10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2023; 19(15):4849-4864. doi:10.7150/ijbs.86384 This issue Cite
Research Paper
Inhibition of Heat Shock-Induced H3K9ac Reduction Sensitizes Cancer Cells to Hyperthermia
1. Key Laboratory of Systems Biomedicine, Shanghai Center for Systems Biomedicine, Shanghai Jiao Tong University, 800 Dong chuan Road, Shanghai 200240, China.
2. Pathology Center, Shanghai General Hospital, Shanghai Jiao Tong University School of Medicine, 100 Hai-Ning Road, Shanghai 200080, China.
Abstract
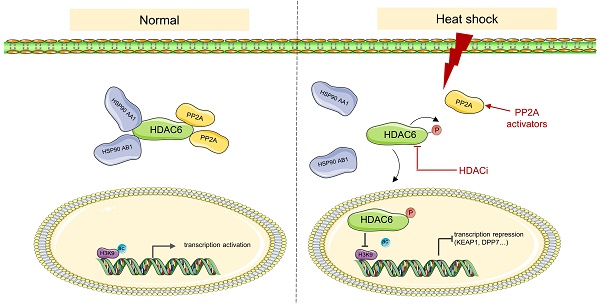
Heat stress, clinically known as hyperthermia, is a promising adjunctive modality in cancer treatment. However, the efficacy of hyperthermia as a monotherapy is limited and the underlying mechanism remains poorly understood. Targeting histone modifications is an emerging strategy for cancer therapy, but little is known regarding the role of heat stress in altering these modifications. Here, we report that heat shock inhibits H3K9 acetylation (H3K9ac) via histone deacetylase 6 (HDAC6) regulation. Heat shock inhibits the interaction between HDAC6 and heat shock protein 90 (HSP90), enhances nuclear localization of HDAC6, and promotes HDAC6 phosphorylation, which is regulated by protein phosphatase 2A (PP2A). Combining hyperthermia with HDAC inhibitors vorinostat or panobinostat leads to better anti-cancer effects compared to monotherapy. KEAP1 and DPP7 as genes affected by heat-induced inhibition of H3K9ac, and combining them with hyperthermia can better induce apoptosis in tumor cells. This study reveals previously unknown mechanisms of H3K9ac decreased by heat shock in cancer cells and highlights a potential combinational therapy involving hyperthermia and targeting of these new mechanisms.
Keywords: heat shock, H3K9 acetylation, hyperthermia, cancer therapy

 Global reach, higher impact
Global reach, higher impact