ISSN: 1449-2288
Int J Biol Sci 2024; 20(8):3156-3172. doi:10.7150/ijbs.95962 This issue Cite
Research Paper
CPEB1 Controls NRF2 Proteostasis and Ferroptosis Susceptibility in Pancreatic Cancer
1. Key University Laboratory of Metabolism and Health of Guangdong, Biochemistry Department, School of Medicine, Southern University of Science and Technology, 1088 Xueyuan Avenue, Shenzhen, Guangdong 518055, P.R. China.
2. Department of Gastroenterology, The First Affiliated Hospital (Shenzhen People's Hospital), Southern University of Science and Technology, 1017 Dongmen North Road, Shenzhen, Guangdong 518020, P.R. China.
3. Department of Geriatrics, and Shenzhen Clinical Research Centre for Geriatrics, The First Affiliated Hospital (Shenzhen People's Hospital), Southern University of Science and Technology, 1017 Dongmen North Road, Shenzhen, Guangdong 518020, P.R. China.
4. School of Medicine, Southern University of Science and Technology, 1088 Xueyuan Avenue, Shenzhen, Guangdong 518055, P.R. China.
5. Clinical laboratory, Southern University of Science and Technology Hospital, 6019 Liuxian Street, Xili Avenue, Shenzhen, Guangdong 518055, P.R. China.
6. Beijing Tsinghua Changgung Hospital & Tsinghua University School of Medicine, 168 Litang Road, Changping District, Beijing 102218, P.R. China.
Abstract
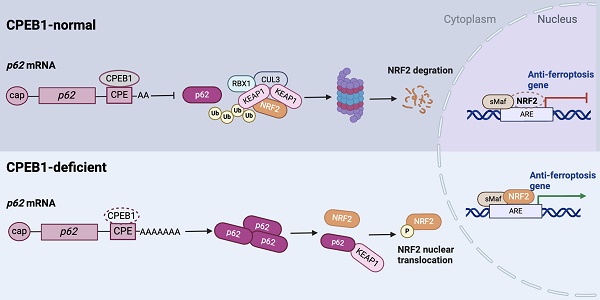
Pancreatic cancer is the deadliest malignancy with a poor response to chemotherapy but is potentially indicated for ferroptosis therapy. Here we identified that cytoplasmic polyadenylation element binding protein 1 (CPEB1) regulates NRF2 proteostasis and susceptibility to ferroptosis in pancreatic ductal adenocarcinoma (PDAC). We found that CPEB1 deficiency in cancer cells promotes the translation of p62/SQSTM1 by facilitating mRNA polyadenylation. Consequently, upregulated p62 enhances NRF2 stability by sequestering KEAP1, an E3 ligase for proteasomal degradation of NRF2, leading to the transcriptional activation of anti-ferroptosis genes. In support of the critical role of this signaling cascade in cancer therapy, CPEB1-deficient pancreatic cancer cells display higher resistance to ferroptosis-inducing agents than their CPEB1-normal counterparts in vitro and in vivo. Furthermore, based on the pathological evaluation of tissue specimens from 90 PDAC patients, we established that CPEB1 is an independent prognosticator whose expression level is closely associated with clinical therapeutic outcomes in PDAC. These findings identify the role of CPEB1 as a key ferroptosis regulator and a potential prognosticator in pancreatic cancer.
Keywords: CPEB1, p62/SQSTM1, NRF2, pancreatic cancer, ferroptosis

 Global reach, higher impact
Global reach, higher impact