10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2023; 19(15):4793-4810. doi:10.7150/ijbs.87492 This issue Cite
Review
Photodynamic Therapy for Inflammatory and Cancerous Diseases of the Intestines: Molecular Mechanisms and Prospects for Application
1. Department of Gastroenterology, Renmin Hospital of Wuhan University, Wuhan, China.
2. Department of General Surgery, Renmin Hospital of Wuhan University, Wuhan, China.
# These authors have contributed equally to this work.
Received 2023-6-26; Accepted 2023-8-21; Published 2023-9-4
Abstract
Photodynamic therapy (PDT) is a minimally invasive treatment that effectively targets cancer and inflammatory diseases. It has gained recognition for its efficacy, low toxicity, and potential for repeated use. Colorectal cancer (CRC) and inflammatory bowel diseases (IBD), including Crohn's disease (CD), and ulcerative colitis (UC), impose a significant burden on global intestinal health, with increasing incidence and prevalence rates. PDT shows promise as an emerging approach for gastrointestinal disease treatment, particularly IBD and CRC. Extensive preclinical research has demonstrated the safety and effectiveness of PDT for IBD and CRC, while clinical studies are currently underway. This review provides an overview of the underlying mechanisms responsible for the anti-inflammatory and anti-tumor effects of PDT, offering insights into the clinical application of PDT in IBD and CRC treatment. It is expected that this review will serve as a valuable reference for future research on PDT for CRC and IBD, contributing to advancements in the treatment of inflammatory and cancerous diseases of the intestines.
Keywords: photodynamic therapy, inflammatory bowel diseases, colorectal cancer, reactive oxygen species, immunology, nanomedicine
1. Introduction
Photodynamic therapy (PDT) is a highly effective and minimally invasive treatment for cancer and inflammatory diseases, known for its recognized efficacy, low toxicity, and suitability for repeated administration [1]. The procedure involves the application of a photosensitizer (PS) to the targeted area, followed by exposure to specific light [2]. The PS selectively targets rapidly proliferating cells, making it a versatile and effective treatment option for various medical conditions [3, 4].
PDT comprises three essential components: excitation light, PS, and oxygen. Table S1 summarized the types of PS and its application in various medical conditions. The choice of PS type and its application in different conditions depends on factors like disease type and stage, tumor location, specific PS properties, and treatment objectives. When a PS is exposed to light of a specific wavelength, it undergoes two types of photochemical reactions that lead to the generation of reactive oxygen species (ROS) [5]. In Type I reactions, the PS transfers energy to biomolecules while in the T1 excited state, causing a transfer of hydrogen or electrons between the photosensitizer and substrate. Subsequently, interacting with oxygen molecules produces superoxide anion radicals, which generate ROS within the cells. Conversely, in Type II reactions, the PS is excited to the triplet state, and its energy directly transfers to ground-state oxygen molecules, resulting in the production of highly oxidizing singlet oxygen [1].
PDT can be categorized into High-dose (HDPDT) and low-dose (LDPDT) based on their efficiency in producing ROS, which is primarily influenced by the power and duration of light exposure [6, 7]. HDPDT can damage cellular structure and function, leading to an anti-vascular effect, and is well-suited for tumor treatment [8-10]. Importantly, elevated levels of ROS can lead to direct photo-damage of proteins, lipids, and other molecules within the PS area, resulting in cell necrosis and apoptosis. Additionally, ROS can cause damage to lysosomes or endoplasmic reticulum, potentially inducing autophagy or ferroptosis [11-13]. Given the potential accumulation of the PS in the adjacent healthy tissues, HDPDT may inadvertently cause harm to normal cells in the intestine while targeting tumor cells [2, 14, 15]. LDPDT shows notable efficacy in preventing mucosal damage [16], and demonstrates excellent immunomodulatory effects [17]. Moreover, it regulates various intestinal bacterial strains and promotes neovascular closure. The successful application of LDPDT in treating inflammatory diseases highlights its favorable safety profile for therapeutic use. [7, 17-19].
Inflammatory bowel disease (IBD), encompassing ulcerative colitis (UC), Crohn's disease (CD), and colorectal cancer (CRC), represents a significant burden on global intestinal health, with increasing incidence and prevalence rates [20-22]. Importantly, IBD is recognized as one of the contributing factors to the development of CRC [23]. IBD-related colorectal cancer (CAC) represents approximately 2% of the total annual mortality from CRC. However, the annual mortality rate among individuals with IBD is significantly higher, ranging from 10% to 15%. Moreover, CAC patients tend to be diagnosed at a younger age compared to those with sporadic CRC, and their 5-year survival rate is 50% [24]. PDT, being one of the established pillars of cancer treatment, is recommended for various types of tumors [25], and has proven to be an effective palliative treatment for advanced CRC [26, 27]. Recent studies have provided evidence of PDT's ability to attenuate IBD through the downregulation of pro-inflammatory cytokines [7], regulation of intestinal microbiota [18], and modulation of miRNAs [16]. The safety and effectiveness of PDT have been extensively demonstrated in preclinical research for IBD and CRC, and clinical studies are currently underway [28-30]. The tubular structure of the intestine allows for precise treatment of intestinal lesions through endoscopic guidance. This significantly mitigates the limitations imposed by PDT's restricted penetration depth, leading to enhanced treatment efficacy [31, 32].
Hence, PDT shows immense promise as a novel approach in the treatment of gastrointestinal diseases, notably IBD and CRC. This review aims to comprehensively explore the mechanisms of PDT in managing inflammatory and neoplastic conditions of the intestines, providing valuable insights to promote its clinical implementation for IBD and CRC management.
2. Advantages of PDT in intestinal-related inflammatory and cancerous diseases
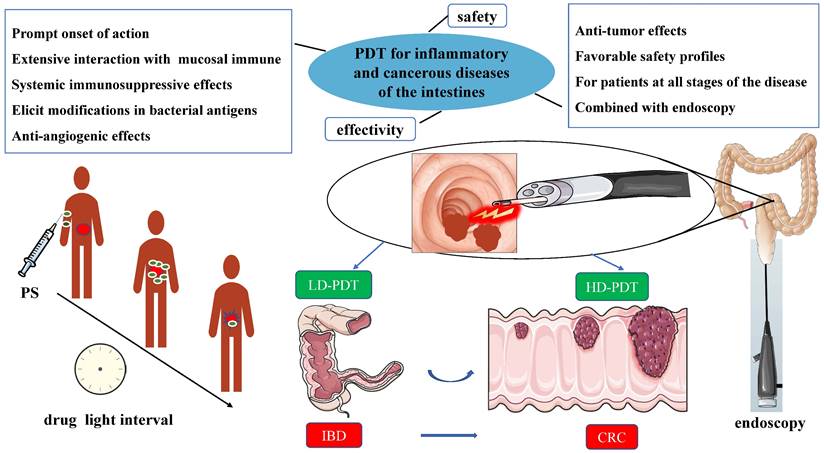
The management of IBD is multifaceted, typically involving prolonged administration of multiple medications and, in some cases, surgical intervention [33]. IBD is a chronic condition without a definitive cure, placing patients at risk of various complications such as intestinal bleeding, perforation, and obstruction. In severe cases, IBD can progress to colorectal cancer [34]. In advanced stages, immunosuppressive drugs may be necessary, although they can induce toxic side effects and impose a financial burden on patients. Close collaboration between healthcare providers is imperative to optimize treatment outcomes [35]. Biologic therapies, including tumor necrosis factor-α (TNF-α) antibodies, have been employed in IBD treatment since the 21st century, demonstrating notable advancements. Nevertheless, the efficacy of these drugs varies among individuals with IBD, with approximately one-third of patients exhibiting unresponsiveness. Consequently, a combination of biologic therapy and immunomodulators is often necessary. However, it is worth noting that studies have indicated an increased risk of severe infections and T-cell lymphomas associated with this treatment. The burden of IBD has been consistently rising in both Western and Asian nations [22]. Consequently, there is a pressing need to explore innovative therapeutic approaches for IBD. Ideally, these novel treatment options should exhibit the following attributes: 1) Fast-acting treatment; 2) Broad-spectrum effects; 3) lack of systemic immunosuppressive effects; 4) ability to induce alterations in bacterial antigens; 5) anti-angiogenic effects; 6) favorable safety profiles; and 7) suitability for patients with IBD at all disease stages.
PDT offers several advantages, including minimally invasive procedures, dual-targeted killing, minimal toxicity and side effects, and the potential for repeated treatments [1]. It fulfills the criteria for the aforementioned new treatment options for IBD [19]. Numerous studies have showcased the considerable advantages of PDT in treating inflammatory diseases, including IBD [4, 36, 37]. PDT can modify cell signal transduction, regulate cytokine generation, and modulate cell surface receptor expression, thereby preserving cell vitality and exerting anti-inflammatory properties [7, 17, 38]. The combination of biologic therapy and immunomodulators represents an essential and indispensable approach for managing IBD, particularly in patients with more severe disease manifestations. Table S2 provides a comparison of the mechanisms of action and therapeutic effects between PDT and this combination therapy. PDT offers a localized and targeted approach with minimal systemic effects, while combination therapy aims to modulate the immune response and control inflammation. The choice between these treatments depends on the specific characteristics and severity of the individual's IBD. Nevertheless, we firmly believe that PDT holds greater potential as a future treatment method for IBD. Moreover, in Table 1, we provide a summary of studies on the successful mitigation of IBD using PDT. Despite notable progress in the diagnosis and treatment of CRC, limited understanding exists regarding the mechanisms through which IBD contributes to CRC and colorectal liver metastasis (CRLM) [39]. Patients with CRLM, particularly those with diffuse liver metastases originating from CRC, frequently encounter unfavorable clinical outcomes, underscoring the necessity for the advancement of novel therapeutic approaches [40]. Promisingly, PDT exhibits substantial potential for application in CRLM [27, 39, 41].
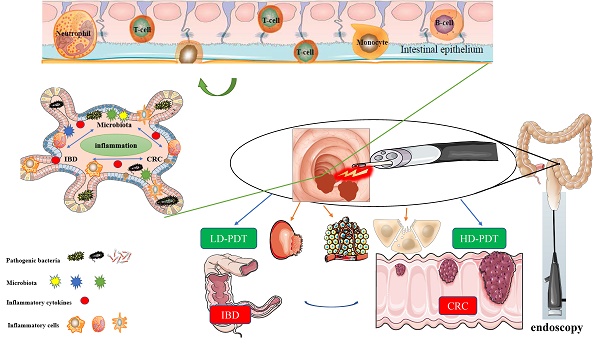
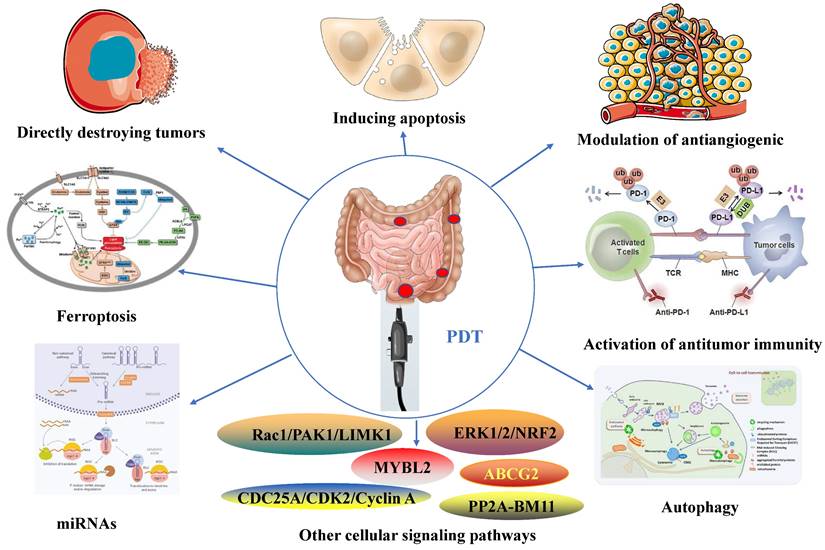
The application of PDT in deep lesions is limited by the tissue penetration ability of the traditionally used near-infrared light source [42]. However, the intestine's natural tubular structure and connection to the outside world through the anus provide a solution. By introducing a fiber optic cable through the anus, the intestinal mucosa can be effectively irradiated, addressing the issue of limited tissue penetration ability in PDT to a significant extent [16]. PDT is particularly suitable for utilization in combination with endoscopes, such as colonoscopes, enabling accurate irradiation and treatment of intestinal lesions while providing direct endoscopic visualization. Moreover, Endoscopy serves as a primary diagnostic and evaluative tool for gastrointestinal diseases. [43]. The direct visual observation and assessment of lesions, along with the ability to intervene, establish endoscopes as the gold standard [44]. Hence, PDT offers significant advantages in the diagnosis and treatment of intestinal diseases, particularly in the context of IBD and CRC [31, 45]. Figure 1 illustrates a schematic description of the application of PDT for inflammatory and cancerous diseases of the intestines.
3. The mechanisms of PDT in intestinal-related inflammatory and cancerous diseases
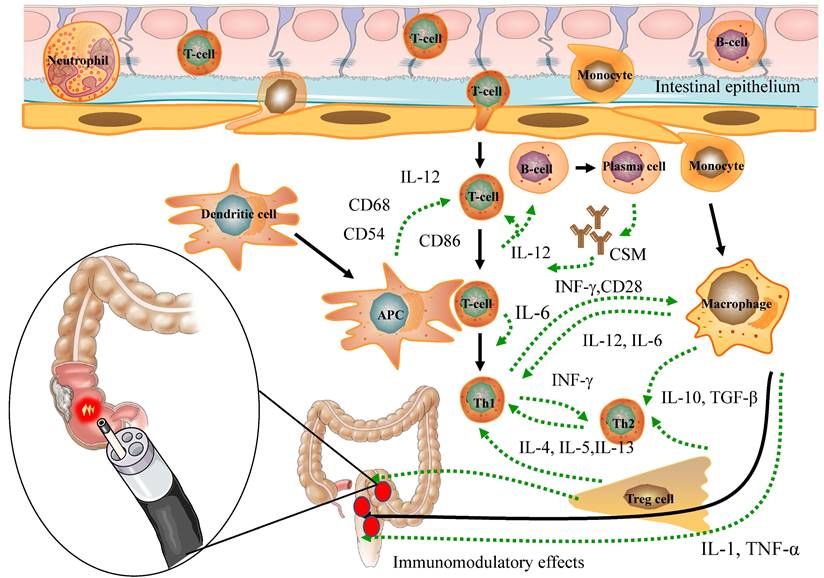
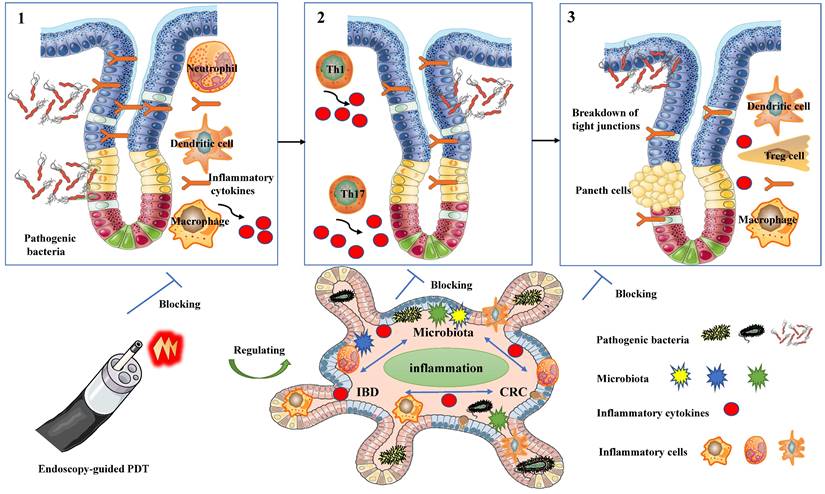
PDT possesses properties that enable the modulation of immune responses (Figure 2), regulation of microbiota homeostasis (Figure 3), and exhibition of anti-tumor effects (Figure 4).
A schematic description of the application of PDT for inflammatory and cancerous diseases of the intestines.
The immunomodulatory effects of PDT for inflammatory and cancerous diseases of the Intestines
The modulation of microbiota homeostasis in PDT for inflammatory and cancerous diseases of the intestines.
The studies related to the effective mitigation of IBD by PDT
| Years | Objects | Photosensitizer and concentration | Wavelength (nm) | Light treatment parameters | Main Findings | Refs. |
|---|---|---|---|---|---|---|
| 1999 | 6 UC patient (2 men) | 5-ALA 10 or 20 mg/Kg | / | / | Inhibiting inflammatory response | [44] |
| 2011 | T cell-mediated colitis model mice | 5-ALA 15mg/Kg | 635 | 5 or 10 J/cm2 at 100 mW/cm2 | Decreasing the proinflammatory cytokines IL-6, IL-17, and INF-γ, and decreasing the number of CD4+ T cells | [7] |
| 2015 | AOM/ DSS-induced UC AND CRC model | Foslip 0.1, 0.05 or 0.001 mg/Kg | 652 | 20 J/cm2 at 100 mW/cm2 | Decreasing the expression of a wide range of inflammatory mediators; lowering neutrophil influx; prevents the onset of a dysbiotic microbiota | [18] |
| 2018 | DSS -induced UC model mice | 5-ALA | 630 | 10 J/cm2 at 300 mW/cm2 | Regulating Neat1-miRNA204-5p-PI3K-AKT axis | [16] |
| 2021 | TNBS-induced UC model rats | LD4 60,120 240 μg/Kg | 650 | 65 J/cm2 | Decreasing the expression of IL-6, IL-1, TNFα, MDA, MPO; increasing the expression of GSH and SOD; inhibition of AOC1 | [19] |
| 2022 | DSS-induced UC model mice | 5-ALA 15mg/Kg | 630 | 10 J/cm2 at 300 mW/cm2 | Regulating miR-301a-3p/ PPARGC1A pathway | [38] |
| 2023 | PBMCs from CD patients | 5-ALA 3 mM | 630 | 180 J/cm2 at 100 mW/cm2 | Decreasing survival of CD3-/CD19+ B cells; suppressing monocytes; inhibiting subcellular levels of inflammatory cytokines and exosomes | [17] |
AOM: azoxymethane; DSS: dextran sulfate sodium; UC: ulcerative colitis; CD: Crohn's disease; TNBS:2,4,6-trinitrobenzene sulfonic acid; 5-ALA: 5-aminolevulinic acid; MDA: malondialdehyde; MPO: myeloperoxidase; GSH: glutathione; SOD: superoxide oxidase; IL: interleukin; IFN-γ: interferon-gamma; PBMCs: peripheral blood mononuclear cells.
The anti-tumor effects of PDT for inflammatory and cancerous diseases of the intestines.
3.1 Immunomodulatory effects of PDT
The influence of PDT on the immune response is intricate and contingent on diverse factors, encompassing the type, pharmacokinetics, and intracellular localization of PS, particularly within immune cells. Moreover, the effectiveness of ROS generation is influenced by factors like the wavelength, intensity, and duration of the activating light, which can impact PDT's regulation of the immune response [46]. HDPDT is typically found to enhance the body's immune response. In particular, photo-induced cytotoxicity directly damages tumor cells, exposing tumor antigens, and thereby regulating tumor immune responses through inflammatory reactions for the treatment of colorectal cancer. CRC [47]. On the other hand, LDPDT demonstrates immunomodulatory effects by altering immune cell functions, including the regulation of immune cell surface molecules and cytokine balance, thereby ameliorating IBD [7, 18] (Figure 2). In conclusion, both HDPDT and LDPDT exhibit remarkable immunomodulatory effects, laying a crucial foundation for the potential application of PDT in the management of inflammatory and neoplastic conditions of the intestines.
3.1.1 Apoptosis of immune cells
During the application of HDPDT for CRC treatment, the PS accumulates in the mitochondria and, upon stimulation by specific light wavelengths, can induce apoptosis in host cells [48-50]. Nonetheless, there is limited research on PDT-induced apoptosis of immune cells, with only a few studies demonstrating apoptosis in leukocytes, macrophages, and T cells following PDT treatment [51-53]. The interaction between PS and immune cells is crucial in PDT-induced immune cell apoptosis. [4, 54, 55] Hydrophobic PS exhibits an affinity for binding with plasma proteins, especially low-density lipoprotein. Moreover, activated immune cells display higher PS absorption than resting immune cells [4, 54], possibly due to upregulated expression levels of lipoprotein receptors [53]. As a result, even in LDPDT, a favorable immunomodulatory function is present. Consequently, the induction of immune cell apoptosis serves as one of the mechanisms by which PDT manifests its immunomodulatory effects. (Figure 2).
3.1.2 Regulation of immune cell surface molecules
Numerous studies have demonstrated that PDT can modify the expression of diverse surface receptors on immune cells, consequently impacting the usual interaction between antigen-presenting cells (APCs) and T cells. This alteration hinders the effective activation of T cells by APCs following PDT treatment [55-57]. Notably, PDT has been observed to downregulate the expression of specific adhesion molecules and major histocompatibility complex (MHC) molecules on immune cells [58]. Furthermore, the expression levels of co-stimulatory molecules are crucial for T cell activation by PDT [59]. Dendritic cells (DCs) are highly efficient APCs that activate immature T lymphocytes through abundant expression of MHC antigens, adhesion molecules, and co-stimulatory molecules. Moreover, they are essential producers of the pro-inflammatory cytokine IL-12, critically involved in regulating T-cell immune responses [57]. PDT results in a substantial reduction of the expression levels of two types of MHC antigens and co-stimulatory molecules in mice with IBD [56]. Furthermore, LDPDT has demonstrated the ability to suppress the production of pro-inflammatory cytokines while promoting the generation of anti-inflammatory cytokines. Additionally, has demonstrated immunomodulatory and anti-inflammatory effects in a murine arthritis model [60]. Notably, both IBD and rheumatoid arthritis share immune-inflammatory mechanisms, and specific drugs are suitable for treating both disorders [61]. The modulation of surface receptor expression by PDT holds significant implications for immune cell function and interactions. Through the alteration of these receptors, PDT can exert influence on immune cell signaling, antigen presentation, and immune responses. Taken together, PDT significantly modifies various surface receptors on immune cells, which in turn influences the interaction between APCs and T cells, ultimately impacting immune cell function and responses. This presents a promising therapeutic approach for conditions characterized by immune dysregulation, including IBD and CRC. (Figure 2).
3.1.3 Modulation of cytokine balance
PDT modulates cytokine balance, thereby influencing immune cell function and interactions, and holds promise as a therapeutic approach for immune-related conditions [25]. Studies have demonstrated that the administration of low-dose PS 5-ALA (15mg/kg) and exposure to light at a wavelength of 635nm, fluence of 10J/cm2, and power density of 100 mW/cm2 significantly reduced the secretion of pro-inflammatory cytokines IL-6, IL-17, and IFN-γ, as well as the number of CD4+ T cells in a murine model of T cell-mediated colitis [7]. Moreover, the administration of low-dose PS Foslip (0.01mg/kg) and exposure to light at a wavelength of 652nm, fluence of 20 J/cm2, and power density of 100 mW/cm2 significantly decreased the expression of pro-inflammatory cytokines TNF-α, IL-1β, IL-12, and IFN-γ, while increasing the expression of the anti-inflammatory cytokine IL-10 in a mouse model of DSS-induced ulcerative colitis [18]. Furthermore, the administration of low-dose PS 5-ALA (15mg/kg) and exposure to light at a wavelength of 630nm, fluence of 10J/cm2, and power density of 300 mW/cm2 significantly downregulated the expression levels of pro-inflammatory cytokines IL-17a, TNF-α, and IL-12a [16].
In addition to effectively destroying malignant colorectal cancer tissue, PDT also exhibits anti-cancer activity by reducing the secretion of IL-6 and IL-10 in CRC cell lines [62]. Furthermore, ALA-LDPDT has the potential to influence the progression and invasion of colorectal cancer cells. It decreases the secretion of IL-6 and IL-10 while increasing the concentration of IL-8. Notably, cell lines with a higher degree of malignancy exhibit higher concentrations of IL-8 [63]. Moreover, PDT regulates nitric oxide, IL-6, and TNF-α to enhance the cytotoxicity of tumor-associated non-resident macrophages in CRC [64].
Notably, the imbalance of cytokines can contribute to the development of cancer, including CRC, in chronic inflammatory diseases like IBD. Growing evidence suggests the involvement of various cytokines released by epithelial and immune cells in the development of CAC. Crucially, cyclooxygenase-2 (COX-2) and nuclear factor kappa B (NF-kB), are crucial genes responsible for regulating cytokine balance and mediating the intricate interplay between inflammation and cancer [65]. Moreover, recent studies have shown that additional factors, such as the IL6/STAT3, IL22/STAT3, and IL23/STAT3/Th17 signaling pathways, contribute to the onset of CAC [66]. Importantly, during the recovery period after PDT, tissue cells maintain the expression of IL-6 or Hyper-IL-6, leading to increased inhibition of proliferation and offering a more effective strategy for tumor control [67]. These studies collectively demonstrate the significant role of PDT in modulating cytokine balance and its potential value in preventing and treating IBD, CAC, and CRC (Figure 2).
3.2 Modulation of microbiota homeostasis
The antibacterial efficacy and the potential of PDT for local infection treatment have been extensively validated over the past few decades [4]. Recently, there has been a growing interest in the role of PDT in modulating microbiota homeostasis [18, 19]. The delicate balance of the gut microbiome is of utmost importance in preserving intestinal health and influencing the progression of both IBD and CRC [68]. Reinhard et al. were the first to investigate the use of LDPDT therapy in preventing the occurrence of dysbiotic microbiota in a CAC model [18]. Research has suggested that Foslip-mediated PDT demonstrates effectiveness, safety, antimicrobial, anti-inflammatory, and anti-carcinogenic properties in IBD [18]. The mechanism of PDT involves enhancing the resilience of the intestinal barrier through the repair of E-cadherin tight junctions and mucin-2 secretion, as well as reshaping the composition of the gut microbiota in a colitis-associated carcinogenesis model [18]. The study showed that LDPDT did not directly affect bacterial symbiosis in mice, but there were significant differences in bacterial composition between the LDPDT treatment group and the disease control group.AOM/DSS mice exhibited lower phylodiversity and higher bacteroidaceae abundance in tumor-bearing animals. Conversely, LDPDT-AOM/DSS mice did not significantly differ from healthy controls, suggesting that LDPDT might modulate the microbiota homeostasis in IBD [18].
Moreover, a recent study reported that LD4-PDT facilitated the healing of colonic mucosa, regulated gut microbiota, and ameliorated clinical symptoms of UC [19]. This study presents compelling evidence supporting the multifaceted effects of LD4-PDT, which include the regulation of inflammatory cytokine balance and modulation of gut microbiota composition. These combined actions result in the restoration of the colonic proteome in TNBS-induced colitis rats, leading to the effective alleviation of acute colitis [19]. Mechanistically, the beneficial effects of LD4-PDT on colitis in rats may be attributed to its regulation of the AOC1 target [19]. Interestingly, LD4-PDT restored the gut microbiota in UC model rats to a state similar to that of the control group, despite significant differences in the TNBS model group. The proportion of Bacteroidetes was higher but less abundant in TNBS model rats, resulting in higher Firmicutes/Bacteroidetes (F/B) values. Following LD4-PDT treatment, Bacteroidetes decreased, highly viscous microorganisms increased, and F/B values decreased, similar to the control group [19]. These findings highlight the significant potential of PDT in modulating microbiota homeostasis.
Research conducted thus far has underscored the significant role of microbiota in shaping the immune system and maintaining the overall health of the host [68-70]. On one hand, an imbalance in Helicobacter or bacteria that produce genotoxins can trigger inflammation and promote tumor growth [71]. On the other hand, patients with IBD or CRC demonstrate distinct microbial compositions compared to healthy individuals. These findings align with observations made in mouse models of IBD and CRC [72], indicating that dysbiosis of the microbiota is crucial in the development of these diseases [69] However, the relationship between the microbiota, inflammation, IBD, and CRC remains complex and not yet fully understood. Inflammation serves as a critical link in this relationship and is essential for dysbiosis and the development of CRC. Both dysbiosis of the microbiota and the development of CRC can trigger sustained inflammation in the intestine. Consequently, it remains uncertain whether dysbiosis of the microbiota in IBD or CRC is the cause, consequence, or a result of causal interaction [73]. Nevertheless, three key factors cannot be disregarded in the intricate network of chronic inflammation in the gut microbiota and IBD and CRC: (1) colonization and invasion by pathogenic bacteria, which elicit inflammation by evading host defenses; (2) dysbiosis resulting from various factors, leading to chronic inflammation involving Th1 and Th17 cells; and (3) impaired host immune responses and immune regulation, resulting in inflammation due to increased antigen exposure and dysfunction of regulatory T cells or anti-inflammatory cytokines [69, 73]. Additionally, accumulating evidence suggests that the gut microbiota influences immunogenic cell death (ICD) and the anti-tumor effects of PDT [68, 69]. Taken together, PDT is crucial in modulating microbiota homeostasis in inflammatory and cancerous diseases of the intestines, demonstrating promising potential as a therapeutic approach for managing these conditions. (Figure 3).
3.3 Anti-tumor effects of PDT
PDT has been widely verified for its potent anti-tumor properties [74]. The localized nature of PDT allows for precise targeting of tumor cells, minimizing harm to healthy tissues. Furthermore, it has the potential to enhance the effectiveness of other treatment modalities [75]. PDT shows promise in the treatment of various tumor types, including CRC, and continues to be an active area of research and clinical development [47]. In this section, we provide a comprehensive overview of the molecular mechanisms underlying the anti- CRC effects of PDT. (Figure 4).
3.3.1 Directly destroying tumors and inducing apoptosis
PDT generates ROS that directly damages tumor cells, resulting in cellular apoptosis and necrosis [25]. Apoptosis is a physiological process that eliminates damaged or unwanted cells in the body. Disruption of this process can lead to uncontrolled cell growth and the formation of cancer. Inducing apoptosis in cancer cells is a commonly employed strategy for cancer treatment [76]. Extensive research has focused on inducing apoptosis in CRC cells through PDT [77]. PDT markedly increases the extent of apoptosis in CRC cells [48]. Moreover, PDT activates apoptosis pathways mediated by mitochondria, endoplasmic reticulum (ER), and lysosomes [49, 78]. Additionally, the involvement of apoptotic pathways such as p38 MAPK/caspase9 [50], Bax [79], and p53 [80] is also crucial in this process. In conclusion, PDT triggers apoptosis in CRC cells through the activation of multiple apoptotic pathways and significant changes in gene expression, as illustrated in Table 2.
3.3.2 Modulation of antiangiogenic
PDT induces the destruction of tumor blood vessels through the production of ROS from the PS. This leads to vasoconstriction, rupture, and thrombosis, ultimately causing tumor ischemia and necrosis [81, 82]. The extent of vascular damage following PDT is influenced by various factors, including the pharmacokinetics of the PS, the intensity and fluence of the excitation light, and the time interval between PS injection and light excitation (drug-light interval). A short drug-light interval primarily results in acute vascular damage, while a long drug-light interval induces greater oxidative damage to the tumor tissue [83]. Moreover, the oxidative stress response following PDT is influenced by the TME, which intricately regulates multiple molecular signaling pathways, leading to the promotion of vascular endothelial growth factor (VEGF) expression [84], This, in turn, impacts the antiangiogenic effect of PDT. VEGF is a critical regulatory factor in the process of angiogenesis during tumor progression. As a tumor grows, it secretes growth factors like VEGF, which stimulate endothelial cells and facilitate extracellular matrix restructuring, leading to the formation of new blood vessels. This process is necessary to maintain an adequate oxygen and nutrient supply for further tumor growth [85].
Contradictory findings exist regarding the effect of PDT on VEGF secretion by other tumor cells. However, studies investigating PDT treatment of CRC consistently report positive outcomes [86, 87]. Kawczyk et al. conducted an in vitro study to examine the impact of PDT on VEGF secretion by CRC cells under aerobic conditions [86]. Their findings revealed that 5-ALA-PDT significantly reduced VEGF secretion in the metastatic CRC SW620 cell line, while the non-metastatic CRC SW480 cell line maintained its VEGF secretion ability unchanged. Moreover, regardless of PDT intervention, the SW620 cell line exhibited significantly higher VEGF secretion capacity than SW480, aligning with recent studies [87]. Therefore, PDT employs a multifaceted mechanism to modulate CRC angiogenesis, involving both direct damage to tumor vasculature and the regulation of oxidative stress response and VEGF expression within the tumor microenvironment. (Table 2).
3.3.3 Activation of antitumor immunity
PDT has been demonstrated to activate the immune system and induce ICD in tumor cells through the production of ROS from the PS. This activation promotes antigen presentation and T cell-mediated immune responses, thereby enhancing antitumor immune responses [88]. Additionally, PDT can increase the expression of tumor antigens, activate immune cells, and deplete T regulatory cells (Tregs), leading to the induction of long-term survival and memory immune responses [89-91]. In a study by Mroz et al., it was observed that PDT induces systemic, antigen-specific anti-tumor immunity in CRC BALB/c mouse models expressing a tumor-specific antigen [92]. Furthermore, the immune response mediated by PDT is associated with Treg depletion, which enhances the immune response and triggers long-term survival and memory immune responses [93]. These findings contribute to the understanding of the complex regulatory mechanisms involving PDT, Tregs, the tumor microenvironment, and different types of tumor antigens. Additional evidence has confirmed the synergistic therapeutic effects of combining NCP@pyrolipid-PDT with anti-PD-L1 therapy, resulting in the inhibition of primary CRC growth and suppression of distant metastasis [94]. Similarly, the combination of Fe-TBP-PDT with anti-PD-L1 treatment can induce synergistic therapy involving CD4+ and CD8+ cytotoxic T cells for CRC treatment [95]. Likewise, UCNP-Ce6-R837-PDT triggers dendritic cell maturation and cytokine secretion to stimulate immune responses. When combined with cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) checkpoint inhibitors, it inhibits Treg cell activity and exhibits a powerful synergistic therapeutic effect. This combined treatment not only eliminates primary CRC tumors and inhibits distant metastasis but also prevents recurrence [96]. Notably, Li et al. reported an innovative approach that combines PDT with immunotherapy using a PS specifically targeting EGFR. This approach demonstrates precise targeting for CRC with remarkable inhibitory effects and no recurrence after treatment [97]. Moreover, the PS SPDMCN is specifically activated in the CRC tumor microenvironment, releasing an immune modulator (DMC) to enhance the effect of PDT [98]. Furthermore, PDT has been shown to inhibit the proliferation of multiple CRC cell lines by inducing ICD [88]. Collectively, these studies provide a foundation for the application of PDT combined with immunotherapy in cancer treatment (Table 2) [95, 99-101].
3.3.4 Modulation of cellular signaling pathways
PDT can disrupt multiple cellular signaling pathways, including autophagy [102], ferroptosis [103], miRNA [104], and inflammatory signaling pathways [105]. These regulatory effects have a direct impact on the viability and proliferation of cancer cells, ultimately contributing to tumor regression.
3.3.4.1 Autophagy
Autophagy is a metabolic process that involves the degradation and recycling of unnecessary or harmful components within cells, providing the energy and nutrients necessary for cell survival. Under normal conditions, autophagy contributes to maintaining cellular homeostasis, preventing DNA damage, and inhibiting abnormal cell proliferation [106-108]. The role of autophagy in cancer is complex and has been shown to exhibit both pro-tumorigenic and anti-tumorigenic effects, depending on various factors including the type of cancer, tumor microenvironment, metabolic status of tumor cells, and regulatory mechanisms of autophagy pathways. Furthermore, the pro-tumorigenic and anti-tumorigenic effects of autophagy can interchange [109-111]. Nonetheless, the importance of autophagy in suppressing CRC through PDT is widely recognized. PDT can induce autophagy in CRC cells, leading to their death. Additionally, PDT can enhance its cytotoxic effects on CRC cells by inhibiting autophagy [112]. Therefore, combining PDT with autophagy inhibitors holds promise as a potential approach for treating CRC [113].
It has been reported that blocking autophagy can enhance the susceptibility of PROM1/CD133+ cells to apoptosis induced by PDT, while also reducing the tumorigenic potential of colorectal cancer stem cells (CSCs) [113]. A combination therapy targeting p38MAPK and autophagy, in conjunction with PDT, may offer an effective treatment for CRC. This is because inhibiting p38MAPK enhances the efficacy of PDT, while autophagy provides protection against photokilling [114]. Ziółkowska et al. conducted an initial analysis of the impact of PDT on the expression of autophagy-related proteins Beclin-1, Atg7, and LC3, which has deepened our understanding of the relationship between autophagy and PDT [115]. Similarly, recent studies have confirmed that the combination of PDT with autophagy enhances the inhibitory effect on CRC [13, 112, 116-119]. Furthermore, PDT triggers autophagy as a survival mechanism, and the activation of the novel HIF-1α/VMP1/autophagic pathway may shed light on the resistance of CRC cells to PDT [120]. Although it is generally believed that inhibiting the expression of the key autophagy substrate p62 can enhance the anti-tumor effect, Kim et al. have shown that overexpressing p62 can increase the effectiveness of PDT. Additionally, they found that CRC cell lines with p62 knocked out exhibit decreased sensitivity to PDT [121]. Inhibiting autophagy can significantly reduce the efficacy of PDT-induced CRC cells by deactivating the ROS/JNK signaling pathway [122]. Corporately, these studies provide a compelling rationale for combining PDT with autophagy inhibitors for the treatment of CRC (Table 2).
3.3.4.2 Ferroptosis
Ferroptosis is a regulated cell death mechanism that triggers the overproduction of lipid-based ROS by inhibiting the cystine/glutamate antiporter system and the synthesis of glutathione (GSH) [123]. Uniquely, ferroptosis increases the cellular labile iron pool (LIP), leading to the continual generation of O2 through the Fenton reaction of H2O2 and Fe (III) [124].
Summary of representative articles on PDT regulating antitumor effects of CRC
| Years | Objects | Photosensitizer and concentration | Wavelength (nm) | Light treatment parameters | Mean Findings | Refs. |
|---|---|---|---|---|---|---|
| PDT inducing apoptosis of CRC | ||||||
| 2007 | Human CRC HCT116 cells | PpIX 2mg/mL | 632.8 | 2J/cm2 | Inducing p53-dependent activation of pro-apoptotic gene expression followed by growth suppression and induction of apoptosis | [80] |
| 2007 | Human CRC HCT116 cells | ATX-S10Na (II) 20 μg/mL | 670 | 2J/cm2 at 167 mW/cm2 | Mediated by p53-Bax network and low levels of Bcl-2 and Bcl-x(L) proteins | [79] |
| 2010 | Human CRC HT29 cells | SiPcGlu 1.5mM | 610 | 48J/cm2 at 40 mW/cm2 | Triggering the apoptotic pathways in both mitochondria and endoplasmic reticulum, but not the lysosome | [199] |
| 2018 | Human CRC LoVo cells | TαPcZn | 600-700 | 53.7J/cm2 | Direct interaction between p38 MAPK and caspase-9 may regulate mitochondria-mediated apoptosis | [50] |
| 2018 | murine CRC CT26 cells | Pc9-T1107 20 mN | 630 | 2.8J/cm2 at 1.17 mW/cm2 | Lysosomal membrane permeabilization, induction of ER stress, and activation of caspase-dependent apoptotic cell death. | [49] |
| PDT regulating antiangiogenic CRC | ||||||
| 2016 | Human CRC SW620 and SW480 cells | 5-ALA 500, 1000,1500 μM | 600-720nm | 10, 30, 60 J/cm2 at 1.5 mW/cm2 | 5-ALA-PDT markedly reduced VEGF secretion in SW620 cell line, while the ability of SW480 cell line to secrete VEGF remained unchanged | [86] |
| 2018 | Human CRC SW620 and SW480 cells | ALA 10000 μM | 600-720nm | 10 J/cm2 at 1.5 mW/cm2 | ALA-PDT reduced the release of VEGF in SW620 cell line, and the VEGF secretion levels of SW620 cells were significantly higher than those of SW480 cells | [87] |
| PDT activating the immune system of CRC | ||||||
| 2010 | CRC BALB/c mouse models expressing b-gal | BPD 1mg/Kg | 690 | 120 J/cm2 at 100 mW/cm2 | The first discovery of the role of antigen expression in PDT immune response. | [92] |
| 2013 | CT26 mouse CRC cells | BPD 1mg/Kg | 690 | 120 J/cm2 at 100 mW/cm2 | Depletion of Treg can enhance the immune response mediated by PDT | [93] |
| 2016 | Human CRC HT29 cells and murine CRC CT26 and MC38 cells | NCP@pyrolipid 2mg/Kg | 670 | 54 J/cm2 at 60 mW/cm2 | PDT combined with anti-PD-L1 inhibited not only the growth of primary CRC but also the distant metastasis of CRC | [94] |
| 2016 | Murine CRC cell CT26 and MC38 | NMOFs 1.5mg/Kg | 650 | 90 J/cm2 at 100 mW/cm2 | PDT significantly increases systemic tumor-specific immune response rates to checkpoint blockade cancer immunotherapy | [99] |
| 2017 | mouse CRC CT26 cells | UCNP-Ce6-R837 Nanoparticles | 980 | 500 mW/cm2 for 20 mins | The combination of PDT and CTLA-4 checkpoint inhibitors shows a powerful synergistic therapeutic effect, which can not only eliminate primary CRC tumors and inhibit distant metastasis of CRC but also prevent CRC recurrence after treatment | [96] |
| 2018 | mouse CRC CT26 cells | Fe-TBP 0.2 µM | 650 | 100 mW/cm2 for 7.5 mins | Combining PDT with anti-PD-L1 treatment can induce the synergistic therapy of CD4+ and CD8+ cytotoxic T cells for the treatment of CRC | [95] |
| 2018 | mouse CRC CT26 cells | EGFR-CPIG | 650 | 500 mW/cm2 for 20 mins | combining PDT with immunotherapy that uses a unique PS targeting EGFR. This approach has precise targeting for CRC and a remarkable inhibitory effect without any recurrence after treatment. | [97] |
| 2021 | mouse CRC CT26 cells | SPDMCN 10-40 μg/mL | 808 | 300 mW/cm2 for 8 mins | The PS SPDMCN was specifically activated in the tumor microenvironment of CRC, releasing DMC to enhance the effect of PDT | [98] |
| 2021 | HT29 et al.13 human CRC cells and murine CRC MC38 cells | IR700DX-6T 100 or 200 mN | 690 | 18 or 28 J/cm2 | PDT can inhibit the proliferation of multiple CRC cell lines by inducing ICD | [88] |
| PDT activating autophagy of CRC | ||||||
| 2014 | PROM1/CD133+ CRC cells | PpIX 1 µg/mL | 633 | 1 or 5 J/cm2 | The elevation of autophagy levels is linked to the enhanced resistance of CSCs to PDT, thus presenting a novel therapeutic strategy for targeting autophagy in the PDT-mediated treatment of CSCs | [102] |
| 2015 | Human CRC SW620 cells | Ce6 2.5mg/ml | 650 | 3 J/cm2 | Autophagy has potential for cancer treatment, with p38MAPK as a promising therapeutic target for boosting efficacy against CRC | [114] |
| 2016 | Human CRC SW620 cells | 5-ALA 3 mM | 610-650 | 4.5 J/cm2 at 60 mW/cm2 | The initial analysis of the impact of PDT on the expression of autophagy-associated proteins Beclin-1, Atg7, and LC3 has improved our understanding of the link between autophagy and PDT | [115] |
| 2017 | Human CRC SW620 and HCT116 cells | PS-II 10 mg/Kg | 630 | 5, 10, 20 J/cm2 at 100 mW/cm2 | combination of PDT with autophagy enhances the inhibitory effect on CRC | [112] |
| 2017 | Human CRC HCT8 and HCT116 cells | Hypericin | 630 | 7.2 J/cm2 | combination of PDT with autophagy enhances the inhibitory effect on CRC | [119] |
| 2017 | Human CRC CaCo2 and SW480 cells | PpIX | 618-652 | 1, 2, 3, 4, 5 J/cm2 at 165 mW/cm2 | PDT triggers autophagy as a means of survival, and the activation of the novel HIF-1α/VMP1/autophagic pathway may shed light on the resistance of CRC cells to PDT | [120] |
| 2018 | Human CRC HCT116 cells | PpIX 1 mg/ml | 630 | 5 or 10 J/cm2 | combination of PDT with autophagy enhances the inhibitory effect on CRC | [13] |
| 2018 | Human CRC SW620 cells | Ce6 0.5 μg/ml | 650 | 6 J/cm2 | combination of PDT with autophagy enhances the inhibitory effect on CRC | [116] |
| 2020 | Human CRC SW480, HCT116, LoVo, and DLD1 cells | Ce6 1.25 mg/Kg | 670 | 4/cm2 at 800 mW/cm2 | overexpressing p62 can instead increase the effectiveness of PDT and CRC cell lines with p62 knocked out were less sensitive to PDT | [121] |
| 2020 | Human CRC SW480 and HCT116 | m-THPC and VP (0.375- 12.0 μmol/L) | 650 | 3/cm2 at 10 mW/cm2 | The anti-cancer efficacy of PDT-mediated CRC cells can be significantly reduced by deactivating the ROS/JNK. signaling pathway through the inhibition of autophagy | [122] |
| 2021 | Human CRC SW480 | Ce6 0.125 -8 µg/mL | 650 | 6J/cm2 | combination of PDT with autophagy enhances the inhibitory effect on CRC | [117] |
| 2022 | Human CRC SW620 | Ce6 0.5 µg/mL | 650 | 6J/cm2 | combination of PDT with autophagy enhances the inhibitory effect on CRC | [118] |
| PDT regulating miRNAs of PDT | ||||||
| Years | Objects | PS | miRNAs | Potential Targets | pathways | Refs. |
| 2017 | Human CRC HT29, HCT116, and RKO cells | mTHPC | miR-140, miR-30b, miR-3151, miR-506, miR-124, miR-30c, miR-663b | P53 | miR124/ iASPP axis | [104] |
| 2018 | Human CRC HT29, HCT116, and LoVo cells | H2TFPC, H2TFPCSGLc | miR-15a, miR-15b, miR-29a, miR-196a, miR-221, miR-25 | TNFAIP3 | LIFR-AS1/miR-29a/TNFAIP3 axis | [136] |
| 2020 | Human CRC CX-1 cells | DVDMS | miR-7112-3p | PERK | PERK/ATF4/ CHOP/caspase cascade pathway | [78] |
| 2021 | Human CRC SW620, RKO, HCT116, and LoVo cells | 5-ALA | miR-124 | P53 | c-Myc/ Neat1/miR-124/ /iASPP/p53 axis | [137] |
b-gal: b-galactosidase; DMC: immune modulator; Ce6: chlorin e6; m-THPC: Meta-tetra hydroxyphenyl chlorin; VP: verteporfin; DVDMS: Sinoporphyrin sodium; PS- II: Photosan-II;
Emerging cancer therapy strategies based on ferroptosis have garnered interest [125, 126]. Increasing the concentration of ROS and O2 in the tumor microenvironment (TME) can enhance the effectiveness of PDT. Fortunately, the characteristics of ferroptosis cells mentioned earlier precisely match what is needed to enhance PDT [127]. There is evidence that PDT can function as a ROS generator, thereby triggering the Fenton reaction. This may intensify the initiation of ferroptosis and increase the effectiveness of PDT in anticancer treatment [127-129]. Inducing ferritin production through PDT could serve as a promising alternative strategy to enhance the efficacy of CRC treatment, especially considering the immunological properties of ferritin [130]. A better understanding of the synergistic interactions between PDT and ferritin may lead to more effective anti-CRC therapy by overcoming resistance to other cell death mechanisms.
Zhou et al. presented a promising treatment for CRC involving copper-cysteamine nanoparticles (Cu-Cy)-mediated PDT to induce ferroptosis in CRC cells. Crucially, in vivo, experiments using an HCT15 tumor-bearing mouse model provided additional confirmation of the superior antitumor efficacy of Cu-Cy-PDT [12]. Additionally, iron administration was found to enhance the anticancer effect of Photolon-based PDT in a CT26 CRC mouse model [131]. Iron-induced oxidative cellular damage might have contributed to the observed increase in the anticancer effect of PDT (Table 2) [131]. These studies suggest that PDT-induced ferroptosis holds promising potential as an avenue to enhance the efficacy of CRC treatment.
3.3.4.3 MiRNAs
MiRNAs exert substantial influence over gene expression and govern diverse cellular processes, encompassing proliferation, apoptosis, and differentiation [132]. Recent studies have demonstrated their involvement in the mechanism of action of PDT [133]. Specifically, miR-21 upregulation is triggered by PDT in cancer cells, and its inhibition enhances PDT-induced cell death [134]. Additionally, miRNAs regulate TME, which is critical for PDT efficacy. For example, miR-155 modulates the TME by regulating the immune response to PDT [135]. These findings suggest that targeting miRNAs can enhance the efficacy of PDT and overcome resistance to therapy.
A study conducted by Liu et al. revealed the role of miR-124 and iASPP in mediating resistance to PDT in CRC cells with p53 mutations or deletions. This finding holds significant importance in enhancing the effectiveness of PDT and optimizing treatment strategies [104]. Furthermore, the long non-coding RNA LIFR-AS1 modulates the resistance of CRC cells to PDT by regulating miR-29a, which in turn regulates TNFAIP3 as a downstream target of miR-19a. This discovery highlights the potential of targeting LIFR-AS1 as a strategy to overcome PDT resistance in CRC [136]. Additionally, PDT reduces the expression of miR7112-3p in CRC cell lines, thereby mitigating the inhibition of PERK. This results in increased PERK expression and induction of apoptosis in CRC cells through endoplasmic reticulum stress [78]. Moreover, recent studies have demonstrated the critical involvement of the c-Myc/NEAT1 axis in mediating the therapeutic efficacy of PDT in CRC through the miR-124/iASPP/p53 pathway.[137] (Table 2). To conclude, the therapeutic mechanism of PDT relies on the involvement of miRNAs. By targeting miRNAs, it is possible to enhance the effectiveness of PDT and surmount treatment resistance.
3.3.4.4 Other signaling pathways
The mechanisms that trigger signaling pathways via PDT may depend on various factors, including cell type, metabolic characteristics [138], subcellular localization of the PS [139], and PDT treatment intensity [140]. PDT for CRC is governed by the intricate involvement of multiple signaling pathways in its regulatory processes. Modulating NRF2 has been shown to enhance PDT sensitivity in various cancer cells, including CRC [105]. Moreover, the activation of the ERK1/2 pathway, mediated by oxidative stress, critically contributes to the positive regulation of HIF-1 transcriptional activity after PDT. Effective strategies to mitigate PDT-induced upregulation of HIF-1 include ROS clearance and inhibition of the MEK/ERK pathway [141]. The Rac1/PAK1/LIMK1/cofilin signaling pathway also exerts a notable impact on inhibiting CRC cells during PDT [142]. Additionally, PDT triggers a toxic response in CRC cells by promoting the PP2A-mediated ubiquitination and degradation of BMI1[143]. Furthermore, the CDC25A/CDK2/Cyclin A pathway and the mitochondrial apoptosis pathway participate in the induction of cell cycle arrest and apoptosis in CRC cells following PDT [144]. MYBL2 loss in CRC cells activates NF-κB, resulting in the upregulation of ABCG2 and subsequent inhibition of the CRC response to PDT [145]. In conclusion, the molecular mechanisms and signaling pathways involved in the PDT treatment of CRC are complex and necessitate further comprehensive investigation (Figure 4).
4. Clinical Application of PDT in intestinal-related inflammatory and cancer diseases
Table 3 comprehensively reviews clinical trials and case reports investigating the use of PDT in intestinal-related inflammatory and cancerous diseases. In a clinical trial of six patients with advanced rectal cancer, Photofrin II-mediated PDT demonstrated remarkable tumor destruction in two patients. Moreover, one patient experienced relief from pain and obstruction symptoms. Importantly, the study reported no significant adverse effects, such as severe bleeding, sepsis, or perforation.[146]. Another study demonstrated the effectiveness of adjuvant intraoperative photodynamic therapy (AIOPDT) in reducing postoperative recurrence rates among patients with CRC [147]. Furthermore, PDT holds promise as a potential technique for treating small gastrointestinal tumors or tumors in patients unsuitable for surgery [148-151]. Importantly, PDT has been demonstrated to be a safe and effective treatment option for unresectable CRLM [152]. Furthermore, Zhang et al. reported a successful case of PDT treatment for R1 rectal cancer [27]. Regrettably, there is currently a lack of available clinical research reports investigating the utilization of PDT for the treatment of IBD (Table 3). Hence, the clinical application of PDT in intestinal-related inflammatory and cancerous diseases still faces significant challenges and requires further advancements before widespread adoption can be achieved.
Summary of clinical trials and case reports investigating the use of PDT for CRC
| Years | Patients | Photosensitizer and concentration | Wavelength (nm) | Light treatment parameters | Research type | Results | Refs. |
|---|---|---|---|---|---|---|---|
| 1991 | 6 advanced rectal cancer patients, (3 males and 3 females), average 66years old (range 37-91) | Photofrin II, 2 mg/kg body weight | 630 ± 3 | 50-200J/cm2 | Phase I/II Study | The tumors in two patients exhibited significant destruction, leading to the alleviation of pain and obstruction symptoms in one patient. | [146] |
| 1994 | 14CRC patients, (10 men and 7 females), | Hpd, 5 or 3 or111 mg /kg body weight | 640 | N/A | Phase I/II Study | AIOPDT leads to a reduction in the postoperative recurrence rate among patients with CRC | [147] |
| 1994 | 33 patients with Tis or T1 cancers of the gastrointestinal tract | Hpd, 5 mg /kg body weight | 632 | 220 J/cm2 | Phase II/Ⅲ Study | 17 patients were observed to exhibit complete eradication of the localized tumor and negative histopathological findings | [148] |
| 1995 | 18 patients with gastrointestinal tumors (12 men and 6 females), average 79years old (range 38-93) | ALA 30-60 mg /kg body weight | 628 | 100J/ cm2 | a pilot study | ALA-PDT may be a promising technique for the treatment of small gastrointestinal tumors. | [149] |
| 1995 | 6 patients with duodenal or rectal tumors | ALA 60 mg /kg body weight or Photofrin 2 mg /kg body weight | 630 | 100J/ cm2 | a pilot study | PDT is a promising treatment for inoperable polyps in patients with familial adenomatous polyposis | [150] |
| 1998 | 22 gastrointestinal tract cancer patients | m-THPc 0.15 mg /kg, Photofrin 2 mg /kg, ALA 60 mg /kg (body weight) | 628 for ALA and Photofrin, 650 for mTHPc | 50-150 J/cm2 for ALA and Photofrin, 10-15 J/cm2 for mTHPc | a pilot study | PDT is a promising treatment for small localized tumors in patients unsuitable for surgery | [151] |
| 2005 | 24 patients with CRLM | Mthpbc 3mg/Kg or 6mg/Kg (body weight) | 740 | 60 J/cm persist 300 to 600 seconds | Phase Ⅰ Study | PDT can safely and effectively treat unresectable CRLM | [152] |
| 2019 | A 56 years old man with low rectal cancer after ultra-low anterior resection | Porphyrin 2mg/kg body weight | 630 | 100 mw/cm2 | Case report | PDT successfully treats rectal cancer R1 | [27] |
PpIX: Protoporphyrin IX; HPD: hematoporphyrin derivative; AIOPDT: adjuvant intraoperative photodynamic therapy; CRLM: colorectal liver metastasis; m-THPC: Meta-tetra hydroxyphenyl chlorin
5. Challenges and Prospects
5.1 Challenges
PDT demonstrates the ability to selectively target and affect diseased cells, including cancerous cells and inflamed tissues while sparing healthy cells. This selectivity significantly reduces damage to healthy tissues and minimizes side effects [153]. Moreover, PDT qualifies as a minimally invasive treatment option that can be administered endoscopically. This characteristic enables direct application to the affected area, resulting in localized treatment and faster recovery times [43]. Therefore, PDT shows promise as an effective approach for treating both inflammatory and cancerous diseases affecting the intestines [4, 47]. However, this therapeutic method presents several challenges [154]. The effectiveness of PDT heavily depends on the selective uptake of the photosensitizing agent by target cells [25]. Achieving specific targeting can be challenging, particularly in cases involving diffuse or widespread lesions [155]. Moreover, PDT protocols and parameters necessitate standardization and optimization for various diseases [156, 157], including inflammatory and cancerous conditions of the intestines [16]. Further research is necessary to determine the optimal photosensitizers, light dosimetry, and treatment regimens for maximum efficacy [158]. Furthermore, PDT is a complex and specialized therapy that requires specific equipment and expertise. The cost and availability of PDT may hinder its widespread adoption, especially in resource-limited settings [159]. Although PDT is generally well-tolerated, it can still result in side effects and complications, such as skin photosensitivity, pain during light activation, and potential damage to adjacent healthy tissues if not precisely targeted [11].
5.2 Prospects
The advancements in science and technology, coupled with a growing understanding of PDT among researchers and practitioners, have significantly contributed to overcoming challenges [160]. The potential of utilizing PDT in the treatment of IBD and CRC continues to exhibit promising prospects. In this article, we discuss several potential directions for future breakthroughs.
5.2.1 Combination therapy
PDT can be combined with other treatment modalities, such as chemotherapy (e.g., 5-FU [161], doxorubicin [74], oxaliplatin [100]), immunotherapy (e.g., anti-PD-L1 antibody [162-164], CTLA-4 inhibitors [101]), targeted therapy (e.g., bevacizumab [165], Cetuximab [166]), or small molecule inhibitors (e.g., inhibitors of COX-1 and COX-2[167], proteasome inhibitors[168], AKT inhibitors[169], autophagy inhibitors[113, 170]), to enhance the overall therapeutic effect. This multimodal approach holds the potential to improve treatment outcomes, especially in cases where diseases have advanced or exhibit resistance to conventional therapies.
5.2.2 Nanomedicine and Carriers in PDT
The application of nanomedicine is instrumental in augmenting the efficacy of PDT by improving the delivery, targeting, imaging, and enabling combination therapies [171]. Nanoparticles effectively encapsulate PS, providing protection and ensuring efficient and sustained delivery to the intended site [172]. Surface-functionalized nanoparticles can bind to cancer cell receptors, enhancing selectivity and reducing side effects [173]. Moreover, nanoparticles incorporating imaging agents enable real-time visualization and precise treatment planning [45]. Additionally, they can carry multiple components, such as chemotherapeutic drugs and immune checkpoint inhibitors, leading to enhanced therapeutic effects [162, 174]. The integration of therapy and diagnostics in theranostic platforms further optimizes the outcomes of PDT [175, 176]. Ultimately, nanomedicine leverages the unique properties of nanoparticles to improve the efficacy and precision of PDT. Importantly, the hypoxic characteristics of the TME can adversely impact the therapeutic efficacy of PDT. However, MnO2 nanoparticles can react with endogenous H2O2 and generate oxygen. Therefore, by encapsulating the PS within the lipid bilayer of liposomes and subsequently coating them with MnO2 nanoparticles, PDT can be enhanced in hypoxic tumor regions [177]. Furthermore, Cu-Cy nanoparticles containing Cu1+ ions can participate in heterogenous Fenton-like activity under acidic conditions, leading to a significant increase in the levels of hydroxyl radicals within cancer cells. This mechanism enables the effective mitigation of low oxygen conditions within the tumor microenvironment, further enhancing the therapeutic outcomes of PDT [178].
The successful implementation of PDT heavily relies on efficiently delivering PS to target cells in complex biological environments. Therefore, developing innovative strategies to enhance PS delivery is of paramount importance [179]. To overcome the challenges associated with direct PS administration, researchers have explored carrier-mediated delivery systems such as liposomes [180], aggregation-induced emission (AIE) luminogen hybrid nanovesicles [181], and extracellular vehicles (EVs) [182] to facilitate PS transport to target cells. Liposomes, which are spherical vesicles composed of lipid bilayers closely resembling biological membranes, serve as versatile carriers for PS [183]. They can encapsulate PS within their hydrophobic core or integrate it into their lipid bilayers. The high surface area-to-volume ratio of liposomes enables efficient drug loading and controlled release. Additionally, surface modifications enhance the targeting specificity and cellular uptake of PS [184]. AIE luminogen hybrid nanovesicles combine the photostability and fluorescence of AIE luminogens with the drug delivery capabilities of nanovesicles [185]. These carriers exhibit excellent stability, high permeability, and tunable surface properties, making them promising vehicles for efficient PS delivery [186]. EVs, naturally released vesicles involved in intercellular communication, have garnered interest as carriers for PS delivery [187]. These vesicles can be loaded with PS and functionalized to achieve targeted delivery. Due to their inherent biocompatibility, low immunogenicity, and ability to traverse biological barriers, EVs are attractive candidates, especially for PS delivery in complex intestinal conditions [188].
5.2.3 Novel Excitation Conditions for PDT
PDT exhibits promise in cancer treatment. Nonetheless, to augment its efficacy, researchers have investigated the integration of PDT with other modalities, such as X-ray, microwave, and sonodynamic therapy.
X-ray radiation possesses the capability to penetrate deep into tissues, rendering it a potential synergistic partner for PDT [189, 190]. Multiple studies have explored the amalgamation of X-ray radiation with PDT, aiming to enhance tumor targeting and treatment outcomes [191,192,193]. It shows potential in treating IBD and CRC. In IBD, X-inducers selectively accumulate in inflamed tissues and, upon X-ray activation, produce ROS, which reduces inflammation [190]. In CRC, X-inducers can be targeted to cancer cells, generating ROS and inducing tumor cell death [193]. X-PDT offers targeted therapy with reduced side effects and can be combined with other treatments [191]. Importantly, PDT allows for multiple repetitions, if necessary, without the risk of cumulative toxicity. This feature proves particularly beneficial in managing chronic conditions, such as IBD, that require long-term treatment [7].
Microwave hyperthermia has been investigated in conjunction with PDT to enhance its therapeutic effects [194]. Microwave-induced hyperthermia can augment blood flow, oxygen supply, and the permeability of tumor blood vessels, which can facilitate the delivery of PS to tumor cells and intensify the generation of reactive oxygen species during PDT [194]. The combination of microwave hyperthermia and PDT has demonstrated improved tumor control and heightened treatment response in specific studies [12].
Sonodynamic therapy involves the use of ultrasound in combination with a sonosensitizer to induce the production of reactive oxygen species and achieve tumor destruction [182]. When combined with PDT, sonodynamic therapy can further enhance the cytotoxic effects on tumor cells [195]. Ultrasound waves can enhance the cellular uptake of PS and improve its localization within the tumor [196]. Moreover, ultrasound-induced cavitation can promote the release of PS from carriers and enhance its intracellular delivery [197]. The combination of sonodynamic therapy and PDT has shown the potential in overcoming the limitations of conventional PDT and improving its therapeutic outcomes [198].
6. Conclusions
PDT holds promise as a minimally invasive and effective treatment for CRC and IBD. Extensive preclinical research provides strong support for its safety and efficacy, while ongoing clinical studies further validate its potential. This review offers valuable insights into the mechanisms underlying the anti-inflammatory and anti-tumor effects of PDT, laying a foundation for its clinical application in CRC and IBD treatment and contributing to advancements in intestinal disease therapy.
Abbreviations
PDT: Photodynamic therapy; PS: photosensitizer; ROS: reactive oxygen species; HDPDT: High-dose Photodynamic therapy; LDPDT: low-dose Photodynamic therapy; IBD: inflammatory bowel disease; UC: ulcerative colitis; CD: Crohn's disease; CRC: colorectal cancer; CAC: inflammatory bowel disease -related colorectal cancer; TNF-α: tumor necrosis factor-α; CRLM: colorectal liver metastasis; APCs: antigen presenting cells; MHC: major histocompatibility complex; DCs: Dendritic cells; CTLA-4: cytotoxic T-lymphocyte-associated protein 4; COX-2: cyclooxygenase-2; NF-KB: nuclear factor kappa B; F/B: Firmicutes/Bacteroidetes; ICD: immunogenic cell death; ER: endoplasmic reticulum; VEGF: Vascular endothelial growth factor; Treg: T regulatory cells; DMC: immune modulator; CSCs: CRC stem cells; GSH: glutathione; LIP: labile iron pool; TME: tumor microenvironment; Cu-Cy: Copper-cysteamine nanoparticles; LPO: lipid peroxides; MDA: malondialdehyde; AIOPDT: adjuvant intraoperative photodynamic therapy; 5-ALA: 5-aminolevulinic acid; BPD: Liposomal benzoporphyrin derivative mono acid ring A; MPO: myeloperoxidase; SOD: superoxide oxidase; IL: interleukin; IFN-γ: interferon-gamma; PBMCs: peripheral blood mononuclear cells; Ce6: chlorin e6; m-THPC: Meta-tetrahydroxyphenylchlorin; VP: verteporfin; DVDMS: Sinoporphyrin sodium; HPD: hematoporphyrin derivative; b-gal: b-galactosidase; AIE: aggregation-induced emission; EVs: extracellular vesicles.
Supplementary Material
Supplementary tables.
Author Contributions
KPW and BYD contributed to conceiving and designing the study. BYD, KPW, LLZ, ZDQ, and WGD performed the article searching. KPW, BYD, LLZ, ZDQ, and WXW extracted the data. BYD and KPW wrote the manuscript. WGD and WXW supervised the manuscript. all authors revised and agree to be accountable for the content of the work.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Kwiatkowski S, Knap B, Przystupski D. et al. Photodynamic therapy - mechanisms, photosensitizers and combinations[J]. Biomed Pharmacother. 2018;106:1098-1107
2. Li X, Lovell J F, Yoon J. et al. Clinical development and potential of photothermal and photodynamic therapies for cancer[J]. Nat Rev Clin Oncol. 2020;17(11):657-674
3. Ji B, Wei M, Yang B. Recent advances in nanomedicines for photodynamic therapy (PDT)-driven cancer immunotherapy[J]. Theranostics. 2022;12(1):434-458
4. Reinhard A, Sandborn W J, Melhem H. et al. Photodynamic therapy as a new treatment modality for inflammatory and infectious conditions[J]. Expert Rev Clin Immunol. 2015;11(5):637-657
5. Zou H, Wang F, Zhou J J. et al. Application of photodynamic therapy for liver malignancies[J]. J Gastrointest Oncol. 2020;11(2):431-442
6. Zhang Z J, Wang K P, Mo J G. et al. Photodynamic therapy regulates fate of cancer stem cells through reactive oxygen species[J]. World J Stem Cells. 2020;12(7):562-584
7. Favre L, Borle F, Velin D. et al. Low dose endoluminal photodynamic therapy improves murine T cell-mediated colitis[J]. Endoscopy. 2011;43(7):604-616
8. Agostinis P, Berg K, Cengel K A. et al. Photodynamic therapy of cancer: an update[J]. CA Cancer J Clin. 2011;61(4):250-281
9. Hodgkinson N, Kruger C A, Abrahamse H. Targeted photodynamic therapy as potential treatment modality for the eradication of colon cancer and colon cancer stem cells[J]. Tumour Biol. 2017;39(10):1393354973
10. Yang L, He J, Wen Y. et al. Nanoscale Photodynamic Agents for Colorectal Cancer Treatment: A Review[J]. J Biomed Nanotechnol. 2016;12(7):1348-1373
11. Zhi D, Yang T, O'Hagan J. et al. Photothermal therapy[J]. J Control Release. 2020;325:52-71
12. Zhou H, Liu Z, Zhang Z. et al. Copper-cysteamine nanoparticle-mediated microwave dynamic therapy improves cancer treatment with induction of ferroptosis[J]. Bioact Mater. 2023;24:322-330
13. Ouyang G, Xiong L, Liu Z. et al. Inhibition of autophagy potentiates the apoptosis-inducing effects of photodynamic therapy on human colon cancer cells[J]. Photodiagnosis Photodyn Ther. 2018;21:396-403
14. Huis I T V R, Heuts J, Ma S. et al. Current Challenges and Opportunities of Photodynamic Therapy against Cancer[J]. Pharmaceutics. 2023 15(2)
15. Ibbotson S H, Wong T H, Morton C A. et al. Adverse effects of topical photodynamic therapy: a consensus review and approach to management[J]. Br J Dermatol. 2019;180(4):715-729
16. Wang K, Zhang Z, Liu K. et al. Neat1-miRNA204-5p-PI3K-AKT axis as a potential mechanism for photodynamic therapy treated colitis in mice[J]. Photodiagnosis Photodyn Ther. 2018;24:349-357
17. Espeland K, Kleinauskas A, Juzenas P. et al. Photodynamic Effects with 5-Aminolevulinic Acid on Cytokines and Exosomes in Human Peripheral Blood Mononuclear Cells from Patients with Crohn's Disease[J]. Int J Mol Sci. 2023 24(5)
18. Reinhard A, Bressenot A, Dassonneville R. et al. Photodynamic therapy relieves colitis and prevents colitis-associated carcinogenesis in mice[J]. Inflamm Bowel Dis. 2015;21(5):985-995
19. Rong Y, Hong G, Zhu N. et al. Photodynamic Therapy of Novel Photosensitizer Ameliorates TNBS-Induced Ulcerative Colitis via Inhibition of AOC(1)[J]. Front Pharmacol. 2021;12:746725
20. Sung H, Ferlay J, Siegel R L. et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries[J]. CA Cancer J Clin. 2021;71(3):209-249
21. Global regional, national burden of colorectal cancer its risk factors, 1990-2019. a systematic analysis for the Global Burden of Disease Study 2019[J]. Lancet Gastroenterol Hepatol. 2022;7(7):627-647
22. The global, regional, national burden of inflammatory bowel disease in 195 countries, territories, 1990-2017. a systematic analysis for the Global Burden of Disease Study 2017[J]. Lancet Gastroenterol Hepatol. 2020;5(1):17-30
23. Shah S C, Itzkowitz S H. Colorectal Cancer in Inflammatory Bowel Disease: Mechanisms and Management[J]. Gastroenterology. 2022;162(3):715-730
24. Keller D S, Windsor A, Cohen R. et al. Colorectal cancer in inflammatory bowel disease: review of the evidence[J]. Tech Coloproctol. 2019;23(1):3-13
25. Rkein A M, Ozog D M. Photodynamic therapy[J]. Dermatol Clin. 2014;32(3):415-425
26. Gu B, Wang B, Li X. et al. Photodynamic therapy improves the clinical efficacy of advanced colorectal cancer and recruits immune cells into the tumor immune microenvironment[J]. Front Immunol. 2022;13:1050421
27. Zhang S Q, Liu K J, Yao H L. et al. Photodynamic therapy as salvage therapy for residual microscopic cancer after ultra-low anterior resection: A case report[J]. World J Clin Cases. 2019;7(6):798-804
28. van Duijnhoven F H, Rovers J P, Engelmann K. et al. Photodynamic therapy with 5,10,15,20-tetrakis(m-hydroxyphenyl) bacteriochlorin for colorectal liver metastases is safe and feasible: results from a phase I study[J]. Ann Surg Oncol. 2005;12(10):808-816
29. Yano T, Muto M, Yoshimura K. et al. Phase I study of photodynamic therapy using talaporfin sodium and diode laser for local failure after chemoradiotherapy for esophageal cancer[J]. Radiat Oncol. 2012;7:113
30. Yoshizawa Y, Sugimoto M, Sato Y. et al. Factors associated with healing of artificial ulcer after endoscopic submucosal dissection with reference to Helicobacter pylori infection, CYP2C19 genotype, and tumor location: Multicenter randomized trial[J]. Dig Endosc. 2016;28(2):162-172
31. Goldstein E S, Rubin P H. Endoscopic Therapy for Inflammatory Bowel Disease[J]. Curr Treat Options Gastroenterol. 2003;6(3):237-243
32. Oxenberg J, Hochwald S N, Nurkin S. Ablative therapies for colorectal polyps and malignancy[J]. Biomed Res Int. 2014;2014:986352
33. Bernstein C N, Fried M, Krabshuis J H. et al. World Gastroenterology Organization Practice Guidelines for the diagnosis and management of IBD in 2010[J]. Inflamm Bowel Dis. 2010;16(1):112-124
34. Nagao-Kitamoto H, Kitamoto S, Kamada N. Inflammatory bowel disease and carcinogenesis[J]. Cancer Metastasis Rev. 2022;41(2):301-316
35. Papamichael K, Stocco G, Ruiz D A A. Challenges in Therapeutic Drug Monitoring: Optimizing Biological Treatments in Patients With Inflammatory Bowel Disease and Other Immune-Mediated Inflammatory Diseases[J]. Ther Drug Monit. 2023
36. Kolbe M F, Ribeiro F V, Luchesi V H. et al. Photodynamic therapy during supportive periodontal care: clinical, microbiologic, immunoinflammatory, and patient-centered performance in a split-mouth randomized clinical trial[J]. J Periodontol. 2014;85(8):e277-e286
37. Rogers L, Sergeeva N N, Paszko E. et al. Lead Structures for Applications in Photodynamic Therapy. 6. Temoporfin Anti-Inflammatory Conjugates to Target the Tumor Microenvironment for In vitro PDT[J]. PLoS One. 2015;10(5):e125372
38. Liu C, Jiang Y, Liu G. et al. PPARGC1A affects inflammatory responses in photodynamic therapy (PDT)-treated inflammatory bowel disease (IBD)[J]. Biochem Pharmacol. 2022;202:115119
39. Zhou H, Liu Z, Wang Y. et al. Colorectal liver metastasis: molecular mechanism and interventional therapy[J]. Signal Transduct Target Ther. 2022;7(1):70
40. Zhang Z J, Huang Y P, Li X X. et al. A Novel Ferroptosis-Related 4-Gene Prognostic Signature for Cholangiocarcinoma and Photodynamic Therapy[J]. Front Oncol. 2021;11:747445
41. Zhou H, Liu Z, Zhang Z. et al. Copper-cysteamine nanoparticle-mediated microwave dynamic therapy improves cancer treatment with induction of ferroptosis[J]. Bioact Mater. 2023;24:322-330
42. Qiu L, Li J, Chen F. et al. Chinese expert consensus on the clinical applications of aminolevulinic acid-based photodynamic therapy in female lower genital tract diseases (2022)[J]. Photodiagnosis Photodyn Ther. 2022;39:102993
43. Klinger A L, Kann B R. Endoscopy in Inflammatory Bowel Disease[J]. Surg Clin North Am. 2019;99(6):1063-1082
44. Messmann H, Knüchel R, Bäumler W. et al. Endoscopic fluorescence detection of dysplasia in patients with Barrett's esophagus, ulcerative colitis, or adenomatous polyps after 5-aminolevulinic acid-induced protoporphyrin IX sensitization[J]. Gastrointest Endosc. 1999;49(1):97-101
45. Shin Y K, Park Y R, Lee H. et al. Real-Time Monitoring of Colorectal Cancer Location and Lymph Node Metastasis and Photodynamic Therapy Using Fucoidan-Based Therapeutic Nanogel and Near-Infrared Fluorescence Diagnostic-Therapy System[J]. Pharmaceutics. 2023 15(3)
46. Nowis D, Stokłosa T, Legat M. et al. The influence of photodynamic therapy on the immune response[J]. Photodiagnosis Photodyn Ther. 2005;2(4):283-298
47. Lyons N J, Giri R, Begun J. et al. Reactive Oxygen Species as Mediators of Disease Progression and Therapeutic Response in Colorectal Cancer[J]. Antioxid Redox Signal. 2023
48. Abrahamse H, Houreld N N. Genetic Aberrations Associated with Photodynamic Therapy in Colorectal Cancer Cells[J]. Int J Mol Sci. 2019 20(13)
49. Chiarante N, García V M, Rey O. et al. Lysosomal permeabilization and endoplasmic reticulum stress mediate the apoptotic response induced after photoactivation of a lipophilic zinc(II) phthalocyanine[J]. Int J Biochem Cell Biol. 2018;103:89-98
50. Wang Y, Xia C, Lun Z. et al. Crosstalk between p38 MAPK and caspase-9 regulates mitochondria-mediated apoptosis induced by tetra-α-(4-carboxyphenoxy) phthalocyanine zinc photodynamic therapy in LoVo cells[J]. Oncol Rep. 2018;39(1):61-70
51. Musser D A, Oseroff A R. Characteristics of the immunosuppression induced by cutaneous photodynamic therapy: persistence, antigen specificity and cell type involved[J]. Photochem Photobiol. 2001;73(5):518-524
52. Simkin G O, Tao J S, Levy J G. et al. IL-10 contributes to the inhibition of contact hypersensitivity in mice treated with photodynamic therapy[J]. J Immunol. 2000;164(5):2457-2462
53. Allison B A, Pritchard P H, Levy J G. Evidence for low-density lipoprotein receptor-mediated uptake of benzoporphyrin derivative[J]. Br J Cancer. 1994;69(5):833-839
54. Jiang H, Granville D J, McManus B M. et al. Selective depletion of a thymocyte subset in vitro with an immunomodulatory photosensitizer[J]. Clin Immunol. 1999;91(2):178-187
55. Jiang H, Granville D J, North J R. et al. Selective action of the photosensitizer QLT0074 on activated human T lymphocytes[J]. Photochem Photobiol. 2002;76(2):224-231
56. King D E, Jiang H, Simkin G O. et al. Photodynamic alteration of the surface receptor expression pattern of murine splenic dendritic cells[J]. Scand J Immunol. 1999;49(2):184-192
57. Hunt D W, Chan A H. Influence of photodynamic therapy on immunological aspects of disease - an update[J]. Expert Opin Investig Drugs. 2000;9(4):807-817
58. Belicha-Villanueva A, Riddell J, Bangia N. et al. The effect of photodynamic therapy on tumor cell expression of major histocompatibility complex (MHC) class I and MHC class I-related molecules[J]. Lasers Surg Med. 2012;44(1):60-68
59. Kimura Y, Aoki H, Soyama T. et al. Photodynamic therapy using mannose-conjugated chlorin e6 increases cell surface calreticulin in cancer cells and promotes macrophage phagocytosis[J]. Med Oncol. 2022;39(5):82
60. Ratkay L G, Chowdhary R K, Neyndorff H C. et al. Photodynamic therapy; a comparison with other immunomodulatory treatments of adjuvant-enhanced arthritis in MRL-lpr mice[J]. Clin Exp Immunol. 1994;95(3):373-377
61. Li H, Zhang Z, Zhang H. et al. Update on the Pathogenesis and Therapy of Atopic Dermatitis[J]. Clin Rev Allergy Immunol. 2021;61(3):324-338
62. Kawczyk-Krupka A, Latos W, Oleś P. et al. The influence of 5-aminolevulinic photodynamic therapy on colon cancer cell interleukin secretion in hypoxia-like condition in vitro[J]. Photodiagnosis Photodyn Ther. 2018;23:240-243
63. Kawczyk-Krupka A, Czuba Z, Latos W. et al. Influence of ALA-mediated photodynamic therapy on secretion of interleukins 6, 8 and 10 by colon cancer cells in vitro[J]. Photodiagnosis Photodyn Ther. 2018;22:137-139
64. Pansa M F, Lamberti M J, Cogno I S. et al. Contribution of resident and recruited macrophages to the photodynamic intervention of colorectal tumor microenvironment[J]. Tumour Biol. 2016;37(1):541-552
65. Kraus S, Arber N. Inflammation and colorectal cancer[J]. Curr Opin Pharmacol. 2009;9(4):405-410
66. Frigerio S, Lartey D A, D'Haens G R. et al. The Role of the Immune System in IBD-Associated Colorectal Cancer: From Pro to Anti-Tumorigenic Mechanisms[J]. Int J Mol Sci. 2021 22(23)
67. Wei L H, Baumann H, Tracy E. et al. Interleukin-6 trans signalling enhances photodynamic therapy by modulating cell cycling[J]. Br J Cancer. 2007;97(11):1513-1522
68. Jackson D N, Theiss A L.Gut bacteria signaling to mitochondria in intestinal inflammation and cancer[J]. Gut Microbes, 2020,11(3):285-304
69. Quaglio A, Grillo T G, De Oliveira E. et al. Gut microbiota, inflammatory bowel disease and colorectal cancer[J]. World J Gastroenterol. 2022;28(30):4053-4060
70. Liu Z, Cao A T, Cong Y. Microbiota regulation of inflammatory bowel disease and colorectal cancer[J]. Semin Cancer Biol. 2013;23(6 Pt B):543-552
71. Papamichael K, Konstantopoulos P, Mantzaris G J. Helicobacter pylori infection and inflammatory bowel disease: is there a link?[J]. World J Gastroenterol. 2014;20(21):6374-6385
72. Chen Y, Wu G, Zhao Y. Gut Microbiota and Alimentary Tract Injury[J]. Adv Exp Med Biol. 2020;1238:11-22
73. Kang M, Martin A. Microbiome and colorectal cancer: Unraveling host-microbiota interactions in colitis-associated colorectal cancer development[J]. Semin Immunol. 2017;32:3-13
74. Chilakamarthi U, Mahadik N S, Koteshwar D. et al. Potentiation of novel porphyrin based photodynamic therapy against colon cancer with low dose doxorubicin and elucidating the molecular signalling pathways responsible for relapse[J]. J Photochem Photobiol B. 2023;238:112625
75. Hao Y, Ma S, Gu Z. et al. Combination of photodynamic therapy and stimulator of interferon genes (STING) agonist inhibits colorectal tumor growth and recurrence[J]. Cancer Commun (Lond). 2023;43(4):513-518
76. Wong R S. Apoptosis in cancer: from pathogenesis to treatment[J]. J Exp Clin Cancer Res. 2011;30(1):87
77. Staneloudi C, Smith K A, Hudson R. et al. Development and characterization of novel photosensitizer: scFv conjugates for use in photodynamic therapy of cancer[J]. Immunology. 2007;120(4):512-517
78. Kong F, Zou H, Liu X. et al. miR-7112-3p targets PERK to regulate the endoplasmic reticulum stress pathway and apoptosis induced by photodynamic therapy in colorectal cancer CX-1 cells[J]. Photodiagnosis Photodyn Ther. 2020;29:101663
79. Mitsunaga M, Tsubota A, Nariai K. et al. Early apoptosis and cell death induced by ATX-S10Na (II)-mediated photodynamic therapy are Bax- and p53-dependent in human colon cancer cells[J]. World J Gastroenterol. 2007;13(5):692-698
80. Zawacka-Pankau J, Issaeva N, Hossain S. et al. Protoporphyrin IX interacts with wild-type p53 protein in vitro and induces cell death of human colon cancer cells in a p53-dependent and -independent manner[J]. J Biol Chem. 2007;282(4):2466-2472
81. He Q, Zhang Z, Liu H. et al. Relieving immunosuppression during long-term anti-angiogenesis therapy using photodynamic therapy and oxygen delivery[J]. Nanoscale. 2020;12(27):14788-14800
82. Wei Z, Liang P, Xie J. et al. Carrier-free nano-integrated strategy for synergetic cancer anti-angiogenic therapy and phototherapy[J]. Chem Sci. 2019;10(9):2778-2784
83. Gallagher-Colombo S M, Maas A L, Yuan M. et al. Photodynamic therapy-induced angiogenic signaling: consequences and solutions to improve therapeutic response[J]. Isr J Chem. 2012;52(8-9):681-690
84. Semenza G L. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics[J]. Oncogene. 2010;29(5):625-634
85. Apte R S, Chen D S, Ferrara N. VEGF in Signaling and Disease: Beyond Discovery and Development[J]. Cell. 2019;176(6):1248-1264
86. Kawczyk-Krupka A, Sieroń-Stołtny K, Latos W. et al. ALA-induced photodynamic effect on vitality, apoptosis, and secretion of vascular endothelial growth factor (VEGF) by colon cancer cells in normoxic environment in vitro[J]. Photodiagnosis Photodyn Ther. 2016;13:308-315
87. Kawczyk-Krupka A, Kwiatek B, Czuba Z P. et al. Secretion of the angiogenic factor VEGF after photodynamic therapy with ALA under hypoxia-like conditions in colon cancer cells[J]. Photodiagnosis Photodyn Ther. 2018;21:16-18
88. Xie Q, Li Z, Liu Y. et al. Translocator protein-targeted photodynamic therapy for direct and abscopal immunogenic cell death in colorectal cancer[J]. Acta Biomater. 2021;134:716-729
89. Donohoe C, Senge M O, Arnaut L G. et al. Cell death in photodynamic therapy: From oxidative stress to anti-tumor immunity[J]. Biochim Biophys Acta Rev Cancer. 2019;1872(2):188308
90. Alzeibak R, Mishchenko T A, Shilyagina N Y. et al. Targeting immunogenic cancer cell death by photodynamic therapy: past, present and future[J]. J Immunother Cancer. 2021 9(1)
91. Mishchenko T, Balalaeva I, Gorokhova A. et al. Which cell death modality wins the contest for photodynamic therapy of cancer?[J]. Cell Death Dis. 2022;13(5):455
92. Mroz P, Szokalska A, Wu M X. et al. Photodynamic therapy of tumors can lead to development of systemic antigen-specific immune response[J]. PLoS One. 2010;5(12):e15194
93. Reginato E, Mroz P, Chung H. et al. Photodynamic therapy plus regulatory T-cell depletion produces immunity against a mouse tumour that expresses a self-antigen[J]. Br J Cancer. 2013;109(8):2167-2174
94. He C, Duan X, Guo N. et al. Core-shell nanoscale coordination polymers combine chemotherapy and photodynamic therapy to potentiate checkpoint blockade cancer immunotherapy[J]. Nat Commun. 2016;7:12499
95. Lan G, Ni K, Xu Z. et al. Nanoscale Metal-Organic Framework Overcomes Hypoxia for Photodynamic Therapy Primed Cancer Immunotherapy[J]. J Am Chem Soc. 2018;140(17):5670-5673
96. Xu J, Xu L, Wang C. et al. Near-Infrared-Triggered Photodynamic Therapy with Multitasking Upconversion Nanoparticles in Combination with Checkpoint Blockade for Immunotherapy of Colorectal Cancer[J]. ACS Nano. 2017;11(5):4463-4474
97. Li Y, Du Y, Liang X. et al. EGFR-targeted liposomal nanohybrid cerasomes: theranostic function and immune checkpoint inhibition in a mouse model of colorectal cancer[J]. Nanoscale. 2018;10(35):16738-16749
98. He S, Li J, Cheng P. et al. Charge-Reversal Polymer Nano-modulators for Photodynamic Immunotherapy of Cancer[J]. Angew Chem Int Ed Engl. 2021;60(35):19355-19363
99. Lu K, He C, Guo N. et al. Chlorin-Based Nanoscale Metal-Organic Framework Systemically Rejects Colorectal Cancers via Synergistic Photodynamic Therapy and Checkpoint Blockade Immunotherapy[J]. J Am Chem Soc. 2016;138(38):12502-12510
100. Zhang J, Li W, Qi Y. et al. PD-L1 Aptamer-Functionalized Metal-Organic Framework Nanoparticles for Robust Photo-Immunotherapy against Cancer with Enhanced Safety[J]. Angew Chem Int Ed Engl. 2023;62(5):e202214750
101. Hao Y, Chung C K, Gu Z. et al. Combinatorial therapeutic approaches of photodynamic therapy and immune checkpoint blockade for colon cancer treatment[J]. Mol Biomed. 2022;3(1):26
102. Wei M F, Chen M W, Chen K C. et al. Autophagy promotes resistance to photodynamic therapy-induced apoptosis selectively in colorectal cancer stem-like cells[J]. Autophagy. 2014;10(7):1179-1192
103. Shui S, Zhao Z, Wang H. et al. Non-enzymatic lipid peroxidation initiated by photodynamic therapy drives a distinct ferroptosis-like cell death pathway[J]. Redox Biol. 2021;45:102056
104. Liu K, Chen W, Lei S. et al. Wild-type and mutant p53 differentially modulate miR-124/iASPP feedback following pohotodynamic therapy in human colon cancer cell line[J]. Cell Death Dis. 2017;8(10):e3096
105. Choi B H, Ryoo I G, Kang H C. et al. The sensitivity of cancer cells to pheophorbide a-based photodynamic therapy is enhanced by Nrf2 silencing[J]. PLoS One. 2014;9(9):e107158
106. Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues[J]. Cell. 2011;147(4):728-741
107. Levine B, Kroemer G. Biological Functions of Autophagy Genes: A Disease Perspective[J]. Cell. 2019;176(1-2):11-42
108. Glick D, Barth S, Macleod K F. Autophagy: cellular and molecular mechanisms[J]. J Pathol. 2010;221(1):3-12
109. Li X, He S, Ma B. Autophagy and autophagy-related proteins in cancer[J]. Mol Cancer. 2020;19(1):12
110. Ferro F, Servais S, Besson P. et al. Autophagy and mitophagy in cancer metabolic remodelling[J]. Semin Cell Dev Biol. 2020;98:129-138
111. White E, Mehnert J M, Chan C S. Autophagy, Metabolism, and Cancer[J]. Clin Cancer Res. 2015;21(22):5037-5046
112. Xiong L, Liu Z, Ouyang G. et al. Autophagy inhibition enhances photocytotoxicity of Photosan-II in human colorectal cancer cells[J]. Oncotarget. 2017;8(4):6419-6432
113. Sun Z, Zhao M, Wang W. et al. 5-ALA mediated photodynamic therapy with combined treatment improves anti-tumor efficacy of immunotherapy through boosting immunogenic cell death[J]. Cancer Lett. 2023;554:216032
114. Xue Q, Wang P, Wang X. et al. Targeted inhibition of p38MAPK-enhanced autophagy in SW620 cells resistant to photodynamic therapy-induced apoptosis[J]. Lasers Med Sci. 2015;30(7):1967-1975
115. Ziółkowska B, Woźniak M, Ziółkowski P. Co-expression of autophagic markers following photodynamic therapy in SW620 human colon adenocarcinoma cells[J]. Mol Med Rep. 2016;14(3):2548-2554
116. Yang K, Niu T, Luo M. et al. Enhanced cytotoxicity and apoptosis through inhibiting autophagy in metastatic potential colon cancer SW620 cells treated with Chlorin e6 photodynamic therapy[J]. Photodiagnosis Photodyn Ther. 2018;24:332-341
117. Luo M, Li H, Han D. et al. Inhibition of autophagy enhances apoptosis induced by Ce6-photodynamic therapy in human colon cancer cells[J]. Photodiagnosis Photodyn Ther. 2021;36:102605
118. Luo M, Ji J, Yang K. et al. The role of autophagy in the treatment of colon cancer by chlorin e6 photodynamic therapy combined with oxaliplatin[J]. Photodiagnosis Photodyn Ther. 2022;40:103082
119. Lin S, Yang L, Shi H. et al. Endoplasmic reticulum-targeting photosensitizer Hypericin confers chemo-sensitization towards oxaliplatin through inducing pro-death autophagy[J]. Int J Biochem Cell Biol. 2017;87:54-68
120. Rodríguez M E, Catrinacio C, Ropolo A. et al. A novel HIF-1α/VMP1-autophagic pathway induces resistance to photodynamic therapy in colon cancer cells[J]. Photochem Photobiol Sci. 2017;16(11):1631-1642
121. Kim J H, Kim I W. p62 manipulation affects chlorin e6-mediated photodynamic therapy efficacy in colorectal cancer cell lines[J]. Oncol Lett. 2020;19(6):3907-3916
122. Song C, Xu W, Wu H. et al. Photodynamic therapy induces autophagy-mediated cell death in human colorectal cancer cells via activation of the ROS/JNK signaling pathway[J]. Cell Death Dis. 2020;11(10):938
123. Li L, Wang K, Jia R. et al. Ferroportin-dependent ferroptosis induced by ellagic acid retards liver fibrosis by impairing the SNARE complexes formation[J]. Redox Biol. 2022;56:102435
124. Shen Z, Liu T, Li Y. et al. Fenton-Reaction-Acceleratable Magnetic Nanoparticles for Ferroptosis Therapy of Orthotopic Brain Tumors[J]. ACS Nano. 2018;12(11):11355-11365
125. Shen Z, Song J, Yung B C. et al. Emerging Strategies of Cancer Therapy Based on Ferroptosis[J]. Adv Mater. 2018;30(12):e1704007
126. Liu T, Liu W, Zhang M. et al. Ferrous-Supply-Regeneration Nanoengineering for Cancer-Cell-Specific Ferroptosis in Combination with Imaging-Guided Photodynamic Therapy[J]. ACS Nano. 2018;12(12):12181-12192
127. Zhu T, Shi L, Yu C. et al. Ferroptosis Promotes Photodynamic Therapy: Supramolecular Photosensitizer-Inducer Nanodrug for Enhanced Cancer Treatment[J]. Theranostics. 2019;9(11):3293-3307
128. Mishchenko T A, Balalaeva I V, Vedunova M V. et al. Ferroptosis and Photodynamic Therapy Synergism: Enhancing Anticancer Treatment[J]. Trends Cancer. 2021;7(6):484-487
129. Pan W L, Tan Y, Meng W. et al. Microenvironment-driven sequential ferroptosis, photodynamic therapy, and chemotherapy for targeted breast cancer therapy by a cancer-cell-membrane-coated nanoscale metal-organic framework[J]. Biomaterials. 2022;283:121449
130. Efimova I, Catanzaro E, Van der Meeren L. et al. Vaccination with early ferroptotic cancer cells induces efficient antitumor immunity[J]. J Immunother Cancer. 2020 8(2)
131. Lee J I, Ahn T G, Choi J H. Effects of Iron on Efficacy of Photodynamic Therapy Using Photolon in a Mouse Model of CT26 Colon Cancer[J]. J Nippon Med Sch. 2023;90(1):41-49
132. Lu T X, Rothenberg M E. MicroRNA[J]. J Allergy Clin Immunol. 2018;141(4):1202-1207
133. El-Daly S M, Abba M L, Gamal-Eldeen A M. The role of microRNAs in photodynamic therapy of cancer[J]. Eur J Med Chem. 2017;142:550-555
134. Zhang P, Ouyang Y, Sohn Y S. et al. miRNA-Guided Imaging and Photodynamic Therapy Treatment of Cancer Cells Using Zn(II)-Protoporphyrin IX-Loaded Metal-Organic Framework Nanoparticles[J]. ACS Nano. 2022;16(2):1791-1801
135. Shang M, Wu Y, Wang Y. et al. Dual antisense oligonucleotide targeting miR-21/miR-155 synergize photodynamic therapy to treat triple-negative breast cancer and inhibit metastasis[J]. Biomed Pharmacother. 2022;146:112564
136. Liu K, Yao H, Wen Y. et al. Functional role of a long non-coding RNA LIFR-AS1/miR-29a/TNFAIP3 axis in colorectal cancer resistance to pohotodynamic therapy[J]. Biochim Biophys Acta Mol Basis Dis. 2018;1864(9 Pt B):2871-2880
137. Liu K, Lei S, Kuang Y. et al. A Novel Mechanism of the c-Myc/NEAT1 Axis Mediating Colorectal Cancer Cell Response to Photodynamic Therapy Treatment[J]. Front Oncol. 2021;11:652831
138. Dos S A, Inague A, Arini G S. et al. Distinct photo-oxidation-induced cell death pathways lead to selective killing of human breast cancer cells[J]. Cell Death Dis. 2020;11(12):1070
139. Turubanova V D, Balalaeva I V, Mishchenko T A. et al. Immunogenic cell death induced by a new photodynamic therapy based on photosens and photodithazine[J]. J Immunother Cancer. 2019;7(1):350
140. Doix B, Trempolec N, Riant O. et al. Low Photosensitizer Dose and Early Radiotherapy Enhance Antitumor Immune Response of Photodynamic Therapy-Based Dendritic Cell Vaccination[J]. Front Oncol. 2019;9:811
141. Lamberti M J, Pansa M F, Vera R E. et al. Transcriptional activation of HIF-1 by a ROS-ERK axis underlies the resistance to photodynamic therapy[J]. PLoS One. 2017;12(5):e177801
142. Wufuer R, Ma H X, Luo M Y. et al. Downregulation of Rac1/PAK1/LIMK1/cofilin signaling pathway in colon cancer SW620 cells treated with Chlorin e6 photodynamic therapy[J]. Photodiagnosis Photodyn Ther. 2021;33:102143
143. Sardoiwala M N, Kushwaha A C, Dev A. et al. Hypericin-Loaded Transferrin Nanoparticles Induce PP2A-Regulated BMI1 Degradation in Colorectal Cancer-Specific Chemo-Photodynamic Therapy[J]. ACS Biomater Sci Eng. 2020;6(5):3139-3153
144. Hu J, Song J, Tang Z. et al. Hypericin-mediated photodynamic therapy inhibits growth of colorectal cancer cells via inducing S phase cell cycle arrest and apoptosis[J]. Eur J Pharmacol. 2021;900:174071
145. Hui Y J, Chen H, Peng X C. et al. Up-regulation of ABCG2 by MYBL2 deletion drives Chlorin e6-mediated photodynamic therapy resistance in colorectal cancer[J]. Photodiagnosis Photodyn Ther. 2023;42:103558
146. Kashtan H, Papa M Z, Wilson B C. et al. Use of photodynamic therapy in the palliation of massive advanced rectal cancer. Phase I/II study[J]. Dis Colon Rectum. 1991;34(7):600-604 604-605
147. Allardice J T, Abulafi A M, Grahn M F. et al. Adjuvant intraoperative photodynamic therapy for colorectal carcinoma: a clinical study[J]. Surg Oncol. 1994;3(1):1-10
148. Foultier M T, Vonarx-Coinsman V, de Brito L X. et al. DNA or cell kinetics flow cytometry analysis of 33 small gastrointestinal cancers treated by photodynamic therapy[J]. Cancer. 1994;73(6):1595-1607
149. Regula J, MacRobert A J, Gorchein A. et al. Photosensitisation and photodynamic therapy of oesophageal, duodenal, and colorectal tumours using 5 aminolaevulinic acid induced protoporphyrin IX-a pilot study[J]. Gut. 1995;36(1):67-75
150. Mlkvy P, Messmann H, Debinski H. et al. Photodynamic therapy for polyps in familial adenomatous polyposis-a pilot study[J]. Eur J Cancer. 1995;31A(7-8):1160-1165
151. Mĺkvy P, Messmann H, Regula J. et al. Photodynamic therapy for gastrointestinal tumors using three photosensitizers-ALA induced PPIX, Photofrin and MTHPC. A pilot study[J]. Neoplasma. 1998;45(3):157-161
152. van Duijnhoven F H, Rovers J P, Engelmann K. et al. Photodynamic therapy with 5,10,15,20-tetrakis(m-hydroxyphenyl) bacteriochlorin for colorectal liver metastases is safe and feasible: results from a phase I study[J]. Ann Surg Oncol. 2005;12(10):808-816
153. Vickerman B M, Zywot E M, Tarrant T K. et al. Taking phototherapeutics from concept to clinical launch[J]. Nat Rev Chem. 2021;5(11):816-834
154. Railkar R, Agarwal P K. Photodynamic Therapy in the Treatment of Bladder Cancer: Past Challenges and Current Innovations[J]. Eur Urol Focus. 2018;4(4):509-511
155. Lan M, Zhao S, Liu W. et al. Photosensitizers for Photodynamic Therapy[J]. Adv Healthc Mater. 2019;8(13):e1900132
156. Bordea I R, Hanna R, Chiniforush N. et al. Evaluation of the outcome of various laser therapy applications in root canal disinfection: A systematic review[J]. Photodiagnosis Photodyn Ther. 2020;29:101611
157. Almadi K H. Impact of antimicrobial photodynamic therapy on the bond-strength and penetration of endodontic sealers: A systematic review[J]. Photodiagnosis Photodyn Ther. 2023;41:103249
158. Chilakamarthi U, Giribabu L. Photodynamic Therapy: Past, Present and Future[J]. Chem Rec. 2017;17(8):775-802
159. Chen J, Fan T, Xie Z. et al. Advances in nanomaterials for photodynamic therapy applications: Status and challenges[J]. Biomaterials. 2020;237:119827
160. Bucharskaya A B, Khlebtsov N G, Khlebtsov B N. et al. Photothermal and Photodynamic Therapy of Tumors with Plasmonic Nanoparticles: Challenges and Prospects[J]. Materials (Basel). 2022 15(4)
161. Sang R, Deng F, Engel A. et al. Lipid-polymer nanocarrier platform enables X-ray induced photodynamic therapy against human colorectal cancer cells[J]. Biomed Pharmacother. 2022;155:113837
162. Emami F, Banstola A, Vatanara A. et al. Doxorubicin and Anti-PD-L1 Antibody Conjugated Gold Nanoparticles for Colorectal Cancer Photochemotherapy[J]. Mol Pharm. 2019;16(3):1184-1199
163. Yuan Z, Fan G, Wu H. et al. Photodynamic therapy synergizes with PD-L1 checkpoint blockade for immunotherapy of CRC by multifunctional nanoparticles[J]. Mol Ther. 2021;29(10):2931-2948
164. She T, Shi Q, Li Z. et al. Combination of long-acting TRAIL and tumor cell-targeted photodynamic therapy as a novel strategy to overcome chemotherapeutic multidrug resistance and TRAIL resistance of colorectal cancer[J]. Theranostics. 2021;11(9):4281-4297
165. Peng C L, Lin H C, Chiang W L. et al. Anti-angiogenic treatment (Bevacizumab) improves the responsiveness of photodynamic therapy in colorectal cancer[J]. Photodiagnosis Photodyn Ther. 2018;23:111-118
166. Hashemkhani M, Demirci G, Bayir A. et al. Cetuximab-Ag(2)S quantum dots for fluorescence imaging and highly effective combination of ALA-based photodynamic/chemo-therapy of colorectal cancer cells[J]. Nanoscale. 2021;13(35):14879-14899
167. Kleban J, Mikes J, Szilárdiová B. et al. Modulation of hypericin photodynamic therapy by pretreatment with 12 various inhibitors of arachidonic acid metabolism in colon adenocarcinoma HT-29 cells[J]. Photochem Photobiol. 2007;83(5):1174-1185
168. Szokalska A, Makowski M, Nowis D. et al. Proteasome inhibition potentiates antitumor effects of photodynamic therapy in mice through induction of endoplasmic reticulum stress and unfolded protein response[J]. Cancer Res. 2009;69(10):4235-4243
169. Wang Y, Chen L, Lai S. et al. Connexin 43 contributes to the sensitization of colorectal cancer cells to photodynamic therapy through Akt inhibition[J]. Photodiagnosis Photodyn Ther. 2022;39:103040
170. Zhu B, Li S, Yu L. et al. Inhibition of Autophagy with Chloroquine Enhanced Sinoporphyrin Sodium Mediated Photodynamic Therapy-induced Apoptosis in Human Colorectal Cancer Cells[J]. Int J Biol Sci. 2019;15(1):12-23
171. Simelane N, Matlou G G, Abrahamse H. Photodynamic Therapy of Aluminum Phthalocyanine Tetra Sodium 2-Mercaptoacetate Linked to PEGylated Copper-Gold Bimetallic Nanoparticles on Colon Cancer Cells[J]. Int J Mol Sci. 2023 24(3)
172. He Y C, Hao Z N, Li Z. et al. Nanomedicine-based multimodal therapies: Recent progress and perspectives in colon cancer[J]. World J Gastroenterol. 2023;29(4):670-681
173. Nkune N W, Kruger C A, Abrahamse H. Possible Enhancement of Photodynamic Therapy (PDT) Colorectal Cancer Treatment when Combined with Cannabidiol[J]. Anticancer Agents Med Chem. 2021;21(2):137-148
174. He H, Liu L, Liang R. et al. Tumor-targeted nanoplatform for in situ oxygenation-boosted immunogenic phototherapy of colorectal cancer[J]. Acta Biomater. 2020;104:188-197
175. Kumar B, Murali A, Bharath A B. et al. Guar gum modified upconversion nanocomposites for colorectal cancer treatment through enzyme-responsive drug release and NIR-triggered photodynamic therapy[J]. Nanotechnology. 2019;30(31):315102
176. Horgan C C, Bergholt M S, Nagelkerke A. et al. Integrated photodynamic Raman theranostic system for cancer diagnosis, treatment, and post-treatment molecular monitoring[J]. Theranostics. 2021;11(4):2006-2019
177. Chudal L, Pandey N K, Phan J. et al. Investigation of PPIX-Lipo-MnO(2) to enhance photodynamic therapy by improving tumor hypoxia[J]. Mater Sci Eng C Mater Biol Appl. 2019;104:109979
178. Chudal L, Pandey N K, Phan J. et al. Copper-Cysteamine Nanoparticles as a Heterogeneous Fenton-Like Catalyst for Highly Selective Cancer Treatment[J]. ACS Appl Bio Mater. 2020;3(3):1804-1814
179. Mariño-Ocampo N, Dibona-Villanueva L, Escobar-Álvarez E. et al. Recent Photosensitizer Developments, Delivery Strategies and Combination-based Approaches for Photodynamic Therapy(†)[J]. Photochem Photobiol. 2023;99(2):469-497
180. Cheng X, Gao J, Ding Y. et al. Multi-Functional Liposome: A Powerful Theranostic Nano-Platform Enhancing Photodynamic Therapy[J]. Adv Sci (Weinh). 2021;8(16):e2100876
181. Zhu D, Duo Y, Suo M. et al. Tumor-Exocytosed Exosome/Aggregation-Induced Emission Luminogen Hybrid Nanovesicles Facilitate Efficient Tumor Penetration and Photodynamic Therapy[J]. Angew Chem Int Ed Engl. 2020;59(33):13836-13843
182. Tong L, Zhang S, Huang R. et al. Extracellular vesicles as a novel photosensitive drug delivery system for enhanced photodynamic therapy[J]. Front Bioeng Biotechnol. 2022;10:1032318
183. Moghassemi S, Dadashzadeh A, Azevedo R B. et al. Photodynamic cancer therapy using liposomes as an advanced vesicular photosensitizer delivery system[J]. J Control Release. 2021;339:75-90
184. Liu X L, Dong X, Yang S C. et al. Biomimetic Liposomal Nanoplatinum for Targeted Cancer Chemophototherapy[J]. Adv Sci (Weinh). 2021;8(8):2003679
185. Li X, Chen L, Huang M. et al. Innovative strategies for photodynamic therapy against hypoxic tumor[J]. Asian J Pharm Sci. 2023;18(1):100775
186. Zha M, Yang G, Li Y. et al. Recent Advances in AIEgen-Based Photodynamic Therapy and Immunotherapy[J]. Adv Healthc Mater. 2021;10(24):e2101066
187. Du J, Wan Z, Wang C. et al. Designer exosomes for targeted and efficient ferroptosis induction in cancer via chemo-photodynamic therapy[J]. Theranostics. 2021;11(17):8185-8196
188. Huis I T V R, Lara P, Jager M J. et al. M1-derived extracellular vesicles enhance photodynamic therapy and promote immunological memory in preclinical models of colon cancer[J]. J Nanobiotechnology. 2022;20(1):252
189. Zou X, Yao M, Ma L. et al. X-ray-induced nanoparticle-based photodynamic therapy of cancer[J]. Nanomedicine (Lond). 2014;9(15):2339-2351
190. Ma L, Zou X, Chen W. A new X-ray activated nanoparticle photosensitizer for cancer treatment[J]. J Biomed Nanotechnol. 2014;10(8):1501-1508
191. Zhang Q, Guo X, Cheng Y. et al. Use of copper-cysteamine nanoparticles to simultaneously enable radiotherapy, oxidative therapy and immunotherapy for melanoma treatment[J]. Signal Transduct Target Ther. 2020;5(1):58
192. Liu Z, Xiong L, Ouyang G. et al. Investigation of Copper Cysteamine Nanoparticles as a New Type of Radiosensitiers for Colorectal Carcinoma Treatment[J]. Sci Rep. 2017;7(1):9290
193. Deng W, McKelvey K J, Guller A. et al. Application of Mitochondrially Targeted Nanoconstructs to Neoadjuvant X-ray-Induced Photodynamic Therapy for Rectal Cancer[J]. ACS Cent Sci. 2020;6(5):715-726
194. Yao M, Ma L, Li L. et al. A New Modality for Cancer Treatment-Nanoparticle Mediated Microwave Induced Photodynamic Therapy[J]. J Biomed Nanotechnol. 2016;12(10):1835-1851
195. Wu T, Liu Y, Cao Y. et al. Engineering Macrophage Exosome Disguised Biodegradable Nanoplatform for Enhanced Sonodynamic Therapy of Glioblastoma[J]. Adv Mater. 2022;34(15):e2110364
196. Bunevicius A, Pikis S, Padilla F. et al. Sonodynamic therapy for gliomas[J]. J Neurooncol. 2022;156(1):1-10
197. Zhang Y, Zhang X, Yang H. et al. Advanced biotechnology-assisted precise sonodynamic therapy[J]. Chem Soc Rev. 2021;50(20):11227-11248
198. Xu M, Zhou L, Zheng L. et al. Sonodynamic therapy-derived multimodal synergistic cancer therapy[J]. Cancer Lett. 2021;497:229-242
199. Chan C M, Lo P C, Yeung S L. et al. Photodynamic activity of a glucoconjugated silicon (IV) phthalocyanine on human colon adenocarcinoma[J]. Cancer Biol Ther. 2010;10(2):126-134
Author contact
![]() Corresponding author: Weiguo Dong AND Weixing Wang. dongweiguoedu.cn and wangwxedu.cn.
Corresponding author: Weiguo Dong AND Weixing Wang. dongweiguoedu.cn and wangwxedu.cn.

 Global reach, higher impact
Global reach, higher impact