antibody ipilimumab in patients with metastatic melanoma after prior anti-PD-1 therapy. Br J Cancer. 2016;114:1084-9
121. Abu-Sbeih H, Ali FS, Naqash AR, Owen DH, Patel S, Otterson GA. et al. Resumption of immune checkpoint inhibitor therapy after immune-mediated colitis. J Clin Oncol. 2019;37:2738-45
122. Amode R, Baroudjian B, Kowal A, Jebali M, Allayous C, Bagot M. et al. Anti-programmed cell death protein 1 tolerance and efficacy after ipilimumab immunotherapy: observational study of 39 patients. Melanoma Res. 2017;27:110-5
123. Desnoyer A, Broutin S, Delahousse J, Maritaz C, Blondel L, Mir O. et al. Pharmacokinetic/pharmacodynamic relationship of therapeutic monoclonal antibodies used in oncology: Part 2, immune checkpoint inhibitor antibodies. Eur J Cancer. 2020;128:119-28
124. Centanni M, Moes DJAR, Trocóniz IF, Ciccolini J, van Hasselt JGC. Clinical Pharmacokinetics and Pharmacodynamics of Immune Checkpoint Inhibitors. Clin Pharmacokinet. 2019;58:835-57
125. Yan T, Yu L, Shangguan D, Li W, Liu N, Chen Y. et al. Advances in pharmacokinetics and pharmacodynamics of PD-1/PD-L1 inhibitors. Int Immunopharmacol. 2023;115:109638
126. Cybulska-Stopa B, Rogala P, Czarnecka AM, Ługowska I, Teterycz P, Galus Ł. et al. Efficacy of ipilimumab after anti-PD-1 therapy in sequential treatment of metastatic melanoma patients - Real world evidence. Adv Med Sci. 2020;65:316-23
127. Fujisawa Y, Yoshino K, Otsuka A, Funakoshi T, Uchi H, Fujimura T. et al. Retrospective study of advanced melanoma patients treated with ipilimumab after nivolumab: Analysis of 60 Japanese patients. J Dermatol Sci. 2018;89:60-6
128. Lin X, Lu X, Luo G, Xiang H. Progress in PD-1/PD-L1 pathway inhibitors: From biomacromolecules to small molecules. Eur J Med Chem. 2020;186:111876
129. Janowska A, Iannone M, Fidanzi C, Romanelli M, Filippi L, Del Re M. et al. The Genetic Basis of Dormancy and Awakening in Cutaneous Metastatic Melanoma. Cancers (Basel). 2022;14:2104
130. Jiang T, Shi T, Zhang H, Hu J, Song Y, Wei J. et al. Tumor neoantigens: from basic research to clinical applications. J Hematol Oncol. 2019;12:93
131. Baxevanis CN, Perez SA. Cancer Dormancy: A Regulatory Role for Endogenous Immunity in Establishing and Maintaining the Tumor Dormant State. Vaccines (Basel). 2015;3:597-619
132. Butts CA. Anti-tumor immune response in early stage non small cell lung cancer (NSCLC): implications for adjuvant therapy. Transl Lung Cancer Res. 2013;2:415-22
133. Nomura M, Otsuka A, Kondo T, Nagai H, Nonomura Y, Kaku Y. et al. Efficacy and safety of retreatment with nivolumab in metastatic melanoma patients previously treated with nivolumab. Cancer Chemother Pharmacol. 2017;80:999-1004
134. Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39:1-10
135. Wu w, Liu Y, Zeng S, Han Y, Shen H. Intratumor heterogeneity: the hidden barrier to immunotherapy against MSI tumors from the perspective of IFN-γ signaling and tumor-infiltrating lymphocytes. J Hematol Oncol. 2021;14:160
136. Creelan BC, Wang C, Teer JK, Toloza EM, Yao J, Kim S. et al. Tumor-infiltrating lymphocyte treatment for anti-PD-1-resistant metastatic lung cancer: a phase 1 trial. Nat Med. 2021;27:1410-8
137. Zhou F. Molecular mechanisms of IFN-gamma to up-regulate MHC class I antigen processing and presentation. Int Rev Immunol. 2009;28:239-60
138. Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR. et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014;124:687-95
139. Blasig H, Bender C, Hassel JC, Eigentler TK, Sachse MM, Hiernickel J. et al. Reinduction of PD1-inhibitor therapy: first experience in eight patients with metastatic melanoma. Melanoma Res. 2017;27:321-5
140. Ito K, Oguri T, Takeda N, Fukumitsu K, Fukuda S, Kanemitsu Y. et al. A case of non-small cell lung cancer with long-term response after re-challenge with nivolumab. Respir Med Case Rep. 2019;29:100979
141. Reinhorn D, Jacobi O, Icht O, Dudnik E, Rotem O, Zer A. et al. Treatment beyond progression with immune checkpoint inhibitors in non-small-cell lung cancer. Immunotherapy. 2020;12:235-43
142. Rangamuwa K, Leong T, Weeden C, Asselin-Labat ML, Bozinovski S, Christie M. et al. Thermal ablation in non-small cell lung cancer: a review of treatment modalities and the evidence for combination with immune checkpoint inhibitors. Transl Lung Cancer Res. 2021;10:2842-57
143. Wei Z, Yang X, Ye X. Rechallenge of camrelizumab in non-small-cell lung cancer patients treated previously with camrelizumab and microwave ablation. J Cancer Res Ther. 2020;16:1191-5
144. Tedbirt B, Pontville MD, Branger P, Picard C, Baroudjian B, Lebbé C. et al. Rechallenge of immune checkpoint inhibitor after pembrolizumab-induced myasthenia gravis. Eur J Cancer. 2019;113:72-4
145. Cabezas-Camarero S, Cabrera-Martin MN, Perez-Segura P. Response to immunotherapy rechallenge after interval chemotherapy in a patient with head and neck cancer. Anticancer Drugs. 2019;30:149-52
146. Gobbini E, Charles J, Toffart AC, Leccia MT, Moro-Sibilot D, Levra MG. Literature meta-analysis about the efficacy of re-challenge with PD-1 and PD-L1 inhibitors in cancer patients. Bull Cancer. 2020;107:1098-1107
147. Wieder T, Eigentler T, Brenner E, Röcken M. Immune checkpoint blockade therapy. J Allergy Clin Immunol. 2018;142:1403-14
Author contact
![]() Corresponding author: Dr. Jin Zhou, Department of Medical Oncology, Sichuan Clinical Research Center for Cancer, Sichuan Cancer Hospital & Institute, Sichuan Cancer Center, Affliated Cancer Hospital of University of Electronic Science and Technology of China, Chengdu, China, E-mail: zhoujinorg.cn.
Corresponding author: Dr. Jin Zhou, Department of Medical Oncology, Sichuan Clinical Research Center for Cancer, Sichuan Cancer Hospital & Institute, Sichuan Cancer Center, Affliated Cancer Hospital of University of Electronic Science and Technology of China, Chengdu, China, E-mail: zhoujinorg.cn.

 Global reach, higher impact
Global reach, higher impact