ISSN: 1449-2288
Int J Biol Sci 2024; 20(9):3530-3543. doi:10.7150/ijbs.94675 This issue Cite
Research Paper
Epsti1 Regulates the Inflammatory Stage of Early Muscle Regeneration through STAT1-VCP Interaction
1. Drug Information Research Institute, Sookmyung Women's University, Seoul 04310, South Korea.
2. Muscle Physiome Research Center, Sookmyung Women's University, Seoul 04310, South Korea.
3. College of Pharmacy, Sookmyung Women's University, Seoul 04310, South Korea.
4. Department of Molecular Cell Biology, Sungkyunkwan University School of Medicine, Suwon 16419, South Korea.
5. Cell and Gene Therapy Products Division, National Institute of Food and Drug Safety Evaluation, Cheongju 28159, South Korea.
6. Research Institute of Aging Related Disease, AniMusCure Inc., Suwon 16419, South Korea.
7. Research Center for Epigenome Regulation, School of Pharmacy, Department of Biochemistry and Molecular Biology, Sungkyunkwan University, Suwon 16419, South Korea.
8. Department of Biomedical Laboratory Science, Far East University, 76-32 Daehakgil, Gamgok-myeon, Eumseong-gun, Chungbuk-do, 27601, Korea.
#These authors contributed equally.
Abstract
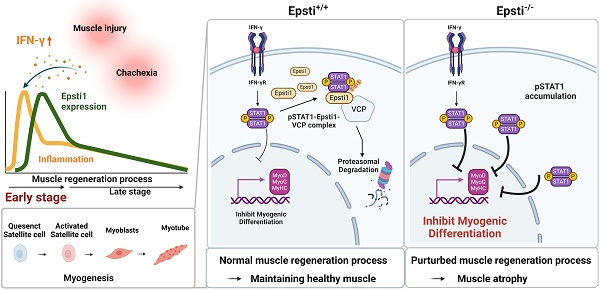
During muscle regeneration, interferon-gamma (IFN-γ) coordinates inflammatory responses critical for activation of quiescent muscle stem cells upon injury via the Janus kinase (JAK) - signal transducer and activator of transcription 1 (STAT1) pathway. Dysregulation of JAK-STAT1 signaling results in impaired muscle regeneration, leading to muscle dysfunction or muscle atrophy. Until now, the underlying molecular mechanism of how JAK-STAT1 signaling resolves during muscle regeneration remains largely elusive. Here, we demonstrate that epithelial-stromal interaction 1 (Epsti1), an interferon response gene, has a crucial role in regulating the IFN-γ-JAK-STAT1 signaling at early stage of muscle regeneration. Epsti1-deficient mice exhibit impaired muscle regeneration with elevated inflammation response. In addition, Epsti1-deficient myoblasts display aberrant interferon responses. Epsti1 interacts with valosin-containing protein (VCP) and mediates the proteasomal degradation of IFN-γ-activated STAT1, likely contributing to dampening STAT1-mediated inflammation. In line with the notion, mice lacking Epsti1 exhibit exacerbated muscle atrophy accompanied by increased inflammatory response in cancer cachexia model. Our study suggests a crucial function of Epsti1 in the resolution of IFN-γ-JAK-STAT1 signaling through interaction with VCP which provides insights into the unexplored mechanism of crosstalk between inflammatory response and muscle regeneration.
Keywords: Muscle regeneration, Inflammatory response, IFN-γ-JAK-STAT1 pathway, Ubiquitin-proteasomal degradation, VCP, Epsti1

 Global reach, higher impact
Global reach, higher impact