10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2023; 19(8):2588-2598. doi:10.7150/ijbs.82362 This issue Cite
Review
Influence of gut microbiota on resilience and its possible mechanisms
1. Beijing Institute of Pharmacology and Toxicology, Beijing 100850, China.
2. State Key Laboratory of Toxicology and Medical Countermeasures, Beijing 100850, China.
3. Department of Pharmacy, The Second Affiliated Hospital of Chongqing Medical University, Chongqing, 400010, China.
# Co-first authors, these two authors contributed equally to this work.
Received 2023-1-4; Accepted 2023-4-28; Published 2023-5-8
Abstract
Excessive stress leads to disruptions of the central nervous system. Individuals' responses to stress and trauma differ from person to person. Some may develop various neuropsychiatric disorders, such as post-traumatic stress disorder, major depression, and anxiety disorders, while others may successfully adapt to the same stressful events. These two neural phenotypes are called susceptibility and resilience. Previous studies have suggested resilience/susceptibility as a complex, non-specific systemic response involving central and peripheral systems. Emerging research of mechanisms underlying resilience is mostly focussing on the physiological adaptation of specific brain circuits, neurovascular impairment of the blood-brain barrier, the role of innate and adaptive factors of the immune system, and the dysbiosis of gut microbiota. In accordance with the microbiota-gut-brain axis hypothesis, the gut microbiome directly influences the interface between the brain and the periphery to affect neuronal function. This review explored several up-to-date studies on the role of gut microbiota implicated in stressful events-related resilience/susceptibility. We mainly focus on the changes in behavior and neuroimaging characteristics, involved brain regions and circuits, the blood-brain barrier, the immune system, and epigenetic modifications, which contribute to stress-induced resilience and susceptibility. The perspective of the gut-brain axis could help to understand the mechanisms underlying resilience and the discovery of biomarkers may lead to new research directions and therapeutic interventions for stress-induced neuropsychiatric disorders.
Keywords: resilience, brain circuits, blood-brain barrier, immune system, epigenetics, gut microbiota
Introduction
The emerging concept of resilience is relatively novel in neuroscience, while psychologists initially defined resilience as "the process of adapting well in the face of adversity, trauma, tragedy, threats, or significant sources of stress" [1]. Individuals' responses to stress and trauma can differ from person to person. Individuals with low resilience might show life-long mental disorders or relatively severe acute responses to negative stressors. However, others with higher resilience may adapt successfully to the same stressful events. As such, resilience can be considered a central role in the maturation of the nervous system and a significant target for neuropsychiatric interventions [2].
Unfortunately, the mechanism of the influence of resilience and related influencing factors is still elusive. Emerging research indicated that the homeostasis of the microbiota-gut-brain axis plays a significant role in the regulation of resilience in multiple pathways. In this paper, we reviewed studies in neuroscience to have a more comprehensive understanding of resilience and the involvement of stress-induced gut microbiota alternations. Deciphering underlying molecular mechanisms by which gut microbiota contributes to stress-induced resilience and susceptibility will help to provide intriguing and novel therapeutic interventions for stress-induced neuropsychiatric disorders.
Implication of gut microbiota in resilience
Definition and features of resilience
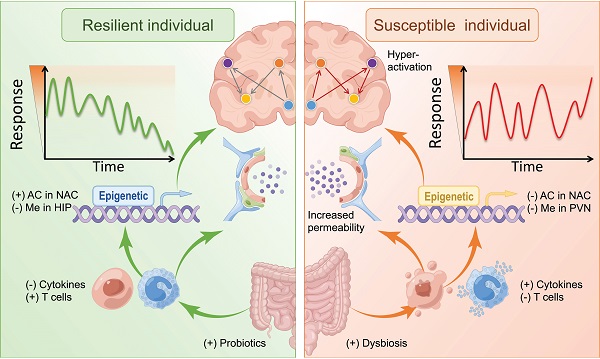
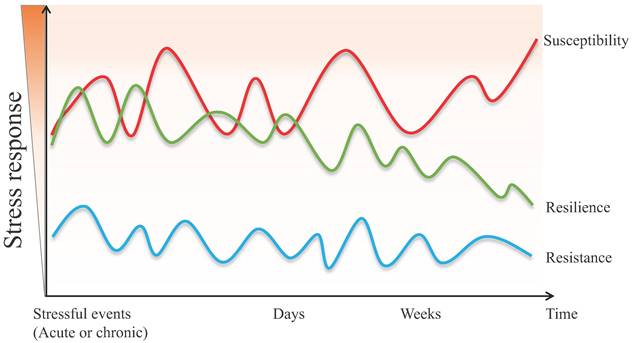
Although the definition of resilience is not conclusive so far, each perspective shares three common characteristics: (1) resilience mainly describes the restoration of equilibrium or steady-state after stress stimulation. (2) resilience is not static, but a dynamic response to the environment, age, psychology, etc. (3) resilience is typically produced within serious but tolerable environmental or physiological stimuli. In other words, the process of being able to adapt well to different types of stressors is called "resilience", while the opposite one is called "susceptibility" [3, 4]. Susceptibility, also referred to as vulnerability, is one in which moderate or severe levels of stress or distress persist and may increase further as more time passes [5]. Furthermore, the individual, whose initial response to the stressful stimulus is absent, is defined as resistant [6]. The main differences between resistant and resilient individuals lie in the initial response to stimulus and post-stress trajectory (Figure 1).
As an adapting process to adversity, resilience is a common and universal physiological phenomenon [7, 8]. Research on resilience emerged in the 1970s, and most of them suggested that intrinsic psychological characteristics, including positive emotionality, were highly associated with the capacity for resilience [9, 10]. In addition, different adaptive strategies may lead to resilience or susceptibility to exposure to stress. For example, an optimistic coping style promotes successful adaptation by intentionally minimizing the physical, psychological, or social damage caused by stress, while passive coping styles (such as avoidance, helplessness, etc.) can conversely lead to vulnerability and susceptibility instead of resilience [11]. Given the possible impact on health, well-being, and quality of life [12, 13], a growing body of research focuses on the measurement methods and biological mechanisms of resilience.
To distinguish between resilient and susceptible individuals, most research scientists primarily measured resilience with scales. For instance, the Ego-Resiliency Scale (ERS) [14], Connor-Davidson Resilience Scale (CD-RISC) [15], Dispositional Resilience Scale (DRS) [16], and Resilience Scale 25 (RS 25) [17]. However, the detailed pathways and mechanisms of resilience can be complex. With the in-depth study of the biological mechanism of resilience, the nervous system, immune system, endocrine system, genetic genes, epigenetics, and intestinal microbes, all play essential roles in the resilience process. Thereinto, stress-induced perturbed microbiota is associated with resilience, and a growing body of research has revealed a cause-effect relationship [18].
Link between the gut microbiota and resilient behavior
Evidence is accumulating that there is a close and bidirectional symbiotic relationship between gut microbiota and the host. Notably, the impact of gut microbiota on resilience-associated behavior has been investigated. Studies revealed that the homeostasis of gut microbiota is intimately involved in the regulation of the host's psychology, emotions, and cognition in response to stressful events [19, 20].
The different psychopathological responses in susceptive, resilient, and resistant individuals when exposed to acute or chronic stressful events.
Animal studies showed that the disordered composition of the gut microbiome contributed to susceptible behavior in rats with the learned helplessness (LH) paradigm [21]. Chronic social defeat stress (CSDS) caused significant anhedonia in the mice with clear changes in microbial diversity and composition, such as Bacteroides spp [22, 23]. Based on the arousal-based individual screening model, the resilient mice showed a decreased volatility of the gut microbiota comparing two-time points (pre-trauma and post-trauma) [24]. The colonization of BALB/c and NIH Swiss mice with microbiota from each other resulted in a switch of anxiety-like phenotypes versus resilient phenotypes [25]. An anhedonia-like phenotype in antibiotic-treated mice was induced by fecal microbiota transplantation (FMT) from susceptible mice [26]. Moreover, germ-free mice showed increased motor activity and decreased anxiety-like behavior, accompanied by an elevated level of norepinephrine (NE), dopamine (DA), and 5-hydroxytryptamine (5-HT) in the striatum compared with control mice with normal gut microbiota [27]. Another study demonstrated that germ-free mice displayed increased depression-like behaviors after the transplantation of fecal microbiota from patients with MDD [28].
Furthermore, in the human study, major depressive disorder (MDD) could exhibit the disruption of the composition of gut microbiota [29, 30]. Supplementation of probiotics can also promote resilience. For example, oral treatment with Bifidobacterium increased the proportion of resilient mice after CSDS compared with controls [31]. Treatment with Lactobacillus rhamnosus led to a reduction of anxiety-like behavior, alleviation of dendritic cell activation, and an increment of IL-10+ Tregs [32]. Intakes of betaine, a dietary nutrient, have been shown to improve an anhedonia-like phenotype and decrease plasma levels of IL-6 in CSDS mice [33]. Similar results were observed for the repeated administration of 3,4-Methylenedioxymethamphetamine (MDMA) [34]. However, further studies are required to elucidate how the disruption of gut microbiota influences the behavior of resilience/susceptibility.
Gut microbiota affected brain functional connectivity
The role of gut microbiota in host-brain functional connectivity has been well established [35]. A growing body of research indicates the influence of the microbiota on brain structural and functional connectivity as well.
In the animal study, treating gut microbiota from attention-deficit/hyperactivity disorder persons showed a decreased functional connectivity between the right motor cortex and right visual cortex [36].
Probiotic administration was correlated with the brain activation patterns in response to emotion-related memory and task of healthy participants [37]. A clinical report revealed that Roseburia spp., a known butyrate producer, positively associated with the adjusted volume of the left hippocampus evaluated by magnetic resonance imaging (MRI), and also accordant negative associations between Bacteroides sp. (Gram-negative bacteria) and the volume of the left hippocampus [38]. Additional functional MRI study demonstrated that the Prevotella genus (Gram-negative bacteria) was associated with the strength of all brain functional connectivity networks, while Bifidobacterium (Gram-positive bacteria, a species of “good bacteria”) was associated with the default mode network (DMN) and frontoparietal-attention network (FPN) [39]. In patients with MDD, the fecal Bacteroides (Gram-negative bacteria) abundance was inversely correlated with the left dorsolateral prefrontal cortex (DLPFC) [40]. Moreover, gut dysfunction also could impact the changes in neuronal integrity and edema quantified by diffusion tensor imaging (DTI) in some studies [41, 42].
Intestinal microbiota influenced resilience-related EEG features
In recent years, electroencephalographic (EEG) signals served as an indicator of a personal capacity to cope with psychological or psychogenic stress. A study indicated higher right prefrontal theta power in resilient participants compared with susceptible participants with PTSD [43]. Additionally, the EEG asymmetry across central cortical regions was found to be able to distinguish between resilience and susceptibility in children [44]. The low-level resilience individual had higher alpha coherence in the right hemisphere [45]. Spectral analysis of the EEG found that resilience rats had significantly greater theta power compared to susceptibility rats [46]. In turn, the neurofeedback training based on the EEG alpha/theta ratio could improve resilience to stress [47].
The data from animal and human clinical studies indicated that altered intestinal microbiomes influenced EEG signals. Lipopolysaccharide intravenous injection remarkably induced excessive theta and delta activity and, these EEG abnormalities could be reversed by FMT in rats [48]. Short-term administration of the probiotic of different Lactobacillus spp. significantly increased the ratio of EEG delta power spectra (CP2305) [49] and decreased the percentage of beta power, alpha power, and theta power in a randomized, double-blind, placebo-controlled pilot trial (PS128) [50]. Gut microbiota depletion by chronic antibiotic treatment reduced the theta power density [51]. In brief, the current studies have revealed the relationship between the gut microbiota and EEG features, denoting that changes to the intestinal microbiome have the potential to improve resilience-related EEG features. However, the exact metabolites that contribute to EEG regulation will be needed to ulteriorly research.
Possible mechanisms of microbiome-mediated resilience and susceptibility
Neural mechanisms
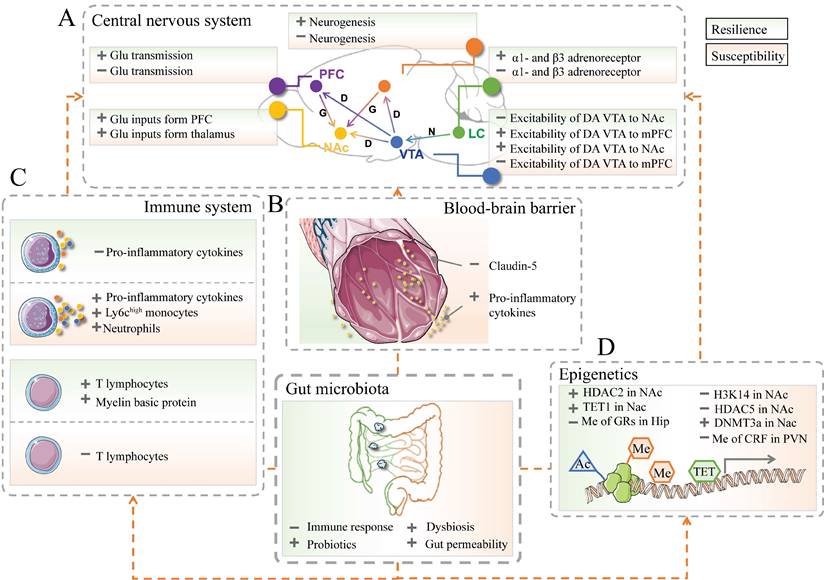
Recent developments in multivariate research enable the discovery of the limbic system and its involvement in resilient and susceptible responses to stressful events. The limbic system includes the prefrontal cortex (PFC), hippocampus, amygdala, VTA-NAc reward circuit (composed of ventral tegmental area, VTA; nucleus accumbens, Nac), hypothalamus, locus coeruleus (LC), and other brain areas [52-55] (Figure 2A). For instance, the expression of brain‑derived neurotrophic factor (BDNF) pro‑peptide, IL-6, and dendritic spine density were regionally different in PFC, hippocampus and Nac, which might contribute to resilience to LH stress or CSDS [56-59].
The dopaminergic pathway in the central nervous system (CNS) is a vital reward circuit consisting of dopamine neurons, those of which located in VTA have projections throughout Nac, hippocampus, mPFC, and other prefrontal areas [60, 61]. The previous literature unveiled that the gut microbiota had a role in the dopaminergic pathway. Gut microbiota composition and the microbial metabolite proved their involvement in the activity of neurotransmitters in the dopamine reward circuit and the alternation of dopamine transporter mRNA levels, which in turn are involved in social behavior deficits and psychiatric disorders [62-65]. Microbiota-based interventions were proven to decrease anxiety-like behavior through the level of dopamine in the Nac and the expression of dopamine D1 and D2 receptors in the PFC [66].
Studies regarding gut microbiota proved that GABA/glutamate can be affected via the microbiota-gut-brain axis. Inhibitory GABA and excitatory glutamate are two major neurotransmitters in CNS, and they normally work together in physiological processes. Notably, imaging studies using DTI and fMRI showed that microbiota-derived fecal metabolites are correlated with functional and anatomical connectivity of the central reward network, including NAc [67]. And the probiotic administration could induce GABA (B1b) mRNA alternations in a brain region-dependent manner and could prevent sCSDS-induced gene alterations that aid in signal transduction or CNS development of the NAc [68, 69]. In addition, transplantation of fecal microbiota from patients with alcoholism could decrease the alpha 1 subunit of GABA type A receptor (α1GABAR) in mPFC and metabotropic glutamate receptors 1 (mGluR1) in NAc and lead to anxiety and depression in mice [70].
The hippocampus is another essential brain area that responds to stress. Numerous studies found that the administration of gut microbial metabolites or probiotics could induce alternations in the hippocampus via the microbiota-gut-brain axis, which raises the possibility that gut microbiota is effective in managing stress-related psychiatric disorders [71-73]. Additionally, fecal microbiota transplantation can relieve brain injury-induced neurological deficits through the elevation of superoxide dismutase and catalase activities in the ipsilateral hippocampus [74]. Gene expression in the hippocampus modified by prebiotic administration was also found to correlate with the reduction of depression- and anxiety-like behavior [75]. Taken together, the emerging scientific data have supported the important role of the intestinal microbiota in the regulation of the CNS and have been highly involved in the pathogenesis of stress-related disorders. However, the precise mechanism of the microbiota in resilience-related neural circuits remained open to debate.
Blood-brain barrier
The blood-brain barrier (BBB) consists of brain microvascular endothelial cells, astrocytes, and surrounding cells. It is a dynamic interface between peripheral circulation and CNS that selectively regulates substance exchange to contribute to the homeostasis of the CNS microenvironment [77, 78] (Figure 2B). Researchers started to realize that stress and gut microbiota could result in BBB disruption [79, 80]. CSDS could induce disruption of BBB integrity via down-regulation of the endothelial tight junction protein (Claudin-5), which in turn led to the infiltration of peripheral cytokine interleukin-6 (IL-6) into the CNS and an increased proportion of stress-susceptible mice [81]. LH-induced mice also showed increased permeability of the BBB. Additionally, the impaired BBB could be repaired by TNF-alpha inhibitor treatment [82]. A recent study revealed that the clearance of the microbiome by antibiotics may also lead to the down-regulation of tight-junction proteins and an increase in BBB permeability. Furthermore, fecal microbiota transplantation could rescue the disruption of BBB [80]. As such, gut microbiota-induced or stressful events-induced BBB impairments may be considered another therapeutic target for resilience research.
The implication of gut microbiota in resilience and susceptibility. (A) The crucial brain regions and circuits in mediating resilient and susceptible stress-induced responses. D or DA: Dopamine; G or Glu: Glutamate; HIP: Hippocampus; LC: Locus coeruleus; mPFC: Medial prefrontal cortex; N: Norepinephrine; NAc: Nucleus accumbens; PFC: Prefrontal cortex; VTA: Ventral tegmental area; light green part represents resilience; light pink part represents susceptibility. (B) The neurovascular impairments of the blood-brain barrier in stress responses. The left part in green represents resilience; the right part in pink represents susceptibility. The images were provided by Servier Medical Art [76]. (C) The innate (top), and adaptive (bottom) immune systems contribute to resilience and susceptibility. (D) The epigenetic processes are associated with resilient responses. Ac: acetylation; Me: methylation; TET: ten-eleven translocation protein; HDAC: histone deacetylase; H3K14: histone 3 lysine 14; DNMTs: DNA methyltransferases; CRF: corticotropin-releasing factor. + represents up-regulation; - represents down-regulation.
The innate and adaptive immune system
Resilience/susceptibility of the nervous system is potentially associated with the immune system. The innate immune system recruits immune cells to infected or inflammatory areas by producing cytokines. As such, they can non-specifically recognize and defect against invading pathogens [83, 84]. Animal or clinical studies suggested that pathogens or repeated psychosocial stress could influence the circulating inflammatory and induce the recruitment of immune cells (e.g., Ly6chigh monocytes and neutrophils) and the production of pro-inflammatory mediators (e.g., IL-1β, IL-6, and TNF-α) [85-88]. The level of peripheral pro-inflammatory cytokines in patients or mice with psychiatric disorders was significantly elevated than that in healthy controls [89, 90].
Furthermore, the inhibition of IL-6 expression in CSDS mice by treatment with IL-6 antibody promotes the recovery of resilience [91, 92]. In addition, lethally irradiated wild-type mice exhibited susceptibility to CSDS after transplantation of hematopoietic stem cells (HSCs) from susceptible C57BL/6J mice. On the contrary, those wild-type mice showed no susceptible phenotype to CSDS after transplanted HSCs from IL-6 knockout mice instead of those from susceptible ones [93]. Chemicals that could prevent cytokine releases, such as dihydrocaffeic acid (DHCA) and malvidin-3′-O-glucoside (Mal-gluc), were proved effective in resilience development [94] (Figure 2C).
Adaptive immunity involves the later stage of infection and can be activated by exposure to invading pathogens [95]. At present, there are relatively few studies on T and B lymphocytes' response when encountering stress [96]. A meta-analysis of depression and immunology identified a reduction of T-cell proportion and elevation in the CD4/CD8 ratio in the blood sample of patients with MDD [97]. Further studies indicated the level of T cells in CNS is positively correlated with resilience improvement in the response to psychological stress [98, 99]. After receiving lymphocytes from mice exposed to CSDS, naïve lymphogenic Rag2-/- mice exerted less anxious behavior and low pro-inflammatory cytokine levels compared with those transplanted with unstressed cells [100] (Figure 2C).
Interestingly, anti-inflammatory therapies may exert antidepressant effects [101]. Although it is still controversial whether traditional antidepressants can decrease the level of peripheral cytokines, a recent meta-analysis showed that serum levels of interleukin-1β (IL-1β) and IL-6 were decreased after antidepressant medication treatment [102]. Another study also proved that the infusion of ketamine, a rapid antidepressant, could reduce the level of pro-inflammatory cytokines [103]. However, whether these inflammatory changes are causally related to the efficacy of antidepressants remains elusive.
Notably, stress-induced alternations of gut microbiota and peripheral immune system led to resilience/susceptibility development. However, the underlying mechanism is still unclear until a recent study revealed that activation of the vagus nerve by gut microbiota-mediated cytokines release could induce pro-rewarding effects in NAc, suggesting a critical role of gut microbiota in immune system-induced psychiatric disorders [104]. It is well known that the α7 subtype of nicotinic acetylcholine receptors (coded by the Chrna7 gene) and soluble epoxide hydrolase (coded by the Ephx2 gene) played key roles in inflammation involved in MDD. FMT from Chrna7 or Ephx2 knock-out mice induced depression-like behaviors in antibiotic-treated mice, which were significantly blocked by the subdiaphragmatic vagotomy (SDV) [26, 105]. The SDV also slowed the development of depression- and anhedonia-like phenotypes induced by ingestion of Lactobacillus intestinalis and Lactobacillus reuteri [106]. These recent findings suggested that the vagus nerve was an essential part of the gut-brain axis [107].
Meanwhile, as we just mentioned above, gut microbiota or stressful events may cause BBB impairment and infiltration of cytokines into the CNS, followed by subsequent disruption of neural function and depression-like behaviors [81]. Meanwhile, microglia from the mice with stressed lymphocytes shifted to an anti-inflammatory phenotype [100]. Antibiotic minocycline can prevent chronic unpredictable stress-induced depressive-like behavior in rats due to inhibition of microglia activation, indicating that interventions for microglia can promote resilience [108]. Since the metabolites of the gut microbiota can not only affect the immune system but also directly influence microglia [109] and BBB [110], the gut microbiota could be a novel therapeutic target for the improvement of resilience and prevent stress-related psychiatric disorders.
Epigenetic mechanisms
Emerging evidence suggests a critical role of gut microbiota and epigenetics in resilience development. Histone modifications and DNA methylation are two major epigenetic markers implicated in stress-related disorders. Enzymes, including acetylases and methylases, are involved in those epigenetic processes (Figure 2D).
However, the role of histone acetylation in resilience is still controversial. The reduction of histone 3 lysine 14 (H3K14) acetylation in NAc was found in patients with depression and CDSD-induced susceptible mice [111]. And the elevation of histone acetylation by delivery of histone deacetylase (HDACs) inhibitor in the hippocampus or NAc exerted an antidepressant effect, which is similar to that of fluoxetine [112, 113]. On the contrary, another study showed the opposite effect of HDACs on resilience. CSDS-induced susceptible mice exhibited a reduction of HDAC5 in NAc instead of elevation. Additionally, chronic administration of antidepressant imipramine could significantly up-regulate HDAC5 mRNA level in the NAc, suggesting upregulation of HDAC5 in NAc may mediate a resilient response [114]. Overexpression of HDAC2 in NAc by AAV-HDAC2 injection prevents social avoidance induced by chronic stress [115]. Albeit the controversial results, those aforementioned findings still indicated that HDACs are highly involved in regulating resilience/susceptibility. Additionally, there is an intriguing finding that the expression of H3K14Ac in NAc is significantly different from that in other brain regions. For example, H3K14Ac levels in the hippocampus were continuously decreased after stress induction, while the levels of which in PFC were oppositely increased [116]. Moreover, researchers found that HDAC inhibitors could enhance the efficacy of antidepressant treatment of the serotonin reuptake inhibitor [117]. A further possibility of HDAC inhibitor treatment for clinical patients with drug-resistant depression has also been proposed [118]. Taken together, HDAC may be developed as a target for the therapeutic intervention of stress-related neuropsychiatric diseases due to its involvement in resilience. Besides acetylation, histone methylation is also closely related to resilience [119].
DNA methylation refers to the process of covalent attaching methyl groups to cytosine by DNA methyltransferases (DNMTs). Methylation is an epigenetic marker for transcriptional repression through chromatin modification and alternation of DNA conformation or stability [120]. Studies found that DNMT3a expression in the brain is highly associated with the separation of resilience/susceptibility phenotypes. Exposure to CSDS and subchronic variable stress (SCVS) may up-regulate the expression of DNMT3a in NAc. In addition, overexpression of DNMT3a in the NAc could result in depression-like behavior in both male and female mice, while knockout DNMAT3a in the NAc of female mice could ameliorate susceptible behavior after SCVS exposure [121, 122]. The role of DNA dioxygenase, ten-eleven translocation protein 1 (TET1), in resilience has been investigated. Research detected a reduction of the level of TET1 in the NAc in CSDS -induced susceptible mice. However, the knockout of TET1 in the NAc can alleviate depression-associated behavior [123]. Furthermore, an increased mRNA expression of corticotropin-releasing factor (CRF) in the paraventricular nucleus (PVN) was found in stress-induced susceptible mice, while methylation of CRF promoter was significantly reduced [124]. Up-regulation of DNA methylation of glucocorticoid receptors in the hippocampus is also associated with depression-like behaviors, especially in individuals who have experienced adversity in childhood [125, 126].
Notably, the activity of those enzymes could be influenced by the host's gut microbiota and its metabolites. Probiotic intervention could restore the decrease of methylation in the hippocampus [127]. Short-chain fatty acids (SCFAs), the main metabolites generated by gut microbiota, were highly involved in gut and brain interaction. Sodium butyrate, one of the SCFAs, could activate the autophagy pathway and inhibit cell proliferation via histone acetylation modification as an HDAC inhibitor [128, 129]. A further study proved that butyrate treatment prevents LPS-induced depressive-like behaviors and activation of microglia in mice hippocampus by the acetylation of histone H3 and H4, suggesting an antidepressant effect [130]. Besides butyrate, methyl donors (choline and folate)-induced global DNA methylation could successfully ameliorate adverse early life-related depressive-like behavior and metabolic disturbance in adult life [131, 132]. The relationship between gut microbiota and epigenetic modification indicates their significant role in epigenetics involved in stress-related resilience.
Outstanding issues
Mounting evidence from both clinical and animal studies presented convincing data that microbiota could influence the neurological, electrophysiological, and immune processes of stress-related resilience. However, our review deemed there two essential questions regarding resilience remained unanswered. Firstly, the measurement method or indicator for distinguishing resilient and susceptible individuals remains open to debate. Most human studies used scales to measure resilience, for instance, ERS, CD-RISC, DRS, RS 25, etc[17]. For animal studies, discrimination has been built on different aspects, including coping strategies [4], social symptoms of depression and anxiety [53], anhedonia [133], and urine scent marking (USM) [134]. Additionally, the peptides Neuropeptide Y [135], orexins [136], sphingosine-1-phosphate 3 receptor [137], and glucocorticoid receptor [138], have been identified to segregate resilient and susceptible animals. Secondly, an explicit conceptual understanding of resilience, irrespective of the terminology being used, is essential to carry out research in this field forward. Thirdly, to determine whether gut microbiota is important in resilience phenotype, it should be researched under different conditions. For instance, if a gut microbiota is exhibited in disorder composition in animals resilient to social stress, is that same gut bacterial genus also significant in response to physical stress or predator odor? Is that same bacterial genus significant in both sexes? Is that same bacterial genus significant for outcomes in multiple response domains? In a nutshell, the causal role of the gut microbiome in genetics, epigenetics, neurobiology, and neuroendocrinology can help researchers promote the understanding of the relationship between gut microbiota and resilience.
Conclusion and future work
An in-depth understanding of resilience could help researchers and clinicians to improve the therapeutic effects on stress-related disorders through individualized targeted therapeutic strategies. However, current studies in this area are still in their infancy, further research is still urgently required to investigate the potential mechanism and to discover potential therapeutic targets in both animal and clinical research. Accumulating evidence suggests gut microbiota influence the development of resilience in multiple pathways. This review recapitulated research on the role of gut microbiota in the stress-induced alternation of behavior, neuroimaging, CNS, BBB, immune system, and epigenetics.
Considerable development has been created in the understanding of the gut-brain axis in preclinical models of neurological disorders and the potential translation of these signs of progress to humans. A growing body of research has confirmed the cross-sectional differentiations in the composition of gut microbiota between defined diseases and controlled healthy populations. Numerous rodent models of neurological diseases have been made that imitate particular disease aspects. The FMTs from patients with certain neurological disorders into antibiotic-treated or GF rodents have led to changed cognitive and social behaviors, mimicking certain features of the human phenotype. To date, FMTs from healthy subjects into patients with neurological disorders have resulted in the amelioration of some symptoms, but not a consistent improvement. There is limited supporting evidence for the efficiency of therapies targeted at the microbiota to data. Hence, the causal relationship between the gut microbiome and resilience remains to be confirmed. Integrative studies of microbiology and neuroscience could lead to novel insight into stress-related MDD, post-traumatic stress disorder, and other neuropsychiatric disorders and effective cross-disciplinary therapeutic approaches.
Abbreviations
5-HT: 5-hydroxytryptamine; BBB: blood-brain barrier; CNS: central nervous system; CRF: corticotropin-releasing factor; CSDS: chronic social defeat stress; DA: dopamine; DHCA: Dihydrocaffeic acid; DNMTs: DNA methyltransferases; H3K14: histone 3 lysine 14; HDACs: histone deacetylase; HPA: hypothalamic-pituitary-adrenal; HSCs: hematopoietic stem cells; IL: interleukin; LC: locus coeruleus; Mal-gluc: malvidin-3′-O-glucoside; MDD: major depressive disorder; MSNs: medium spiny neurons; NAc: nucleus accumbens; NE: norepinephrine; PFC: prefrontal cortex; PVN: paraventricular nucleus; SCVS: subchronic variable stress; TET1: ten-eleven translocation protein 1; vSub: ventral subiculum; VTA: ventral tegmental area.
Acknowledgements
Funding
This review was supported by the National Natural Science Foundation of China (82174006) and the Beijing Municipal Natural Science Foundation (7222292).
Author Contributions
Conceptualization, W.Z., Z.X.; resources, J.W., T.Z.; data curation, F.L., Y.H.; writing-original draft preparation, J.W., T.Z.; writing-review and editing, J.W., T.Z.; supervision, W.Z., Z.X. All authors have read and agreed to the published version of the manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Southwick SM, Charney DS. The science of resilience: implications for the prevention and treatment of depression. Science (New York, NY). 2012;338:79
2. Masten AS. Global perspectives on resilience in children and youth. Child Dev. 2014;85:6-20
3. Southwick SM, Vythilingam M, Charney DS. The psychobiology of depression and resilience to stress: implications for prevention and treatment. Annu Rev Clin Psychol. 2005;1:255-91
4. Wood SK, Bhatnagar S. Resilience to the effects of social stress: evidence from clinical and preclinical studies on the role of coping strategies. Neurobiol Stress. 2015;1:164-73
5. Bhatnagar S. Rethinking stress resilience. Trends Neurosci. 2021;44:936-45
6. Monika Fleshner, Steven F, Maier David. et al. The neurobiology of the stress-resistant brain. Stress. 2011
7. Garmezy N. Vulnerability research and the issue of primary prevention. Am J Orthopsychiatry. 1971;41:101-16
8. Masten AS. Ordinary magic. Resilience processes in development. Am Psychol. 2001;56:227-38
9. Feder A, Nestler EJ, Charney DS. Psychobiology and molecular genetics of resilience. Nat Rev Neurosci. 2009;10:446-57
10. Ozbay F, Fitterling H, Charney D, Southwick S. Social support and resilience to stress across the life span: a neurobiologic framework. Curr Psychiatry Rep. 2008;10:304-10
11. Koolhaas JM, Korte SM, De Boer SF, Van Der Vegt BJ, Van Reenen CG, Hopster H. et al. Coping styles in animals: current status in behavior and stress-physiology. Neurosci Biobehav Rev. 1999;23:925-35
12. Ungar M, Theron L. Resilience and mental health: how multisystemic processes contribute to positive outcomes. Lancet Psychiatry. 2020;7:441-8
13. Di Monte C, Monaco S, Mariani R, Di Trani M. From Resilience to Burnout: Psychological Features of Italian General Practitioners During COVID-19 Emergency. Front Psychol. 2020;11:567201
14. Block J, Kremen AM. IQ and ego-resiliency: conceptual and empirical connections and separateness. J Pers Soc Psychol. 1996;70:349-61
15. Connor KM, Davidson JR. Development of a new resilience scale: the Connor-Davidson Resilience Scale (CD-RISC). Depress Anxiety. 2003;18:76-82
16. Bartone PT. Test-retest reliability of the dispositional resilience scale-15, a brief hardiness scale. Psychol Rep. 2007;101:943-4
17. Konaszewski K, Skalski S, Surzykiewicz J. The Polish Version of the Resilience Scale 25: Adaptation and Preliminary Psychometric Evaluation. Front Psychol. 2021;12:668800
18. Isingrini E, Perret L, Rainer Q, Amilhon B, Guma E, Tanti A. et al. Resilience to chronic stress is mediated by noradrenergic regulation of dopamine neurons. Nature neuroscience. 2016;19:560-3
19. Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13:701-12
20. Sekirov I, Russell SL, Antunes LC, Finlay BB. Gut microbiota in health and disease. Physiol Rev. 2010;90:859-904
21. Zhang K, Fujita Y, Chang L, Qu Y, Pu Y, Wang S. et al. Abnormal composition of gut microbiota is associated with resilience versus susceptibility to inescapable electric stress. Translational Psychiatry. 2019;9:231
22. Wang S, Qu Y, Chang L, Pu Y, Zhang K, Hashimoto K. Antibiotic-induced microbiome depletion is associated with resilience in mice after chronic social defeat stress. Journal of Affective Disorders. 2020;260:448-57
23. Dos Santos Guilherme M, Valeri F, Winter J, Müller MB, Schwiertz A, Endres K. Resilience and the Gut Microbiome: Insights from Chronically Socially Stressed Wild-Type Mice. Microorganisms. 2022;10:1077
24. Laudani S, Torrisi SA, Alboni S, Bastiaanssen TFS, Benatti C, Rivi V. et al. Gut microbiota alterations promote traumatic stress susceptibility associated with p-cresol-induced dopaminergic dysfunctions. Brain, Behavior, and Immunity. 2023;107:385-96
25. Bercik P, Denou E, Collins J, Jackson W, Lu J, Jury J. et al. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology. 2011;141:599-609 e1-3
26. Wang S, Ishima T, Qu Y, Shan J, Chang L, Wei Y. et al. Ingestion of Faecalibaculum rodentium causes depression-like phenotypes in resilient Ephx2 knock-out mice: A role of brain-gut-microbiota axis via the subdiaphragmatic vagus nerve. Journal of Affective Disorders. 2021;292:565-73
27. Diaz Heijtz R, Wang S, Anuar F, Qian Y, Bjorkholm B, Samuelsson A. et al. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci U S A. 2011;108:3047-52
28. Zheng P, Zeng B, Zhou C, Liu M, Fang Z, Xu X. et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host's metabolism. Mol Psychiatry. 2016;21:786-96
29. Jiang H, Ling Z, Zhang Y, Mao H, Ma Z, Yin Y. et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav Immun. 2015;48:186-94
30. Naseribafrouei A, Hestad K, Avershina E, Sekelja M, Linlokken A, Wilson R. et al. Correlation between the human fecal microbiota and depression. Neurogastroenterol Motil. 2014;26:1155-62
31. Yang C, Fujita Y, Ren Q, Ma M, Dong C, Hashimoto K. Bifidobacterium in the gut microbiota confer resilience to chronic social defeat stress in mice. Sci Rep. 2017;7:45942
32. Bharwani A, Mian MF, Surette MG, Bienenstock J, Forsythe P. Oral treatment with Lactobacillus rhamnosus attenuates behavioural deficits and immune changes in chronic social stress. BMC Med. 2017;15:7
33. Qu Y, Zhang K, Pu Y, Chang L, Wang S, Tan Y. et al. Betaine supplementation is associated with the resilience in mice after chronic social defeat stress: a role of brain-gut-microbiota axis. Journal of Affective Disorders. 2020;272:66-76
34. Qu Y, Eguchi A, Wan X, Ma L, Chang L, Shan J. et al. Repeated use of 3,4-methylenedioxymethamphetamine is associated with the resilience in mice after chronic social defeat stress: A role of gut-microbiota-brain axis. Psychiatry Research. 2022;320:115020
35. Morais LH, Schreiber HLt, Mazmanian SK. The gut microbiota-brain axis in behaviour and brain disorders. Nat Rev Microbiol. 2021;19:241-55
36. Tengeler AC, Dam SA, Wiesmann M, Naaijen J, van Bodegom M, Belzer C. et al. Gut microbiota from persons with attention-deficit/hyperactivity disorder affects the brain in mice. Microbiome. 2020;8:44
37. Bagga D, Reichert JL, Koschutnig K, Aigner CS, Holzer P, Koskinen K. et al. Probiotics drive gut microbiome triggering emotional brain signatures. Gut Microbes. 2018;9:486-96
38. Arnoriaga-Rodríguez M, Mayneris-Perxachs J, Burokas A, Contreras-Rodríguez O, Blasco G, Coll C. et al. Obesity Impairs Short-Term and Working Memory through Gut Microbial Metabolism of Aromatic Amino Acids. Cell Metab. 2020;32:548-60.e7
39. Kohn N, Szopinska-Tokov J, Llera Arenas A, Beckmann CF, Arias-Vasquez A, Aarts E. Multivariate associative patterns between the gut microbiota and large-scale brain network connectivity. Gut Microbes. 2021;13:2006586
40. Strandwitz P, Kim KH, Terekhova D, Liu JK, Sharma A, Levering J. et al. GABA-modulating bacteria of the human gut microbiota. Nat Microbiol. 2019;4:396-403
41. Ahluwalia V, Betrapally NS, Hylemon PB, White MB, Gillevet PM, Unser AB. et al. Impaired Gut-Liver-Brain Axis in Patients with Cirrhosis. Sci Rep. 2016;6:26800
42. Liu P, Peng G, Zhang N, Wang B, Luo B. Crosstalk Between the Gut Microbiota and the Brain: An Update on Neuroimaging Findings. Front Neurol. 2019;10:883
43. Cowdin N, Kobayashi I, Mellman TA. Theta frequency activity during rapid eye movement (REM) sleep is greater in people with resilience versus PTSD. Exp Brain Res. 2014;232:1479-85
44. Curtis WJ, Cicchetti D. Emotion and resilience: a multilevel investigation of hemispheric electroencephalogram asymmetry and emotion regulation in maltreated and nonmaltreated children. Dev Psychopathol. 2007;19:811-40
45. Lee JY, Choi JS, Kwon JS. Neurophysiological Mechanisms of Resilience as a Protective Factor in Patients with Internet Gaming Disorder: A Resting-State EEG Coherence Study. J Clin Med. 2019 8
46. Sweeten BLW, Sutton AM, Wellman LL, Sanford LD. Predicting stress resilience and vulnerability: brain-derived neurotrophic factor and rapid eye movement sleep as potential biomarkers of individual stress responses. Sleep. 2020 43
47. Keynan JN, Cohen A, Jackont G, Green N, Goldway N, Davidov A. et al. Electrical fingerprint of the amygdala guides neurofeedback training for stress resilience. Nat Hum Behav. 2019;3:63-73
48. Li S, Lv J, Li J, Zhao Z, Guo H, Zhang Y. et al. Intestinal microbiota impact sepsis associated encephalopathy via the vagus nerve. Neurosci Lett. 2018;662:98-104
49. Nishida K, Sawada D, Kuwano Y, Tanaka H, Rokutan K. Health Benefits of Lactobacillus gasseri CP2305 Tablets in Young Adults Exposed to Chronic Stress: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients. 2019 11
50. Ho YT, Tsai YC, Kuo TBJ, Yang CCH. Effects of Lactobacillus plantarum PS128 on Depressive Symptoms and Sleep Quality in Self-Reported Insomniacs: A Randomized, Double-Blind, Placebo-Controlled Pilot Trial. Nutrients. 2021 13
51. Ogawa Y, Miyoshi C, Obana N, Yajima K, Hotta-Hirashima N, Ikkyu A. et al. Gut microbiota depletion by chronic antibiotic treatment alters the sleep/wake architecture and sleep EEG power spectra in mice. Sci Rep. 2020;10:19554
52. Russo SJ, Murrough JW, Han MH, Charney DS, Nestler EJ. Neurobiology of resilience. Nat Neurosci. 2012;15:1475-84
53. Han MH, Nestler EJ. Neural Substrates of Depression and Resilience. Neurotherapeutics. 2017;14:677-86
54. Rodman AM, Jenness JL, Weissman DG, Pine DS, McLaughlin KA. Neurobiological Markers of Resilience to Depression Following Childhood Maltreatment: The Role of Neural Circuits Supporting the Cognitive Control of Emotion. Biol Psychiatry. 2019;86:464-73
55. Baratta MV, Maier SF. New tools for understanding coping and resilience. Neurosci Lett. 2019;693:54-7
56. Yang B, Yang C, Ren Q, Zhang J-C, Chen Q-X, Shirayama Y. et al. Regional differences in the expression of brain-derived neurotrophic factor (BDNF) pro-peptide, proBDNF and preproBDNF in the brain confer stress resilience. European Archives of Psychiatry and Clinical Neuroscience. 2016;266:765-9
57. Qu Y, Yang C, Ren Q, Ma M, Dong C, Hashimoto K. Regional differences in dendritic spine density confer resilience to chronic social defeat stress. Acta Neuropsychiatrica. 2018;30:117-22
58. Yang C, Shirayama Y, Zhang J-C, Ren Q, Hashimoto K. Regional differences in brain-derived neurotrophic factor levels and dendritic spine density confer resilience to inescapable stress. The International Journal of Neuropsychopharmacology. 2015;18:pyu121
59. Yang C, Shirayama Y, Zhang J-C, Ren Q, Hashimoto K. Peripheral interleukin-6 promotes resilience versus susceptibility to inescapable electric stress. Acta Neuropsychiatrica. 2015;27:312-6
60. Nestler EJ, Carlezon WA Jr. The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59:1151-9
61. Russo SJ, Nestler EJ. The brain reward circuitry in mood disorders. Nat Rev Neurosci. 2013;14:609-25
62. Fuenzalida C, Dufeu MS, Poniachik J, Roblero JP, Valenzuela-Perez L, Beltran CJ. Probiotics-Based Treatment as an Integral Approach for Alcohol Use Disorder in Alcoholic Liver Disease. Front Pharmacol. 2021;12:729950
63. Bermudez-Martin P, Becker JAJ, Caramello N, Fernandez SP, Costa-Campos R, Canaguier J. et al. The microbial metabolite p-Cresol induces autistic-like behaviors in mice by remodeling the gut microbiota. Microbiome. 2021;9:157
64. Gonzalez-Arancibia C, Urrutia-Pinones J, Illanes-Gonzalez J, Martinez-Pinto J, Sotomayor-Zarate R, Julio-Pieper M. et al. Do your gut microbes affect your brain dopamine? Psychopharmacology (Berl). 2019;236:1611-22
65. Nettleton JE, Klancic T, Schick A, Choo AC, Shearer J, Borgland SL. et al. Low-Dose Stevia (Rebaudioside A) Consumption Perturbs Gut Microbiota and the Mesolimbic Dopamine Reward System. Nutrients. 2019 11
66. Agusti A, Campillo I, Balzano T, Benitez-Paez A, Lopez-Almela I, Romani-Perez M. et al. Bacteroides uniformis CECT 7771 Modulates the Brain Reward Response to Reduce Binge Eating and Anxiety-Like Behavior in Rat. Mol Neurobiol. 2021;58:4959-79
67. Osadchiy V, Labus JS, Gupta A, Jacobs J, Ashe-McNalley C, Hsiao EY. et al. Correlation of tryptophan metabolites with connectivity of extended central reward network in healthy subjects. PLoS One. 2018;13:e0201772
68. Maehata H, Kobayashi Y, Mitsuyama E, Kawase T, Kuhara T, Xiao JZ. et al. Heat-killed Lactobacillus helveticus strain MCC1848 confers resilience to anxiety or depression-like symptoms caused by subchronic social defeat stress in mice. Biosci Biotechnol Biochem. 2019;83:1239-47
69. Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG. et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A. 2011;108:16050-5
70. Zhao W, Hu Y, Li C, Li N, Zhu S, Tan X. et al. Transplantation of fecal microbiota from patients with alcoholism induces anxiety/depression behaviors and decreases brain mGluR1/PKC epsilon levels in mouse. Biofactors. 2020;46:38-54
71. Wang H, Braun C, Murphy EF, Enck P. Bifidobacterium longum 1714 Strain Modulates Brain Activity of Healthy Volunteers During Social Stress. Am J Gastroenterol. 2019;114:1152-62
72. Juan Z, Chen J, Ding B, Yongping L, Liu K, Wang L. et al. Probiotic supplement attenuates chemotherapy-related cognitive impairment in patients with breast cancer: a randomised, double-blind, and placebo-controlled trial. Eur J Cancer. 2022;161:10-22
73. Chen Y, Wan M, Zhong Y, Gao T, Zhang Y, Yan F. et al. Partially Hydrolyzed Guar Gum Modulates Gut Microbiota, Regulates the Levels of Neurotransmitters, and Prevents CUMS-Induced Depressive-Like Behavior in Mice. Mol Nutr Food Res. 2021;65:e2100146
74. Du D, Tang W, Zhou C, Sun X, Wei Z, Zhong J. et al. Fecal Microbiota Transplantation Is a Promising Method to Restore Gut Microbiota Dysbiosis and Relieve Neurological Deficits after Traumatic Brain Injury. Oxid Med Cell Longev. 2021;2021:5816837
75. Burokas A, Arboleya S, Moloney RD, Peterson VL, Murphy K, Clarke G. et al. Targeting the Microbiota-Gut-Brain Axis: Prebiotics Have Anxiolytic and Antidepressant-like Effects and Reverse the Impact of Chronic Stress in Mice. Biol Psychiatry. 2017;82:472-87
76. Art SM. smart.servier.com
77. Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37:13-25
78. Banks WA. Blood-brain barrier transport of cytokines: a mechanism for neuropathology. Curr Pharm Des. 2005;11:973-84
79. Friedman A, Kaufer D, Shemer J, Hendler I, Soreq H, Tur-Kaspa I. Pyridostigmine brain penetration under stress enhances neuronal excitability and induces early immediate transcriptional response. Nat Med. 1996;2:1382-5
80. Sun N, Hu H, Wang F, Li L, Zhu W, Shen Y. et al. Antibiotic-induced microbiome depletion in adult mice disrupts blood-brain barrier and facilitates brain infiltration of monocytes after bone-marrow transplantation. Brain Behav Immun. 2021;92:102-14
81. Menard C, Pfau ML, Hodes GE, Kana V, Wang VX, Bouchard S. et al. Social stress induces neurovascular pathology promoting depression. Nat Neurosci. 2017;20:1752-60
82. Cheng Y, Desse S, Martinez A, Worthen RJ, Jope RS, Beurel E. TNFalpha disrupts blood brain barrier integrity to maintain prolonged depressive-like behavior in mice. Brain Behav Immun. 2018;69:556-67
83. Mogensen TH. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin Microbiol Rev. 2009;22:240-73 Table of Contents
84. Medzhitov R, Janeway C Jr. Innate immunity. N Engl J Med. 2000;343:338-44
85. Dantzer R. Neuroimmune Interactions: From the Brain to the Immune System and Vice Versa. Physiol Rev. 2018;98:477-504
86. Steptoe A, Hamer M, Chida Y. The effects of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis. Brain Behav Immun. 2007;21:901-12
87. Heidt T, Sager HB, Courties G, Dutta P, Iwamoto Y, Zaltsman A. et al. Chronic variable stress activates hematopoietic stem cells. Nat Med. 2014;20:754-8
88. Powell ND, Sloan EK, Bailey MT, Arevalo JM, Miller GE, Chen E. et al. Social stress up-regulates inflammatory gene expression in the leukocyte transcriptome via beta-adrenergic induction of myelopoiesis. Proc Natl Acad Sci U S A. 2013;110:16574-9
89. Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK. et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67:446-57
90. Kohler CA, Freitas TH, Maes M, de Andrade NQ, Liu CS, Fernandes BS. et al. Peripheral cytokine and chemokine alterations in depression: a meta-analysis of 82 studies. Acta Psychiatr Scand. 2017;135:373-87
91. Rojas M, Rodriguez Y, Monsalve DM, Pacheco Y, Acosta-Ampudia Y, Rodriguez-Jimenez M. et al. Cytokine imbalance in patients with systemic sclerosis and resilience: the key role of interleukin-6. Clin Exp Rheumatol. 2019;37(Suppl 119):15-22
92. Hodes GE, Pfau ML, Leboeuf M, Golden SA, Christoffel DJ, Bregman D. et al. Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proc Natl Acad Sci U S A. 2014;111:16136-41
93. Hodes GE, Kana V, Menard C, Merad M, Russo SJ. Neuroimmune mechanisms of depression. Nat Neurosci. 2015;18:1386-93
94. Wang J, Hodes GE, Zhang H, Zhang S, Zhao W, Golden SA. et al. Epigenetic modulation of inflammation and synaptic plasticity promotes resilience against stress in mice. Nat Commun. 2018;9:477
95. Bonilla FA, Oettgen HC. Adaptive immunity. J Allergy Clin Immunol. 2010;125:S33-40
96. Miller AH. Depression and immunity: a role for T cells? Brain Behav Immun. 2010;24:1-8
97. Zorrilla EP, Luborsky L, McKay JR, Rosenthal R, Houldin A, Tax A. et al. The relationship of depression and stressors to immunological assays: a meta-analytic review. Brain Behav Immun. 2001;15:199-226
98. Lewitus GM, Schwartz M. Behavioral immunization: immunity to self-antigens contributes to psychological stress resilience. Mol Psychiatry. 2009;14:532-6
99. Lewitus GM, Cohen H, Schwartz M. Reducing post-traumatic anxiety by immunization. Brain Behav Immun. 2008;22:1108-14
100. Brachman RA, Lehmann ML, Maric D, Herkenham M. Lymphocytes from chronically stressed mice confer antidepressant-like effects to naive mice. J Neurosci. 2015;35:1530-8
101. Kohler O, Benros ME, Nordentoft M, Farkouh ME, Iyengar RL, Mors O. et al. Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects: a systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry. 2014;71:1381-91
102. Hannestad J, DellaGioia N, Bloch M. The effect of antidepressant medication treatment on serum levels of inflammatory cytokines: a meta-analysis. Neuropsychopharmacology. 2011;36:2452-9
103. Chen MH, Li CT, Lin WC, Hong CJ, Tu PC, Bai YM. et al. Rapid inflammation modulation and antidepressant efficacy of a low-dose ketamine infusion in treatment-resistant depression: A randomized, double-blind control study. Psychiatry Res. 2018;269:207-11
104. Ezquer F, Quintanilla M, Moya-Flores F, Morales P, Munita J, Olivares B. et al. Innate gut microbiota predisposes to high alcohol consumption. Addiction biology. 2021;26:e13018
105. Pu Y, Tan Y, Qu Y, Chang L, Wang S, Wei Y. et al. A role of the subdiaphragmatic vagus nerve in depression-like phenotypes in mice after fecal microbiota transplantation from Chrna7 knock-out mice with depression-like phenotypes. Brain, Behavior, and Immunity. 2021;94:318-26
106. Wang S, Ishima T, Zhang J, Qu Y, Chang L, Pu Y. et al. Ingestion of Lactobacillus intestinalis and Lactobacillus reuteri causes depression- and anhedonia-like phenotypes in antibiotic-treated mice via the vagus nerve. Journal of Neuroinflammation. 2020;17:241
107. Chang L, Wei Y, Hashimoto K. Brain-gut-microbiota axis in depression: A historical overview and future directions. Brain Research Bulletin. 2022;182:44-56 https://www.sciencedirect.com/science/article/pii/S0361923022000375
108. Kreisel T, Frank MG, Licht T, Reshef R, Ben-Menachem-Zidon O, Baratta MV. et al. Dynamic microglial alterations underlie stress-induced depressive-like behavior and suppressed neurogenesis. Mol Psychiatry. 2014;19:699-709
109. Rothhammer V, Borucki DM, Tjon EC, Takenaka MC, Chao CC, Ardura-Fabregat A. et al. Microglial control of astrocytes in response to microbial metabolites. Nature. 2018;557:724-8
110. Braniste V, Al-Asmakh M, Kowal C, Anuar F, Abbaspour A, Toth M. et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med. 2014;6:263ra158
111. Bagot R, Labonté B, Peña C, Nestler E. Epigenetic signaling in psychiatric disorders: stress and depression. Dialogues in clinical neuroscience. 2014;16:281-95
112. Covington HE 3rd, Maze I, LaPlant QC, Vialou VF, Ohnishi YN, Berton O. et al. Antidepressant actions of histone deacetylase inhibitors. J Neurosci. 2009;29:11451-60
113. Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci. 2006;9:519-25
114. Renthal W, Maze I, Krishnan V, Covington HE 3rd, Xiao G, Kumar A. et al. Histone deacetylase 5 epigenetically controls behavioral adaptations to chronic emotional stimuli. Neuron. 2007;56:517-29
115. Uchida S, Hara K, Kobayashi A, Otsuki K, Yamagata H, Hobara T. et al. Epigenetic status of Gdnf in the ventral striatum determines susceptibility and adaptation to daily stressful events. Neuron. 2011;69:359-72
116. Covington HE 3rd, Vialou VF, LaPlant Q, Ohnishi YN, Nestler EJ. Hippocampal-dependent antidepressant-like activity of histone deacetylase inhibition. Neurosci Lett. 2011;493:122-6
117. Schmauss C. An HDAC-dependent epigenetic mechanism that enhances the efficacy of the antidepressant drug fluoxetine. Sci Rep. 2015;5:8171
118. Fuchikami M, Yamamoto S, Morinobu S, Okada S, Yamawaki Y, Yamawaki S. The potential use of histone deacetylase inhibitors in the treatment of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2016;64:320-4
119. Network Pathway Analysis Subgroup of Psychiatric Genomics C. Psychiatric genome-wide association study analyses implicate neuronal, immune and histone pathways. Nat Neurosci. 2015;18:199-209
120. Fuks F. DNA methylation and histone modifications: teaming up to silence genes. Curr Opin Genet Dev. 2005;15:490-5
121. Hodes GE, Pfau ML, Purushothaman I, Ahn HF, Golden SA, Christoffel DJ. et al. Sex Differences in Nucleus Accumbens Transcriptome Profiles Associated with Susceptibility versus Resilience to Subchronic Variable Stress. J Neurosci. 2015;35:16362-76
122. LaPlant Q, Vialou V, Covington HE 3rd, Dumitriu D, Feng J, Warren BL. et al. Dnmt3a regulates emotional behavior and spine plasticity in the nucleus accumbens. Nat Neurosci. 2010;13:1137-43
123. Feng J, Pena CJ, Purushothaman I, Engmann O, Walker D, Brown AN. et al. Tet1 in Nucleus Accumbens Opposes Depression- and Anxiety-Like Behaviors. Neuropsychopharmacology. 2017;42:1657-69
124. Elliott E, Ezra-Nevo G, Regev L, Neufeld-Cohen A, Chen A. Resilience to social stress coincides with functional DNA methylation of the Crf gene in adult mice. Nat Neurosci. 2010;13:1351-3
125. Weaver IC, Cervoni N, Champagne FA, D'Alessio AC, Sharma S, Seckl JR. et al. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847-54
126. McGowan PO, Sasaki A, D'Alessio AC, Dymov S, Labonte B, Szyf M. et al. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci. 2009;12:342-8
127. Xiao J, Wang T, Xu Y, Gu X, Li D, Niu K. et al. Long-term probiotic intervention mitigates memory dysfunction through a novel H3K27me3-based mechanism in lead-exposed rats. Translational psychiatry. 2020;10:25
128. Donohoe D, Holley D, Collins L, Montgomery S, Whitmore A, Hillhouse A. et al. A gnotobiotic mouse model demonstrates that dietary fiber protects against colorectal tumorigenesis in a microbiota- and butyrate-dependent manner. Cancer discovery. 2014;4:1387-97
129. Zhang Y, Xu S, Qian Y, He X, Mo C, Yang X. et al. Sodium butyrate attenuates rotenone-induced toxicity by activation of autophagy through epigenetically regulating PGC-1alpha expression in PC12 cells. Brain Res. 2022;1776:147749
130. Yamawaki Y, Yoshioka N, Nozaki K, Ito H, Oda K, Harada K. et al. Sodium butyrate abolishes lipopolysaccharide-induced depression-like behaviors and hippocampal microglial activation in mice. Brain research. 2018;1680:13-38
131. Paternain L, Martisova E, Campión J, Martínez J, Ramírez M, Milagro F. Methyl donor supplementation in rats reverses the deleterious effect of maternal separation on depression-like behaviour. Behavioural brain research. 2016;299:51-8
132. McCoy CR, Rana S, Stringfellow SA, Day JJ, Wyss JM, Clinton SM. et al. Neonatal maternal separation stress elicits lasting DNA methylation changes in the hippocampus of stress-reactive Wistar Kyoto rats. Eur J Neurosci. 2016;44:2829-45
133. Febbraro F, Svenningsen K, Tran TP, Wiborg O. Neuronal substrates underlying stress resilience and susceptibility in rats. PLoS One. 2017;12:e0179434
134. Lehmann ML, Geddes CE, Lee JL, Herkenham M. Urine scent marking (USM): a novel test for depressive-like behavior and a predictor of stress resiliency in mice. PLoS One. 2013;8:e69822
135. Zenz G, Farzi A, Fröhlich EE, Reichmann F, Holzer P. Intranasal Neuropeptide Y Blunts Lipopolysaccharide-Evoked Sickness Behavior but Not the Immune Response in Mice. Neurotherapeutics. 2019;16:1335-49
136. Ji MJ, Zhang XY, Chen Z, Wang JJ, Zhu JN. Orexin prevents depressive-like behavior by promoting stress resilience. Mol Psychiatry. 2019;24:282-93
137. Corbett BF, Luz S, Arner J, Pearson-Leary J, Sengupta A, Taylor D. et al. Sphingosine-1-phosphate receptor 3 in the medial prefrontal cortex promotes stress resilience by reducing inflammatory processes. Nat Commun. 2019;10:3146
138. Gross M, Romi H, Miller A, Pinhasov A. Social dominance predicts hippocampal glucocorticoid receptor recruitment and resilience to prenatal adversity. Sci Rep. 2018;8:9595
Author contact
![]() Corresponding author: Tel: +8610-66931625, Fax: +8610-68211656, E-mail: Yan Qian: cqqianyancqmu.edu.cn; Wenxia Zhou: zhouwxac.cn.
Corresponding author: Tel: +8610-66931625, Fax: +8610-68211656, E-mail: Yan Qian: cqqianyancqmu.edu.cn; Wenxia Zhou: zhouwxac.cn.

 Global reach, higher impact
Global reach, higher impact