10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2024; 20(5):1927-1946. doi:10.7150/ijbs.91543 This issue Cite
Research Paper
mtDNA-cGAS-STING axis-dependent NLRP3 inflammasome activation contributes to postoperative cognitive dysfunction induced by sevoflurane in mice
1. Department of Physiology, School of Basic Medical Science, Central South University, Changsha, Hunan 410078, China.
2. Key Laboratory of General University of Hunan Province, Basic and Clinic Research in Major Respiratory Disease, Changsha, Hunan 410078, China.
3. National Experimental Teaching Demonstration Center for Medical Function, Changsha, Hunan 410013, China.
4. Department of Anesthesiology, Liuzhou People's Hospital, Liuzhou 545000, China.
Abstract
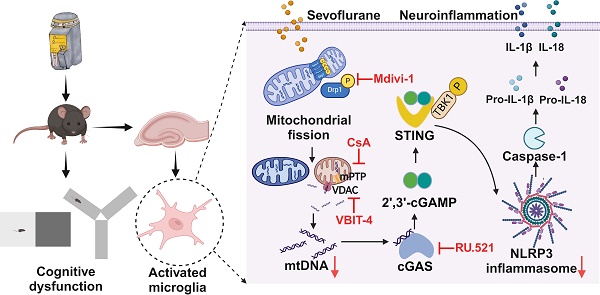
The activation of NLRP3 inflammasome in microglia is critical for neuroinflammation during postoperative cognitive dysfunction (POCD) induced by sevoflurane. However, the molecular mechanism by which sevoflurane activates the NLRP3 inflammasome in microglia remains unclear. The cGAS-STING pathway is an evolutionarily conserved inflammatory defense mechanism. The role of the cGAS-STING pathway in sevoflurane-induced NLRP3 inflammasome-dependent neuroinflammation and the underlying mechanisms require further investigation. We found that prolonged anesthesia with sevoflurane induced cognitive dysfunction and triggered the neuroinflammation characterized by the activation of NLRP3 inflammasome in vivo. Interestingly, the cGAS-STING pathway was activated in the hippocampus of mice receiving sevoflurane. While the blockade of cGAS with RU.521 attenuated cognitive dysfunction and NLRP3 inflammasome activation in mice. In vitro, we found that sevoflurane treatment significantly activated the cGAS-STING pathway in microglia, while RU.521 pre-treatment robustly inhibited sevoflurane-induced NLRP3 inflammasome activation. Mechanistically, sevoflurane-induced mitochondrial fission in microglia and released mitochondrial DNA (mtDNA) into the cytoplasm, which could be abolished with Mdivi-1. Blocking the mtDNA release via the mPTP-VDAC channel inhibitor attenuated sevoflurane-induced mtDNA cytosolic escape and reduced cGAS-STING pathway activation in microglia, finally inhibiting the NLRP3 inflammasome activation. Therefore, regulating neuroinflammation by targeting the cGAS-STING pathway may provide a novel therapeutic target for POCD.
Keywords: postoperative cognitive dysfunction, neuroinflammation, cGAS-STING, mitochondrial fission, NLRP3 inflammasome.

 Global reach, higher impact
Global reach, higher impact