10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2024; 20(12):4872-4887. doi:10.7150/ijbs.98806 This issue Cite
Review
Elucidating the evolving role of cuproptosis in breast cancer progression
1. Department of Plastic Surgery, Renmin Hospital of Wuhan University, No. 238 Jiefang Road, Wuhan, 430060, Hubei Province, China.
2. Department of Plastic and Cosmetic Surgery, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, 430030, Hubei, China.
3. Department of Thyroid and Breast Surgery, Shenzhen Qianhai Shekou Free Trade Zone Hospital, Shenzhen, 518067, China.
4. Department of Wound Repair Surgery, Liyuan Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, 430062, Hubei Province, China.
5. Xianning Medical College, Hubei University of Science & Technology, Xianning, 437000, Hubei, China.
# Co-first author.
Received 2024-5-23; Accepted 2024-8-20; Published 2024-9-9
Abstract
Breast cancer (BC) persists as a highly prevalent malignancy in females, characterized by diverse molecular signatures and necessitating personalized therapeutic approaches. The equilibrium of copper within the organism is meticulously maintained through regulated absorption, distribution, and elimination, underpinning not only cellular equilibrium but also various essential biological functions. The process of cuproptosis is initiated by copper's interaction with lipoylases within the tricarboxylic acid (TCA) cycle, which triggers the conglomeration of lipoylated proteins and diminishes the integrity of Fe-S clusters, culminating in cell demise through proteotoxic stress. In BC, aberrations in cuproptosis are prominent and represent a crucial molecular incident that contributes to the disease progression. It influences BC cell metabolism and affects critical traits such as proliferation, invasiveness, and resistance to chemotherapy. Therapeutic strategies that target cuproptosis have shown promising antitumor efficacy. Moreover, a plethora of cuproptosis-centric genes, including cuproptosis-related genes (CRGs), CRG-associated non-coding RNAs (ncRNAs), and cuproptosis-associated regulators, have been identified, offering potential for the development of risk assessment models or diagnostic signatures. In this review, we provide a comprehensive exposition of the fundamental principles of cuproptosis, its influence on the malignant phenotypes of BC, the prognostic implications of cuproptosis-based markers, and the substantial prospects of exploiting cuproptosis for BC therapy, thereby laying a theoretical foundation for targeted interventions in this domain.
Keywords: breast cancer, cuproptosis, copper, cuproptosis-related genes, diagnosis, therapy
Introduction
Breast cancer (BC) remains the most prevalent and high-mortality type of female tumor worldwide, imposing a heavy social burden (1). With the intensive development of diagnostic and therapeutic technologies, individualized treatment strategies based on molecular typing have led to a significant improvement in the prognosis of BC patients (2,3). However, the prognosis of some high-risk BC types or advanced BC is still poor (4). The genetics, pathology, and molecular biology of patients are profoundly altered, and continued exploration will bring about profound transformations in the management of BC (5).
Cell death, as one of the metabolic processes, is of importance for the maintenance of biological life, along with cell proliferation and differentiation (6,7). Different programmed cell deaths (PCDs) are extensively involved in tumor evolution, including apoptosis, necroptosis, pyroptosis, ferroptosis, and cuproptosis (8). PCDs are cellular suicide mechanisms that have arisen in living organisms during a long evolutionary process and are closely related to a variety of pathophysiological phenomena, including cell fate, immune regulation, tumors and infectious diseases, among others (9). Notably, PCD involves specific intracellular gene regulatory molecular programs, and many neoplastic diseases are associated with defects in one or several of the PCD processes (10). In particular, copper homeostasis in the body is sustained by modulating copper absorption, transport, and excretion (11). Copper homeostasis is fundamental to cellular homeostasis since the accumulation of intracellular copper induces oxidative stress and disrupts many cellular functions (12).
Cuproptosis begins with the binding of copper to lipolysis in the tricarboxylic acid (TCA) cycle, contributing to consequential protein aggregation, proteotoxic stress, and eventually cell death (13). Cuproptosis is dysregulated in BC tissues, and cancer cells have a higher copper requirement compared to undivided cells. High heterogeneity in the expression variation of cuproptosis-related genes (CRGs) in BC reveals that the imbalance of CRG expression plays a pivotal role in BC development. Abnormal copper accumulation promotes the malignant transformation of cells (14). CRGs and BC progression are inextricably linked (15). For example, ATP7B and dihydrolipoamide S-acetyltransferase (DLAT) protein expression showed a high expression pattern in BC samples and had an impact on the tumor cell cycle. The novel constructed risk signatures of CRGs have predictive properties for the prognosis, tumor microenvironment (TME), and immunotherapy of BR patients (16). Some CRGs, DLAT, solute carrier family 31 member 1 (SLC31A1), ATP7A, and ATP7B, are distinctively associated with overall survival (OS) in BC patients (17). Zou et al. established a 12-gene cell death index (CDI), and patients in the triple negative breast cancer (TNBC) cohort with a high index had worse prognostic outcomes (18). The CDI score correlated with ICs and sensitivity to standard adjuvant chemotherapy and palbociclib.
Given the importance of cuproptosis and its involvement in multiple aspects of BC, we systematically review in this article the basic concepts of cuproptosis, the impact of cuproptosis on the malignant biological behaviors of BC, the prognostic value of cuproptosis based on cuproptosis, as well as the huge potential of cuproptosis for BC treatment. An in-depth understanding of cuproptosis and its underlying molecular mechanisms is beneficial for appreciating the evolution of BC and screening cuproptosis-targeted therapeutic strategies.
Copper metabolism and cuproptosis in vivo
Copper, an essential trace element in the animal body, is primarily found in muscle, bone, and liver (19). Owing to its redox properties, copper can serve as a cofactor for key enzymes involved in a variety of biological processes, including energy conversion, antioxidant, intracellular oxidative metabolism, and epigenetic modifications (20).
The main source of copper in the body is absorbed from food through the duodenum and small intestine (21). The six-transmembrane epithelial antigen of the prostate (STEAP) can reduce Cu2+ from food to Cu+, which then enters the cells through the mediation of copper transport protein 1 (CTR1) (22). Cu2+ can also be transported into the cells via divalent metal transporter 1 (DMT1), but it cannot be utilized directly (23). After being absorbed by the epithelial cells, Cu can be transported to the other side of the epithelium via the copper molecular chaperone antioxidant 1 (ATOX1), then secreted into the bloodstream through the ATPase copper transporting alpha (ATP7A) (24). Soluble carriers in the blood that bind to copper ions, such as albumin, copper-green proteins, and histidine, can bind to copper and then transport it to the liver, which is the primary organ for storing and excreting copper (25,26). Copper storage proteins, such as metallothionein and glutathione, can mediate Cu storage by chelating with Cu+, and excess Cu is secreted into the bile, mainly through the ATPase copper transporting beta (ATP7B) on hepatocytes, and then gets excreted out of the body (27,28).
Collectively, these processes mediate copper homeostasis at the macroscopic level in vivo. At the cellular level, copper enzymes, copper molecular chaperones, and membrane transport proteins, synergistically regulate the uptake, efflux, and utilization of copper, thus maintaining copper homeostasis (29,30). Copper transporter 1 (CTR1) and STEAP are cooperatively responsible for transporting copper into the cells, while copper molecular chaperones, such as CCS, Atox 1, and Cox17, are in charge of intracellular copper transport (31,32). CCS delivers copper to superoxide dismutase 1 (SOD1), which is involved in reactive oxygen species (ROS) scavenging and maintenance of copper homeostasis (33). ATOX1 binds Cu+ and transfers it to ATP7A and ATP7B in the trans-Golgi network, enhancing the synthesis of cuproenzymes (34). When intracellular copper levels are overly high, ATP7A and ATP7B can facilitate the efflux of excess copper through translocation to the cell membrane (35). Cox17 can target Cu+ to the mitochondrial membrane gap and insert it into cytochrome oxidase C (CCO) via COX11 or SCO1, participating in cellular metabolism (36,37). Abnormalities in these processes lead to disturbed copper metabolism and disrupted copper homeostasis.
Maintaining normal copper homeostasis is necessary for normal life activities. Lack of copper ions may lead to deficiencies in the activity of multiple enzymes and the development of disease (38). However, excessive copper accumulation can also harm cells. Copper ions undergo redox reactions accompanied by electron transfer and ROS production, capable of damaging a variety of cellular biomolecules (39,40). Elevated copper levels are also linked with the severity and progression of several cancers. Copper can promote tumorigenesis and progression by enhancing cell proliferation, inducing drug resistance in tumor cells, stimulating angiogenesis, and promoting tumor cell migration (41,42). On the other hand, the application of copper alone or together with ion carriers can induce cancer cell death, such as cuproptosis (43,44).
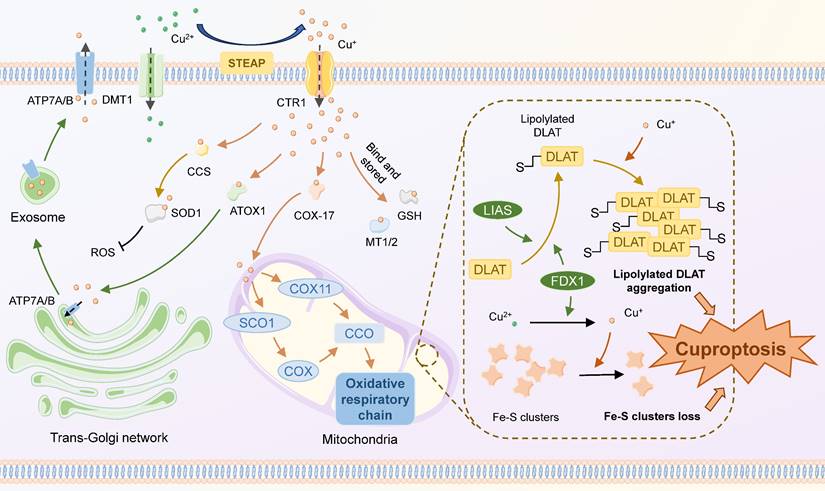
Cuproptosis is a regulatory cell death pattern distinct from other oxidative stress-related cell death patterns (45). The morphological features of cuproptosis are similar to those of apoptosis, characterized by mitochondrial crumpling, cell membrane rupture, endoplasmic reticulum damage, and chromosome breaks (46). In this process, Cu+ directly binds to lipoylated mitochondrial protein fractions of the tricarboxylic acid (TCA) cycle and acts on Fe-S clusters, inducing the aggregation of lipoylated proteins and the reduction of Fe-S clusters, ultimately resulting in cellular death mediated by proteotoxic stress (47) (Figure 1). Some critical components play important roles in this process. For example, as a key gene in cuproptosis, FDX 1 is involved in proteolipid acylation and reduction of Cu2+, but its exact mechanism still requires further interpretation. Several other CRGs, including those encoding LIPT 1, LIAS, DLD, DLAT, PDHA 1, and PDHB, are also significantly involved in this procedure.
Mechanisms of cuproptosis. Extracellular copper exists primarily in the form of Cu2+, which can directly enter the cell via DMT1 or travel through CTR1 after being reduced to Cu+ by STEAP. Cu+ enters the cell and binds to different copper ion carriers, thus avoiding its cytotoxicity, and there are four main destinations: 1) Binds to GSH or MT1/2 to be stored, forming an unstable copper pool; 2) Binds to CCS, then translocates to and inserts into SOD1, which participates in the detoxification of ROS; 3) Binds to ATOX1 and is targeted for translocation to ATP7A/B in the trans-Golgi network, contributing to copper enzyme synthesis or copper efflux; 4) Binds to COX17 and targets for transporting to the mitochondrial membrane interstitials, inserting into CCO and engaging in the cytosolic oxidative respiratory chain. The classical mechanism of cuproptosis primarily involves the aggregation of lipoylated TCA cycle proteins and the loss of Fe-S clusters in the mitochondria. FDX1 and LIAS regulate the lipoylation of mitochondrial proteins. High intracellular concentrations of Cu+ can bind to lipoylated proteins and foster their aggregation. Cu+ can also destabilize the Fe-S Cluster, leading to a reduction in the Fe-S Cluster. These can lead to Cu-induced proteotoxic stress and ultimately cell death.
The roles and mechanisms of cuproptosis in remodelling BC progression
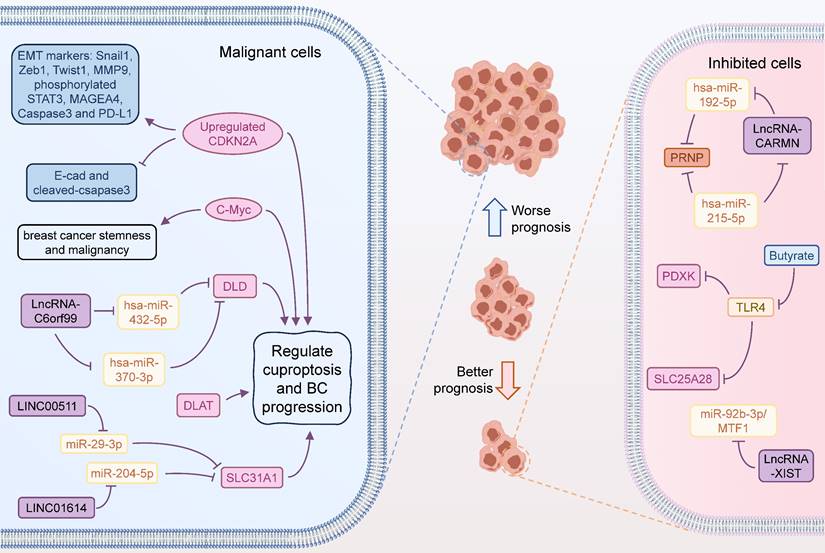
As an oncogene, cyclin-dependent kinase inhibitor 2A (CDKN2A) can encode p16 and p14, often expressed as homozygous deletions in a variety of cancers, such as oral squamous cell carcinoma and melanoma (48,49). Deletions, point mutations, promoter hypermethylation, and transcriptional abnormalities of the CDKN2A gene, are responsible for the inactivation of the CDKN2A gene, resulting in cell cycle dysregulation. CDKN2A is identified as a key CRG whose high expression was predictive of poor outcomes through model screening constructed by artificial intelligence (50). Knockdown of CDKN2A using siRNA technology resulted in a significant decrease in Zeb1, vimentin, and MMP9, indicators related to BC cell migration and metastasis, as well as down-regulation of the expression of MEGEA4, phosphorylated STAT3, PD-L1, and caspase-3. Hence, CDKN2A/MAGEA4 was a pathway that was critically associated with BC chemosensitivity, invasion, and metastasis. In another similar bioinformatic study, CDKN2A was also recognized as a pivotal modulator influencing TNBC course by driving ferroptosis and cuproptosis (51). Histochemical results of multiple samples showed that c-Myc was strongly associated with BC stemness and significantly correlated with cuproptosis-induced cell death (52). Cuproptosis is an underlying downstream of c-Myc-mediated malignant changes in tumors. DLAT fulfils a similar role in BC. Sha et al. found that high level of DLAT was associated with resistance to HER2-targeted therapy, and BC sensitivity to trastuzumab was enhanced after knocking down DLAT (53). Dihydrolipoyl dehydrogenase (DLD) is another critical CRG, but its role in BC prognosis remains unclear. Xu et al. investigated the potential impact of DLD on BC progression (54). Knockdown of DLD in BC cell lines MDA-MB-468 and SK-BR-3, significantly inhibited cell migration, invasion, and proliferation. High DLD expression in tumor regions was associated with PD-L1 and macrophages, while stromal regions showed an increase in CD4+ T cells and macrophages.
Alternatively, some metabolites may affect the progression of BC by interfacing with CRGs. For example, butyrate, a common short-chain fatty acid (SCFA), has been shown to regulate the development of BC, though its underlying mechanisms were previously unclear. Zhang et al. found that SCFA levels were lower in fecal samples from BC patients compared to controls (55). Butyrate significantly inhibited the viability, migration, and invasion of T47D cells in a dose-dependent manner and effectively suppressed tumor growth in animal models. Mechanistically, butyrate inhibited the expression of Toll-like receptor 4 (TLR4), and promoted the expression of CRGs PDXK and SLC25A28. Overexpression of TLR4 reversed the effects of butyrate on PDXK and SLC25A28 expression and on the malignant behavior of T47D cells. These findings suggest that CRGs are important potential prognostic markers in BC, offering new therapeutic targets and avenues for BC treatment.
CeRNA is a complex regulatory network between ncRNAs and coding RNAs in the cell via miRNAs (56,57). The core principle of ceRNA network is to form a competitive relationship between mRNAs, lncRNAs, circRNAs, through the competitive binding mechanism of miRNAs (58,59). This interaction of multiple makes the intracellular regulatory network more flexible and helpful in adapting to different biological environments (60). By using bioinformatic analysis of multiple databases, Lian et al. revealed that SLC31A1 was abundantly expressed in BC tissues and cell lines and was intimately connected to both recurrence-free survival (RFS) and distant metastasis-free survival (DMFS) (61). Functionally, SLC31A1, which was involved in copper ion transport, was found to be associated with m6A-related genes, especially YTHDF3. In-depth, the up-regulation of SLC31A1 expression mediated by the LINC00511-miR-29-3p axis was implicated in the modulation of copper ion transport and poor prognosis of BC, and was positively linked to the tumor immune cell infiltration, immune markers, and cancer-associated fibroblasts (CAFs) (61). Similarly, lncRNA XIST was also found to regulate the miR-92b-3p/MTF1 axis for shaping BC progression, deserving further exploration by in vitro and in vivo assays (62). In another multi-method bioinformatic study, LINC01614 likewise facilitated the expression of SLC31A1 by inhibiting miR-204-5p and was involved in BC progression. Thus, LINC01614/miR-204-5p/SLC31A1 is also a regulatory chain related to cuproptosis and was critical for BC (63). In addition, PRNP was identified as a core CRG involved in tumor progression in BC. hsa-miR-192-5p and hsa-miR-215-5p were confirmed to target regulatory PRNP by PPI and immunoassay, and a ceRNA regulatory network was identified, including mRNAPRNP/miRNA hsa-miR-215-5p and hsa-miR-192-5p/lncRNA CARMN axis (64). Zhang et al. constructed a risk model for Estrogen receptor-positive (ER+) BC consisting of 4 CRGs, including DLD, DBT, DLAT, and ATP7A, that predicted immune infiltration, immune function, ICs, characteristic gene changes, and pathway activation in different scoring risk subgroups (65). In this system, the authors identified two ceRNA action networks lnc RNA C6orf99/hsa-miR-370-3p and hsa-miR-432-5p/DLD, and that DLD was a core target associated with resistance to ET through cuproptosis (Figure 2).
Cuproptosis-related functions in BC progression. Some CRGs are involved in the development and progression of BC. Inhibition of CDKN2A downregulates the expression of a series of proteins associated with tumor proliferation and migration, leading to a better prognosis of BC. LncRNA-C6orf99 can enhance the expression of DLD by regulating the expression of hsa-miR-370-3p and hsa-miR-432-5p, thus strengthening the tolerance of BC cells to endocrine therapy. LINC00511 and LINC01614 could act on miR-29-3p and miR-204-5p, respectively, to enhance the expression of SLC31A1, which was associated with worse prognosis of BC. Increased c-Myc expression may also promote BC progression through cuproptosis. LncRNA-XIST can inhibit BC progression by targeting the miR-92b-3p/MTF1 axis. LncRNA-CARMN can promote PRNP expression by regulating hsa-miR-192-5p and hsa-miR-215-5p, thereby inhibiting the development of BC. Butyrate promotes the expression of PDXK and SLC25A28 by inhibiting TLR4, thereby promoting cuproptosis in tumour cells.
Cuproptosis-related applications in BC diagnosis
Mammography imaging and magnetic resonance imaging, histopathologic testing, and serologic screening methods, have been common clinical screening tools for BC in the present (66,67). Several novel techniques, including the applications of microwave imaging and artificial intelligence, offer new possibilities for the early diagnosis and treatment of BC (68,69). However, postoperative failure, metastasis, relapse, and chemoresistance, are the primary factors contributing to therapeutic failure in BC patients, especially TNBC. Currently, desirable predictive models for these factors are still lacking (17,70,71). Cuproptosis regulates a wide range of biological functions and possesses a contributory function in shaping the complexity and diversity of the TME, then its associated hallmarks are potentially the basic unit of BC prediction (72,73). Models based on multiple CRGs and signatures are capable of responding to a variety of clinical prognostic evaluations, tumor mutation burden (TMB), immune cell infiltration and activity, immune checkpoint, and chemotherapeutic drug sensitivity (74-76). Here, we divide this section into single CRG-based, multiple CRGs, and combined CRG-related genes.
Models based on single CRG
SLC31A1 is a critically endorsed class of copper transporters that impacts copper uptake at the cell membrane by functioning as a homotrimer, and its overexpression promotes greater copper uptake (77). As an influential player in the cuproptosis gene family, SLC31A1 has been confirmed to be highly correlated with poor prognosis in a range of tumor types, including BC, cervical cancers, head and neck squamous cell carcinoma, and esophageal carcinoma (ESCA) (63,78). Li et al. integrated SLC31A1 with other clinical parameters, such as age, T-stage, N-stage, and clinical staging to construct a predictive model, demonstrating favorable predictive effectiveness for both near-term and long-term OS (17). High expression of SLC31A1 was statistically associated with immunity, metabolic dysregulation, and treatment responsiveness to paclitaxel and CTLA4.
Pyruvate dehydrogenase E1 component subunit alpha (PDHA1) is a key gene involved in cuproptosis and consequently in the glucose metabolism reprogram of cancer cells (79). PDHA1 is implicated in a variety of signaling pathways in the malignant evolution of tumors, including DNA damage, cell invasion, immunosuppression, and angiogenesis (80). PDHA1 is potentially a prognostic and immune-related parameter for multiple cancers. For example, Huang et al. demonstrated through multiple validation and characterization that PDHA1 was a regulator of BC malignant progression and that PDHA1 expression was tightly correlated with the infiltration of a variety of immune cells, including CD4+ T cells, macrophage subsets, and mast cells (81). Thus, this study demonstrated that PDHA1 was an independent factor in BC prediction and was expected to be a novel immunotherapeutic target.
Models based on multiple CRGs
Models based on multiple differentially expressed CRGs are able to capture more BC patient information than a single CRG and may be more efficient in terms of accuracy (82,83). After discovering the pivotal role of DLAT in BC, Sha et al. constructed a risk score model for HER 2+ BC patients based on CRGs, and confirmed DLAT as an independent prognostic factor for HER2+ BC patients in further analysis (53). Xia et al. matched 21 known CRGs with differentially expressed ncRNAs in BC, subsequently screening them with machine learning algorithm (84). They ultimately chose five key CRGs from these to construct a diagnostic model together, which was able to predict the molecular subtypes of BC accurately, providing a new perspective on personalized treatment for BC patients. Zhu et al. constructed 6 CRGs-comprising risk signatures for BC prediction (85). The model demonstrated excellent capabilities to delineate high-risk and low-risk groups, to evaluate higher than TNM staging, and to assess immune responsiveness. Zheng et al. reported a signature based on CRGs, and emphasized that patients in the high-risk group classified according to the model had lower OS, and there were significant differences between the high- and low-risk groups in terms of OS, immune status, and sensitivity to chemotherapy (86). Jiang et al. successfully built a risk model based on 6 CRGs, comprising DKN2A, MTF1, PDHA1, DLD, LIPT1, and FDX1. The model line was predictive of pre-pTNM, MSI, drug sensitivity, and immune infiltration characteristics in BC patients (62).
ER+ BC is the most common subtype of BC and is accompanied by features such as immune stress and epigenetic modifications (87). The treatment of ER+ BC consists of interventions that inhibit estrogen secretion and/or directly target the ER, improving patient outcomes (88). However, the resistance and immune profile of such therapies require clearer predictive modalities and indications. Fan et al. constructed a risk scoring system by using CRGs for ER+ early BC, showing that High expression of FDX1, LIAS, LIPT1, DLD, PDHB, and ATP7B and low expression of CDKN2A were related to better RFS (89). This model specified a higher risk of recurrence with a high CRG score and therefore could be used synergistically as a complement to conventional clinical parameters for long-term prognostic evaluation of ER+ early BC.
Models based on combined CRG-related genes
Models constructed based on multiple composite genes are also common approaches nowadays, including those based on PCD-associated ncRNAs, PCD-associated mRNAs, or Mendelian randomization (90,91). In BC, cuproptosis-related lncRNAs (CRLs) are the most common type of ncRNAs used to construct BC prediction models. In contrast, cuproptosis-related miRNAs and circRNAs based on cuproptosis have barely been mentioned.
CRLs are instrumental in mediating the biological functions of BC with the potential to predict BC prognosis and sensitivity to various therapies (92). The resistance biomarker expressions of CDK4/6 (CCNE1, E2F1, and E2F2) and PARP (BRCA1/BRCA2) were lower in low-risk patients, suggesting that low-risk patients were more prone to the application of these two inhibitors. Utilizing the TCGA database in combination with machine learning, Li et al. constructed a cuproptosis-associated lncRNA-based prediction model, which exhibited excellent performance in predicting the prognosis, tumor mutation burden, and responsiveness to immunotherapy in BC patients (93). Similarly, Sun et al. also screened differentially expressed cuproptosis-associated lncRNAs in BC samples (94). Cellular experiments confirmed that AC104211.1 and LINC01863 influenced BC cell proliferation, while the screened drugs, Trametinib, 5-fluorouracil, and AICAR significantly inhibited the viability of MCF-7 cells. Xu et al. found the cuproptosis genes FDX1, PDHA1, and DLAT were remarkably down-regulated in BC tissues, furthermore, they identified a unique model BCCuS based on cuproptosis-related 2-lncRNAs USP2-AS1 and NIFK-AS1 for BC prediction (95). The BCCuS model had high prognostic predictive performance and was strongly associated with high TMB and immune assessment.
Li et al. also constructed a characteristic predictive model using 5 CRLs, C2orf91 (LINC02898), PRKAR1B-AS1, AC012213.3, AL137847.1, MFF-DT, and the pathways of these CRLs were closely related to immune responsiveness (96). The model was able to respond well to the high sensitivity of multiple drugs to high risk, including Lapatinib, Sunitinib, Phenformin, and Idelalisib. Li et al. used 10 CRLs for constructing a risk signature for BC risk stratification, which obtained desirable predictive capabilities (97). Yu et al. constructed a risk model based on 11 CRLs, demonstrating satisfactory prognostic prediction performance in BC (98). Besides, this model was also related to the assessment of immune checkpoint inhibitors (ICIs), TMB, m6A, and agent sensitization. Guo et al. reported a cuproptosis-associated risk model with 9 CRLs as an effective tool for independent prognostic prediction of BC (99). Moreover, the model also possessed immuno-predictive efficacy, and the low-risk group was able to respond better to immunotherapy, showing higher CD8+ T-cell infiltration and activation, and more TMB. Jiang et al. reported a novel signature based on 11 CRLs, and this signature could independently predict the prognosis and TMB (100). Furthermore, the high score cataloged by this signature showed high drug sensitivity for anti-CD276 therapy and imatinib, lapatinib, and pazopanib. Pan et al. also mined a model constructed from 10 CRLs and had similar predictive functions as independent prognostic predictors for BC (101). Zhang et al. adopted cuproptosis-related ferroptosis-related genes to investigate their expression patterns in predicting OS of BC. This study posed the capability of the pattern, and its relationship with steroid biosynthesis, ABC transporters, and drug sensitivity of AKT inhibitor VIII and cisplatin (102).
There is probably a robust link between cuproptosis and tumour immunity. Wang et al. explored the intricate relationship between cuproptosis and tumor immunity in TNBC to establish prognostic models based on miRNA and mRNA (103). By comparing the expression levels of CRGs between normal individuals and the TCGA-TNBC cohort, they identified 5 prognostic miRNAs (miR-203a-3p, miR-1277-3p, miR-135b-5p, miR-200c-3p, and miR-592) and 3 biomarkers (DENND5B, IGF1R, and MEF2C) that were significantly associated with TNBC prognosis. These factors might influence TNBC progression by affecting adipogenesis, inflammatory response, hormone metabolism, and the immune microenvironment. The cancer immunity cycle (CIC) is a crucial component of the cancer immune microenvironment, and might interact with cuproptosis to exert biological functions. Liu et al. utilized the TIP database and machine learning to identify 4 key cuproptosis-CIC interactions in BC and constructed a prognostic prediction model (104). Further experiments confirmed that HSPA9 was a critical protein that restricted BC growth and migration. These studies provide insights into the complex relationship between the cuproptosis-CIC network and the BC immune microenvironment (Table 1).
Cuproptosis-targeted therapy in BC
Evidence suggests that tumor cells undergoing regulatory death can remodel TME immunogenicity and intensity of immune effects, demonstrating the potential to inhibit cancer metastasis and recurrence (105). Other stromal and immune-type cells and components in the TME also undergo a definite degree of PCD effects, all of which exert a certain positive regulatory effect on tumor immunity (106,107). Currently, therapeutic modalities utilizing cuproptosis-targeted induced copper ion carriers in combination with small molecule drugs have demonstrated some tumor therapeutic potential (108).
Some TNBC-related CRGs, including ATP7A, PIK3CA, LIAS, and LIPT, were closely associated with some mutations and immune infiltration (71). Moreover, several drugs targeting cuproptosis were screened for the treatment of TNBC, including dasatinib in combination with ABT-737, Erastin or methotrexate, docetaxel/isprinib combination. Zinc pyrithione (ZnPT), an antifungal drug for seborrheic dermatitis, contributes to DNA damage and PARP-dependent energetic crisis (109). ZnPT is capable of exerting certain anticancer effects, including inhibition of PDAC tumor progression through suppression of SDCBP (110). Yang et al. demonstrated that ZnPT could induce TNBC cell death by disrupting the homeostasis of copper metabolism as well as triggering DLAT aggregation (111). Moreover, ZnPT inhibited TNBC cell proliferation, motility, stemness, and sensitized TNBC to chemotherapeutic effects, and ultimately inhibited TNBC development by inhibiting EGFR-PI3K-AKT and activating the MAPK signaling pathway. Deng et al. also constructed a risk model based on 4 CRLs, including C9orf163, PHC2-AS1, AC087741.1, and AL109824.1 after multiple analyses, and utilized this model to innovatively propose that the Hsp90 inhibitor 17-AAG was a candidate with potential therapeutic effects in high-risk groups (64).
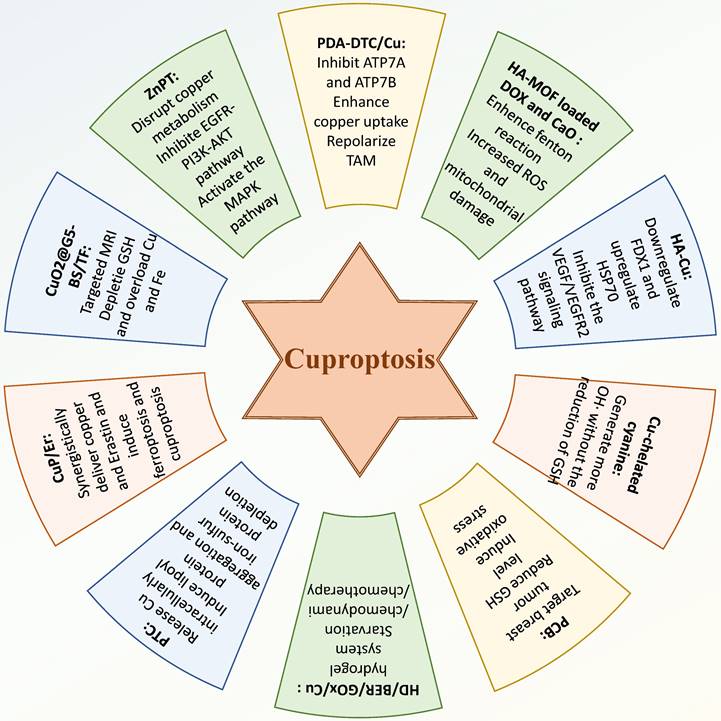
Nano-complex-based drug-carrying systems can fully utilize the advantages of targeting, multiple synergistic functions, and safety (112-114). The induction and enhancement of PCD through nanocomplexes, such as cuproptosis, can not only promote tumor cell death, but also promote the release of immunogenic substances, promote immune activation, and amplify the therapeutic effect of the tumor (115). Of late, researchers focus on combining this advanced delivery method with cuproptosis induction, and have developed a series of novel drugs for BC. Inducing or enhancing cuproptosis by introducing high concentrations of copper and other agents is a common form. For instance, Chang et al. designed a polydopamine nanostructure (PDA-DTC/Cu) loaded with high concentrations of copper ions, which could enhance copper uptake and inhibit the expression of ATP7A and ATP7B, thereby reducing copper export and increasing intracellular copper accumulation to promote cuproptosis (116).
Cuproptosis-related applications in Breast Cancer diagnosis.
| Related Molecule | Diagnosis application | Reference |
|---|---|---|
| SLC31A1 | Integrated SLC31A1 with other clinical parameters, such as age, T-stage, N-stage, and clinical staging. | Ref.17 |
| PDHA1 | PDHA1 was a regulator of BC malignant progression and its expression was tightly correlated with the infiltration of a variety of immune cells. | Ref.81 |
| DLAT, SLC31A2, SLC25A3, ATOX1 | Classifying HER2+ BC patients into low-risk and high-risk groups by risk scores, effectively assess the prognosis of HER2+ BC patients. | Ref.53 |
| CASC 8, LINC 02188, LINC 00511, GRIK 1-AS 1, ROCR | The AUC of the model constructed for the prediction of breast cancer subtypes was 0.816 and the F1 score was 0.57. In addition, the precision of the model was 0.56, the specificity was 0.81, and the sensitivity (Sn) was 0.61. | Ref.84 |
| PTPRN2, SCARB1, SLC37A2, YES1, LY6D, and NOTCH3 | With a high AUC score over 0.85 in TCGA dataset, high- and low-risk groups distinguished by the model had distinct prognosis and immune infiltration. | Ref.85 |
| TFF1, GREB1, SCUBE2, MMP7, SUSD3, CHI3L1, STC2, SLC7A5, MAPT, FABP7 and CHAD | Significant differences existed between the high- and low-risk groups in terms of OS, immune status, and sensitivity to chemotherapy. | Ref.86 |
| DKN2A、MTF1、PDHA1、DLD、LIPT1 and FDX1 | The model predicted the OS rate with an accuracy that ranged from medium to high. | Ref.62 |
| FDX1, LIAS, LIPT1, DLD, PDHB, ATP7B and CDKN2A | The model specified a higher risk of recurrence with a high CRG score. | Ref.89 |
| PPIC-AS1, MME-AS1, AC012676.3, MFF-DT, OTUD6B-AS1, AL109936.9, AL807757.2, AL118556.1, AL137847.1 | The predictive model classified patients into high-risk and low-risk groups, with a significantly higher mortality rate in the high-risk group. The results of the C-index curves indicated that the predictive model possesses a higher degree of accuracy in assessing prognosis than the clinicopathological features. | Ref.93 |
| AC104211.1, AL162595.1, LINC02166, AC007996.1, AC005865.2, AC007493.1, AL449423.1, AC004847.1, AL161910.1, LINC01863, AC018978.1, AC092384.3, AL356740.2 | The model grouped patients according to thier scores, and the AUC values of the risk scores for predicting 1-, 3-, and 5-year survival of BC in the validation set were 0.73, 0.68, and 0.71, respectively. | Ref.94 |
| USP2-AS1, NIFK-AS1 | BCCuS-high group and BCCuS-low group showed significant differences in gene mutation frequency, immune function, TIDE (tumor immune dysfunction and exclusion) score and other phenotypes. | Ref.95 |
| C2orf91(LINC02898), PRKAR1B-AS1, AC012213.3, AL137847.1, MFF-DT | These CRLs were closely related to immune responsiveness, the OS of patients in the high-risk group was lower than that in the low-risk group. | Ref.96 |
| AL139241.1、MFF-DT、AL451123.1、AC009120.5、AL137847.1、HECW 2-AS 1、LINC 01031、NIFK-AS 1、AL592301.1、U73166.1 | The model categorized patients into high- and low-risk groups, the risk score was significantly relevant to the survival of BRCA patients and demonstrated a better predictive ability for 1- and 3-year survival with the AUC of approximately 0.74, as well as the potential of predicting immunotherapy responsiveness | Ref.97 |
| AL023882.1, AC091588.1, AC138028.2, AC027514.1, AL592301.1, LRRC8C-DT, MFF-DT, NIFK-AS1, MECOM-AS1, OTUD6B-AS1 and RNF32-AS1 | Patients in the high-risk group distinguished by this model face heavier tumor mutational load (TMB), have suppressed anti-tumor immunity, and possess a worse prognosis. | Ref.98 |
| LRRC8C-DT, TDRKH-AS1, SAMMSON, SIAH2-AS1, WDFY3-AS2, LINC00393, ARHGAP28-AS1, PCAT18, and LINC01711 | Patients with BC in the low-risk groups showed better clinical outcomes different immune cell infiltrations, with better response to immunotherapies. | Ref.99 |
| GORAB-AS1, AC 079922.2, AL 589765.4, AC 005696.4, Cytor, ZNF 197-AS1, AC 002398.1, AL 451085.3, YTH DF 3-AS1, AC 008771.1, LINC 02446 | The model showed the ability to independently predict the prognosis and TMB, with the AUC values for ROC of 1-, 3-, and 5-year risk were 0.849, 0.779, and 0.794, respectively. | Ref.100 |
| AL118556.1, AL451123.1, MFF-DT, AL133243.2, ZKSCAN7-AS1, AC012676.3, AC009506.1, AC079766.1, MIR1915HG, AC138028.2 | ROC and PCA showed that the model has accurate prediction ability, with higher tumor mutation burden and high TMB-resulted lower survival in high-risk group. | Ref.101 |
| ANO6, CHAC1, CHMP6, CS, EMC2, G6PD, GPX4, PANX1, PIK3CA, SLC7A5, and SOCS1 | The model demonstrated differences in steroid biosynthesis, ABC transporters, and drug sensitivity to AKT inhibitor VIII and cisplatin between subgroups. | Ref.102 |
| miR-203a-3p, miR-1277-3p, miR-135b-5p, miR-200c-3p, miR-592, DENND5B, IGF1R, MEF2C | Patients were categorised into high- and low-risk groups based on risk scores from the model, which combined the cancer status and pathological staging to predict 1/3/5-year patient survival and provide new therapeutic targets for BC. | Ref.103 |
| CXCL13, HSPA2, HSPA9, MICB | A nomogram model was developed to predict survival in BC patients by linking risk scores to clinicopathological features and revealed that targeting HSPA9 could be a potential means of improving breast cancer outcomes. | Ref.104 |
Additionally, tumor-associated macrophages were also stimulated to repolarize, alleviating immunosuppression within the tumor microenvironment. Overall, PDA-DTC/Cu demonstrated excellent tumor inhibitory effects by inducing cuproptosis as the core mechanism. Du et al. developed a self-reinforced bimetallic Mito-Jammer by constructing a hyaluronic acid -modified metal-organic framework loaded with doxorubicin and calcium peroxide (117). In 4T1 cell and 4T1 tumor-bearing models, the dissociation of calcium peroxide into hydrogen peroxide and Ca2+ enhanced the Cu2+-mediated Fenton reaction. This led to increased ROS production and mitochondrial damage, thereby significantly enhancing the sensitivity of tumor cells to cuproptosis and inhibiting tumor metastasis. In another study, Xu et al. synthesized disulfonamide-dimethylpyrimidine-phenanthroline-metal complexe HA-Cu, which demonstrated superior inhibitory effects on TNBC both in vitro and in vivo (118). HA-Cu effectively suppressed tumor survival and progression through a synergistic mechanism involving antiproliferative, antiangiogenic, anti-inflammatory, pro-apoptotic, and cuproptosis-inducing effects. HA-Cu inhibited the VEGF/VEGFR2 signaling pathway and enhanced cuproptosis in MDA-MB-231 cells by downregulating FDX1 expression and upregulating HSP70 expression. He et al. fabricated a type of Cu-chelated cyanine dye to deliver copper ions in different oxidation states to 4T1 cells and 4T1 tumor-bearing mice to investigate their roles in combating TNBC (119). They found that Cu+, in comparison to Cu2+, exhibited improved antitumor performance by generating more hydroxyl radicals through a faster pathway that did not require glutathione reduction. Zhang et al. developed a BPTES-loaded biomimetic Cu-doped polypyrrole nanoparticle (CuP) nanosystem (PCB), utilising various components to actively target tumor sites, reduce GSH levels, and induce oxidative stress and cuproptosis (120). PCB effectively inhibited the growth of both primary and distal tumors. By introducing glucose oxidase (GOx) and Cu sulfate, Lee et al. constructed a HD/BER/GOx/Cu hydrogel system for converting accumulating glucose into hydroxyl radicals and starvation/chemodynamic therapy by introducing glucose oxidase (GOx) and copper sulfate (121). The system enabled a multitude of tumor-killing effects through sustained release of drugs, including, notably, cupping-induced chemotherapy. In another study, Ning et al. designed a nanodelivery platform based on multifaceted induction of cupping, consisting of platelet vesicle (PV)-coated cuprous oxide nanoparticles (Cu2O)/TBP-2 cupping sensitization system (PTC) (122). This PTC system was able to release copper ions intracellularly, inhibit copper efflux, and further lead to lipoyl protein aggregation and iron-sulfur protein depletion, resulting in proteotoxic stress and cuproptosis. In this mechanism, PTC ultimately inhibited BC lung metastasis and enhanced T-cell immune effects.
Some studies have attempted to combine inducing cuproptosis with other antitumor therapies to enhance therapeutic efficacy. For example, Li et al. designed a core-shell nanoparticle, CuP/Er, which was capable of co-delivering copper and Erastin to cancer cells, thereby synergistically inducing ferroptosis and cuproptosis (123). Furthermore, CuP/Er was found to promote T cell proliferation and infiltration, enhancing the efficacy of immune checkpoint blockade therapy, resulted in inhibited survival and metastasis of murine colorectal adenocarcinoma and TNBC. Huang et al. developed a poly (amidoamine) dendrimer modified with p-carboxybenzenesulfonamide, loaded with copper peroxide nanoparticles, and combined with iron (Fe)-tannic acid (TF) networks to create a nanocomposite (CuO2@G5-BS/TF) (124). This nanocomposite effectively targeted and was internalized by 4T1 cells for targeted MRI. Furthermore, CuO2@G5-BS/TF induced ferroptosis and cuproptosis in 4T1 cells by depleting GSH and overloading copper and iron, while also alleviating the acidity of the tumor microenvironment, thereby inhibiting tumor metastasis. This modality offerd an attractive new method for targeted MRI and treatment of TNBC (Figure 3).
The re-thinking of cuproptosis in BC
Researchers have long noted the association between copper and cancer (125,126). As an essential transition metal in the body, the dysregulation of copper metabolism can lead to a range of cellular metabolic dysfunctions. The normal function of copper ions in cancer cells depends on the interactions between various copper-related proteins. These include proteins involved in copper transmembrane transport such as CTR1, SLC25A3, ATP7A/B, proteins responsible for binding and storing copper ions like MT and glutathione, and copper chaperone proteins such as ATOX1, CCS, and COX17. The interaction of these proteins is fundamental in maintaining intracellular copper homeostasis (127-130). By engaging key molecules in these pathways, intracellular copper can directly participate in regulating multiple signaling pathways in tumor cells, including receptor tyrosine kinase (RTK)-related signaling pathways, phosphoinositide 3-kinase (PI3K)-AKT signaling pathway, mitogen-activated protein kinase (MAPK) signaling pathway, and Notch pathway (131-134). These pathways influence tumor metabolism, proliferation, angiogenesis, and metastasis.
Elevated copper levels have been found in the tumors and serum of various animal models and cancer patients, including prostate cancer, BC, thyroid cancer, lung cancer, and gastric cancer. In lung cancer patients, higher serum copper ion concentrations are associated with worse clinical staging in northeast china (135). Based on the major discovery that copper can induce cell death, cuproptosis is considered a promising direction for cancer therapy. By regulating copper levels in tumour cells, normal biochemical processes can be disrupted, thereby inhibiting the proliferation and survival of cancer cells. Consequently, the profound interconnection between cuproptosis and cancer represents a burgeoning area of interest. Tsvetkov et al. identified 10 genes significantly associated with the risk of cuproptosis through genome-wide knockout screening. Further analysis revealed that SLC31A1, ATP7A, and ATP7B were deeply involved in regulating intracellular copper homeostasis. These 13 genes were considered critical cuproptosis genes (CKGs) (28). Researchers have focused on the expression levels and clinical significance of these CKGs and some CRGs in different tumors. Interestingly, CKGs and CRGs seem to play different roles in various tumors. For instance, FDX1 encodes Ferredoxin1, which participates in multiple redox reactions and enhances the toxicity of copper ions by reducing them, playing a central role in cuproptosis (136,137). Elevated levels of FDX1 are observed in glioblastoma and female reproductive tumors, whereas its expression is downregulated in solid tumors like lung adenocarcinoma and hepatocellular carcinoma (138). High levels of FDX1 are associated with poor prognosis in patients with head and neck squamous cell carcinoma and low-grade glioma, but predict better prognosis in patients with cervical squamous cell carcinoma and clear cell renal cell carcinoma. Similar effects can be observed in another CKG, namely LIAS. The high expression of LIAS is associated with poor prognosis in lung cancer, whereas in KIRC and ovarian cancer, high expression of LIAS indicates better prognosis (139).
Given that research on cuproptosis is still in its early stages, further studies are needed to elucidate the reasons for these differential expressions in various tumors and the specific mechanisms by which these differences impact prognosis. On one hand, this may be because these CKGs are involved not only in cuproptosis-related pathways but also in regulating biological processes, such as immune infiltration, energy metabolism, and methylation. In pan-cancer analyses, these genes exhibit different characteristics in various tumors (140). On the other hand, it may also be related to the inherent characteristics of some tumors. For example, there is a significant correlation between cuproptosis and mitochondrial metabolic levels, and certain tumors inherently exhibit higher levels of mitochondrial metabolism, such as melanoma, BC, and leukemia (141,142). Therefore, inducing cuproptosis as a means to treat tumors is more likely to yield ideal therapeutic effects in these types of cancers.
BC exhibits significant heterogeneity, both among different patients and within tumors from the same individual, primarily characterized by variations in hormone receptor status, HER2 expression, mutational landscape, and intratumoral heterogeneity (143,144). Hormone receptor-positive (ER+ and/or PR+) and TNBC differ markedly in their biological characteristics, treatment responses, and prognoses (145,146). HER2-positive tumors are particularly responsive to HER2-targeted therapies like trastuzumab but may exhibit reduced sensitivity to other treatments (147). Common genetic mutations in BC include TP53, PIK3CA, and GATA3, which influence the malignancy and therapeutic responses of the tumors (148,149). Additionally, intra-tumor heterogeneity is evident, with different cellular subpopulations within a single breast tumor displaying variations in cell morphology, proliferative capacity, and gene expression patterns (150). This extensive molecular heterogeneity not only dictates the biological behaviors of BC but also directly impacts its response to various therapeutic strategies.
BC cells exhibit a unique copper metabolism, requiring higher amounts of copper, which makes them more sensitive to cuproptosis inducers. Research indicates that dysregulation in copper metabolism is closely related to malignant progression of BC. High expression of copper transport proteins, such as SLC31A1, facilitates copper uptake, leading to the occurrence of cuproptosis (151). Dysregulation of copper metabolism-related genes affects copper homeostasis, causing intracellular copper ion accumulation and triggering cuproptosis. By regulating the expression of these genes, the growth and proliferation of BC cells can be effectively controlled. For instance, TNBC shows a high copper demand and sensitivity to cuproptosis inducers like ZnPT (111). This disrupts copper metabolic homeostasis and induces cell death by DLAT aggregation, thereby inhibiting proliferation, migration, and stemness. The unique relationship between cuproptosis and BC highlights the role of molecular heterogeneity in determining therapeutic responses. Different BC subtypes exhibit distinct responses to cuproptosis-inducing therapies (152). Hormone receptor-positive BC cells are sensitive to hormone therapy but may respond weakly to cuproptosis inducers; however, this can be improved through combination treatments. HER2-positive BC cells, while responsive to HER2-targeted therapies like trastuzumab, require further investigation to understand their response to cuproptosis inducers. Combining HER2-targeted therapies with cuproptosis inducers could be an effective strategy, as copper metabolism may interact with the HER2 signaling pathway, influencing cell growth and proliferation. TNBC, with its high dependency on copper metabolism, presents significant potential for the application of cuproptosis inducers, offering a promising approach for its treatment.
Discussion
In this study, we systematically elucidated the cuproptosis mechanism, the regulatory mechanism of cuproptosis on BC, the application of cuproptosis in the diagnosis and prediction of BC, and the therapeutic strategy of BC based on cuproptosis. However, there are some limitations and outlooks that need close attention in the current field.
Therapy targeting cuproptosis in BC. Researchers have developed some drugs that can inhibit the development of BC by modulating cuproptosis in BC cells. These drugs, mostly in nano form, can induce cuproptosis in BC cells in a variety of ways or combine the induction of cuproptosis with other therapeutic approaches, yielding promising results in the treatment of BC.
First, cuproptosis has been poorly studied in BC in general, and most of these studies are still in the data mining related to cuproptosis. The biological processes of BC involve many different types of PCD modalities, including apoptosis, ferroptosis, autophagy, and cuproptosis (153,154). Compared to other forms of PCD, experiments related to cell, animal, and drug mining for cuproptosis are less reported. This implies that the roles and mechanisms of cuproptosis-related molecules in BC that have been mined so far are still full of mysteries. Different PCD patterns are co-existed in the BC progression, as evidenced by that simultaneous inductions of cuproptosis and ferroptosis in BC cells inhibit BC cell malignant behaviors (124). Furthermore, BC is a highly heterogeneous solid tissue with a complex composition, which also encompasses complex pathological and molecular typing. The responsiveness of different BC subtypes to cuproptosis is significantly different. Previous studies of cuproptosis often did not give a clear qualification of the subtypes in BC, and only in a few studies did they indicate the subtypes as TNBC or ER+ BC. The possibility that different subtypes may differ in their responsiveness to cuproptosis is a subsequent point that needs to be addressed urgently. It is also important to note that most of the cuproptosis focuses on the tumor cells themselves in the TME, while the cuproptosis effects of other stromal and immune cells have been reported. Overall, cuproptosis, as an emerging research hotspot, still lacks a large number of high-quality basic studies to confirm its role and mechanism in BC.
Cuproptosis has shown significant potential in the diagnosis of BC. In BC diagnostics, CRGs and CRLs provided valuable key information, such as rich tumor immunoprediction and clinical prediction. Risk models constructed from different CRGs have been widely recognized. BC is a heterogeneous entity composed of a variety of immune cells, stromal cells, and tumor cells (155,156). CRG-related model is not only capable of determining the prognosis, but also analyzing the characteristics of the cellular components of the entity microenvironment, the intensity of immunity, the TMB, and the assessment of the responsiveness to treatment. These provide rational therapeutic windows and strategies for precise therapies of BC. Risk models constructed based on multiple CRGs or combining CRGs and other ncRNAs together demonstrated excellent predictive capabilities. However, cuproptosis-based markers are still in the very early stages of validation. Nost of the studies are still at the stage of basic research and data mining, lacking large-scale clinical validation. In addition, the clinical translation of CRG models faces challenges, including the need for standardized detection methods and large-scale clinical trials. More in-depth retrospective and prospective studies are necessary to establish cuproptosis as a useful adjunct to traditional diagnostic methods in clinical practice.
Finally, in tumor therapy, the antitumor potential and safety of a large number of copper chelators and copper complexes have been preliminarily demonstrated in preclinical cell and animal studies. Copper-related drugs represented by DSF and TTM can produce synergistic therapeutic effects with other chemotherapeutic agents, inhibiting tumor resistance, suppressing tumor recurrence as well as sensitizing drug effects (157,158). However, compared with copper homeostasis-related genetic diseases, the application of cuproptosis-targeted therapy for tumors is not well established. In BC, the relevant therapeutic applications are in the cellular and animal studies of cuproptosis induction, and the specific mechanisms and applicable individuals have not been fully analyzed. Moreover, the number of these studies is relatively small, and continued exploration is necessary for the treatment of BC. In addition, since curpoptosis is a novel PCD mechanism that has been relatively little and superficially studied in BC, the modulation of tumor immunity has not been given a clear exploration. However, PCD produces damage-associated molecular patterns that contribute to antitumor immunotherapy, stimulate inflammatory responses, and mediate the activation of a variety of immune cells and factors, making the induction of PCD a potential immunotherapeutic strategy (159,160). Moreover. These types of cell death are not independent, but are closely cascaded or coordinated, and the use of nanomedicines can induce a combination of PCDs for treatment.
Finally, the increased copper requirement of tumour cells may be related to the role of copper in cell proliferation and metabolic activities. However, excess copper confers an anti-tumour effect by inducing copper toxicity, which is toxic to cancer cells. Copper is also an ion essential for normal physiological cellular activity, and the concentration must be maintained within a specific physiological range. To achieve therapeutic copper overload while minimizing damage to normal tissues, precise control over copper delivery and concentration is crucial. Additionally, targeted delivery systems or selective activation mechanisms could help specifically induce cuproptosis within the tumor microenvironment, thereby reducing adverse effects on normal cells.
Conclusion
Collectively, the delicate equilibrium of copper ions within the human body is essential for maintaining cellular homeostasis and is intricately linked to the pathogenesis and progression of BC. Cuproptosis has gradually emerged to play an increasingly prominent role in BC, including influencing the proliferation, invasion, prognosis, and treatment of BC. CRGs and CRLs based on cuproptosis have also demonstrated rich predictive information for BC. In addition, therapeutic strategies targeting cuproptosis are capable of capabilities to elicit positive therapeutic effects. Compared with other PCD types, cuproptosis currently has a long way to go in BC, which will provide a theoretical basis for cuproptosis-targeted therapy. Lasly, cuproptosis sheds light on the copper dependency and metabolic characteristics of BC, providing new avenues for precision medicine and personalized therapeutic strategies.
Abbreviations
ATOX1: copper molecular chaperone antioxidant 1
ATP7A: ATPase copper transporting alpha
ATP7B: ATPase copper transporting beta
BC: breast cancer
CAFs: cancer-associated fibroblasts
CCO: cytochrome oxidase C
CDI: cell death index
CDKN2A: cyclin-dependent kinase inhibitor 2A
CIC: cancer immunity cycle
CKGs: critical cuproptosis genes
CRGs: cuproptosis-related genes
CRLs: cuproptosis-related lncRNAs
CTR1: copper transport protein 1
DLAT: dihydrolipoamide S-acetyltransferase
DLD: dihydrolipoyl dehydrogenase
DMFS: distant metastasis-free survival
ER+: estrogen receptor-positive
GOx: glucose oxidase
ICIs: immune checkpoint inhibitors
MAPK: mitogen-activated protein kinase
OS: overall survival
PV: platelet vesicle
PCDs: programmed cell deaths
PDHA1: pyruvate dehydrogenase E1 component subunit alpha
PI3K: phosphoinositide 3-kinase
ROS: reactive oxygen species
RFS: recurrence-free survival
RTK: receptor tyrosine kinase
SCFA: short-chain fatty acid
STEAP: six-transmembrane epithelial antigen of the prostate
SLC31A1: solute carrier family 31 member 1
SOD1: superoxide dismutase 1
TCA : tricarboxylic acid
TLR4: Toll-like receptor 4
TME: tumor microenvironment
TMB: tumor mutation burden
TNBC: triple negative breast cancer
ZnPT: zinc pyrithione
Acknowledgements
Funding
This work was financially supported by Natural Science Foundation of Hubei Province (2022CFB298), Shenzhen Nanshan District Health Bureau (NSZD2024042), and Wuhan University Education and Development Foundation (No. 2002330).
Author contributions
ZZ and KZ performed the literature search and prepared the original draft. YZ, QZ and JZ conceived the project, reviewed and revised the manuscript. All authors participated in the manuscript and approved the final version.
Consent for publication
All the authors agreed to be published.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Burstein HJ, Curigliano G, Thürlimann B, Weber WP, Poortmans P, Regan MM, Senn HJ, Winer EP, Gnant M, Aebi S. et al. Customizing local and systemic therapies for women with early breast cancer: the St. Gallen International Consensus Guidelines for treatment of early breast cancer 2021. Annals of Oncology. 2021;32:1216-1235
2. Ye F, Dewanjee S, Li Y, Jha NK, Chen Z-S, Kumar A, Vishakha, Behl T, Jha SK, Tang H. Advancements in clinical aspects of targeted therapy and immunotherapy in breast cancer. Mol Cancer. 2023;22:105
3. Li H, Shi W, Shen T, Hui S, Hou M, Wei Z, Qin S, Bai Z, Cao J. Network pharmacology-based strategy for predicting therapy targets of Ecliptae Herba on breast cancer. Medicine. 2023;102:E35384
4. Xi G, Guo W, Kang D, Ma J, Fu F, Qiu L, Zheng L, He J, Fang N, Chen J. et al. Large-scale tumor-associated collagen signatures identify high-risk breast cancer patients. Theranostics. 2021;11:3229-3243
5. Bertheloot D, Latz E, Franklin BS. Necroptosis, pyroptosis and apoptosis: an intricate game of cell death. Cell Mol Immunol. 2021;18:1106-1121
6. Newton K, Strasser A, Kayagaki N, Dixit VM. Cell death. Cell. 2024;187:235-256
7. Christgen S, Tweedell RE, Kanneganti TD. Programming inflammatory cell death for therapy. Pharmacol Ther. 2022;232:108010
8. Xu L, Wang S, Zhang D, Wu Y, Shan J, Zhu H, Wang C, Wang Q. Machine learning- and WGCNA-mediated double analysis based on genes associated with disulfidptosis, cuproptosis and ferroptosis for the construction and validation of the prognostic model for breast cancer. J Cancer Res Clin Oncol. 2023;149:16511-16523
9. Li M, Wang ZW, Fang LJ, Cheng SQ, Wang X, Liu NF. Programmed cell death in atherosclerosis and vascular calcification. Cell Death Dis. 2022;13(5):467
10. Hadian K, Stockwell BR. The therapeutic potential of targeting regulated non-apoptotic cell death. Nat Rev Drug Discov. 2023;22:723-742
11. Chen X, Cai Q, Liang R, Zhang D, Liu X, Zhang M, Xiong Y, Xu M, Liu Q, Li P. et al. Copper homeostasis and copper-induced cell death in the pathogenesis of cardiovascular disease and therapeutic strategies. Cell Death Dis. 2023;14(2):105
12. De Luca A, Barile A, Arciello M, Rossi L. Copper homeostasis as target of both consolidated and innovative strategies of anti-tumor therapy. J Trace Elem Med Biol. 2019;55:204-213
13. Chen L, Min J, Wang F. Copper homeostasis and cuproptosis in health and disease. Signal Transduct Target Ther. 2022;7:378
14. Kim JH, Matsubara T, Lee J, Fenollar-Ferrer C, Han K, Kim D, Jia S, Chang CJ, Yang H, Nagano T. et al. Lysosomal SLC46A3 modulates hepatic cytosolic copper homeostasis. Nat Commun. 2021;12(1):290
15. Liu Y, Wang J, Jiang M. Copper-related genes predict prognosis and characteristics of breast cancer. Front Immunol. 2023;14:1145080
16. Shen L, He Y, Fang C, Qiu H, Chen Q, Huang F, Wu Z. Cuproptosis-associated genes and immune microenvironment characterization in breast cancer. Medicine (United States). 2022;101:E32301
17. Li L, Li L, Sun Q. High expression of cuproptosis-related SLC31A1 gene in relation to unfavorable outcome and deregulated immune cell infiltration in breast cancer: an analysis based on public databases. BMC Bioinformatics. 2022;23:350
18. Zou Y, Xie J, Zheng S, Liu W, Tang Y, Tian W, Deng X, Wu L, Zhang Y, Wong CW. et al. Leveraging diverse cell-death patterns to predict the prognosis and drug sensitivity of triple-negative breast cancer patients after surgery. International Journal of Surgery. 2022;107:106936
19. Festa RA, Thiele DJ. Copper: An essential metal in biology. Current Biology. 2011;21:R877-R883
20. Jin X, Liu W, Miao J, Tai Z, Li L, Guan P, Liu J. Copper ions impair zebrafish skeletal myofibrillogenesis via epigenetic regulation. The FASEB Journal. 2021;35:e21686
21. Cholewińska E, Marzec A, Sołek P, Fotschki B, Listos P, Ognik K, Juśkiewicz J. The Effect of Copper Nanoparticles and a Different Source of Dietary Fibre in the Diet on the Integrity of the Small Intestine in the Rat. Nutrients. 2023;15(7):1588
22. Ohgami RS, Campagna DR, McDonald A, Fleming MD. The Steap proteins are metalloreductases. Blood. 2006;108:1388-1394
23. Pezacki AT, Matier CD, Gu X, Kummelstedt E, Bond SE, Torrente L, Jordan-Sciutto KL, DeNicola GM, Su TA, Brady DC. et al. Oxidation state-specific fluorescent copper sensors reveal oncogene-driven redox changes that regulate labile copper(II) pools. Proceedings of the National Academy of Sciences. 2022;119:e2202736119
24. Lönnerdal B. Intestinal regulation of copper homeostasis: a developmental perspective. Am J Clin Nutr. 2008;88:846S-850S
25. Ramos D, Mar D, Ishida M, Vargas R, Gaite M, Montgomery A, Linder MC. Mechanism of Copper Uptake from Blood Plasma Ceruloplasmin by Mammalian Cells. PLoS One. 2016;11:e0149516
26. Moriya M, Ho YH, Grana A, Nguyen L, Alvarez A, Jamil R, Ackland ML, Michalczyk A, Hamer P, Ramos D. et al. Copper is taken up efficiently from albumin and alpha2-macroglobulin by cultured human cells by more than one mechanism. Am J Physiol Cell Physiol. 2008;295(3):C708-21
27. Lutsenko S. Dynamic and cell-specific transport networks for intracellular copper ions. J Cell Sci. 2021;134:jcs240523
28. Tsvetkov P, Coy S, Petrova B, Dreishpoon M, Verma A, Abdusamad M, Rossen J, Joesch-Cohen L, Humeidi R, Spangler RD. et al. Copper induces cell death by targeting lipoylated TCA cycle proteins. Science. 2022;375:1254-1261
29. Chen L, Li N, Zhang M, Sun M, Bian J, Yang B, Li Z, Wang J, Li F, Shi X. et al. APEX2-based Proximity Labeling of Atox1 Identifies CRIP2 as a Nuclear Copper-binding Protein that Regulates Autophagy Activation. Angew Chem Int Ed Engl. 2021;60:25346-25355
30. Fujieda N, Umakoshi K, Ochi Y, Nishikawa Y, Yanagisawa S, Kubo M, Kurisu G, Itoh S. Copper-Oxygen Dynamics in the Tyrosinase Mechanism. Angew Chem Int Ed Engl. 2020;59:13385-13390
31. Das A, Ash D, Fouda AY, Sudhahar V, Kim YM, Hou Y, Hudson FZ, Stansfield BK, Caldwell RB, McMenamin M. et al. Cysteine oxidation of copper transporter CTR1 drives VEGFR2 signalling and angiogenesis. Nat Cell Biol. 2022;24:35-50
32. Xue Q, Kang R, Klionsky DJ, Tang D, Liu J, Chen X. Copper metabolism in cell death and autophagy. Autophagy. 2023;19:2175-2195
33. Sturtz LA, Diekert K, Jensen LT, Lill R, Culotta VC. A Fraction of Yeast Cu,Zn-Superoxide Dismutase and Its Metallochaperone, CCS, Localize to the Intermembrane Space of Mitochondria. Journal of Biological Chemistry. 2001;276:38084-38089
34. Lutsenko S, Barnes NL, Bartee MY, Dmitriev OY. Function and regulation of human copper-transporting ATPases. Physiol Rev. 2007;87:1011-1046
35. Horn N, Wittung-Stafshede P. ATP7A-Regulated Enzyme Metalation and Trafficking in the Menkes Disease Puzzle. Biomedicines. 2021;9(4):391
36. Cobine PA, Pierrel F, Winge DR. Copper trafficking to the mitochondrion and assembly of copper metalloenzymes. Biochim Biophys Acta Mol Cell Res. 2006;1763:759-772
37. Banci L, Bertini I, Ciofi-Baffoni S, Hadjiloi T, Martinelli M, Palumaa P. Mitochondrial copper(I) transfer from Cox17 to Sco1 is coupled to electron transfer. Proc Natl Acad Sci U S A. 2008;105:6803-6808
38. Bertini I, Rosato A. Menkes disease. Cellular and Molecular Life Sciences. 2008;65:89-91
39. McCauley SR, Clark SD, Quest BW, Streeter RM, Oxford EM. Erratum: Review of canine dilated cardiomyopathy in the wake of diet-associated concerns. J Anim Sci. 2020;98(7):skaa209
40. Beinhardt S, Leiss W, Stättermayer AF, Graziadei I, Zoller H, Stauber R, Maieron A, Datz C, Steindl-Munda P, Hofer H. et al. Long-term outcomes of patients with Wilson disease in a large Austrian cohort. Clin Gastroenterol Hepatol. 2014;12:683-689
41. Ge EJ, Bush AI, Casini A, Cobine PA, Cross JR, DeNicola GM, Dou QP, Franz KJ, Gohil VM, Gupta S. et al. Connecting copper and cancer: from transition metal signalling to metalloplasia. Nat Rev Cancer. 2022;22:102-113
42. Guan D, Zhao L, Shi X, Ma X, Chen Z. Copper in cancer: From pathogenesis to therapy. Biomed Pharmacother. 2023;163:114791
43. Xu Y, Liu S, Zeng L, Ma H, Zhang Y, Yang H, Liu Y, Fang S, Zhao J, Xu Y. et al. An Enzyme-Engineered Nonporous Copper(I) Coordination Polymer Nanoplatform for Cuproptosis-Based Synergistic Cancer Therapy. Advanced Materials. 2022;34:e2204733
44. Michniewicz F, Saletta F, Rouaen JRC, Hewavisenti R V, Mercatelli D, Cirillo G, Giorgi FM, Trahair T, Ziegler D, Vittorio O. Copper: An Intracellular Achilles' Heel Allowing the Targeting of Epigenetics, Kinase Pathways, and Cell Metabolism in Cancer Therapeutics. ChemMedChem. 2021;16:2315-2329
45. Wang Y, Zhang L, Zhou F. Cuproptosis: a new form of programmed cell death. Cell Mol Immunol. 2022;19:867-868
46. Cobine PA, Brady DC. Cuproptosis: Cellular and molecular mechanisms underlying copper-induced cell death. Mol Cell. 2022;82:1786-1787
47. Xiong C, Ling H, Hao Q, Zhou X. Cuproptosis: p53-regulated metabolic cell death? Cell Death Differ. 2023;30:876-884
48. Xing X, Cai W, Shi H, Wang Y, Li M, Jiao J, Chen M. The prognostic value of CDKN2A hypermethylation in colorectal cancer: A meta-analysis. Br J Cancer. 2013;108:2542-2548
49. Kreuger IZM, Slieker RC, van Groningen T, van Doorn R. Therapeutic Strategies for Targeting CDKN2A Loss in Melanoma. J Invest Dermatol. 2023;143:18-25.e1
50. Wan H, Yang X, Sang G, Ruan Z, Ling Z, Zhang M, Liu C, Hu X, Guo T, He J. et al. CDKN2A was a cuproptosis-related gene in regulating chemotherapy resistance by the MAGE-A family in breast cancer: based on artificial intelligence (AI)-constructed pan-cancer risk model. Aging. 2023;15:11244-11267
51. Cheng T, Wu Y, Liu Z, Yu Y, Sun S, Guo M, Sun B, Huang C. CDKN2A-mediated molecular subtypes characterize the hallmarks of tumor microenvironment and guide precision medicine in triple-negative breast cancer. Front Immunol. 2022;13:970950
52. Wang R, Xu K, Chen Q, Hu Q, Zhang J, Guan X. Cuproptosis engages in c-Myc-mediated breast cancer stemness. J Transl Med. 2023;21:409
53. Sha R, Dong X, Yan S, Dai H, You L, Guo Z, Sun A. Cuproptosis-related genes predict prognosis and trastuzumab therapeutic response in HER2-positive breast cancer. Sci Rep. 2024;14(1):2908
54. Xu L, Yang L, Zhang D, Wu Y, Shan J, Zhu H, Lian Z, He G, Wang C, Wang Q. Multi-omics analysis reveals the unique landscape of DLD in the breast cancer tumor microenvironment and its implications for immune-related prognosis. Comput Struct Biotechnol J. 2024;23:1201-1213
55. Zhang L, Huang S, Yuan Y. Butyrate inhibits the malignant biological behaviors of breast cancer cells by facilitating cuproptosis-associated gene expression. J Cancer Res Clin Oncol. 2024;150(6):287
56. Yang S, Wang X, Zhou X, Hou L, Wu J, Zhang W, Li H, Gao C, Sun C. ncRNA-mediated ceRNA regulatory network: Transcriptomic insights into breast cancer progression and treatment strategies. Biomed Pharmacother. 2023;162:114698
57. Zhu H, Shi J, Li W. Bioinformatics analysis of ceRNA network of autophagy-related genes in pediatric asthma. Medicine. 2023;102(48):e36343
58. Dong N, Li D, Cai H, Shi L, Huang L. Expression of lncRNA MIR193BHG in serum of preeclampsia patients and its clinical significance. J Gynecol Obstet Hum Reprod. 2022;51(5):102357
59. Braga EA, Fridman M V, Moscovtsev AA, Filippova EA, Dmitriev AA, Kushlinskii NE. LncRNAs in Ovarian Cancer Progression, Metastasis, and Main Pathways: ceRNA and Alternative Mechanisms. Int J Mol Sci. 2020;21:1-44
60. Kong Z, Han Q, Zhu B, Wan L, Feng E. Circ_0069094 regulates malignant phenotype and paclitaxel resistance in breast cancer cells via targeting the miR-136-5p/YWHAZ axis. Thorac Cancer. 2023;14:1831-1842
61. Lian W, Yang P, Li L, Chen D, Wang C. A ceRNA network-mediated over-expression of cuproptosis-related gene SLC31A1 correlates with poor prognosis and positive immune infiltration in breast cancer. Front Med (Lausanne). 2023;10:1194046
62. Jiang B, Zhu H, Feng W, Wan Z, Qi X, He R, Xie L, Li Y. Database Mining Detected a Cuproptosis-Related Prognostic Signature and a Related Regulatory Axis in Breast Cancer. Dis Markers. 2022;2022:9004830
63. Wu J, Cheng T, Zhu B, Gao H, Zheng L, Chen W. Identification of cuproptosis-related gene SLC31A1 and upstream LncRNA-miRNA regulatory axis in breast cancer. Sci Rep. 2023;13:18390
64. Deng J, Fu F, Zhang F, Xia Y, Zhou Y. Construct ceRNA Network and Risk Model of Breast Cancer Using Machine Learning Methods under the Mechanism of Cuproptosis. Diagnostics. 2023;13:1203
65. Zhang D, Lu W, Zhuo Z, Wang Y, Zhang W, Zhang M. Comprehensive analysis of a cuproptosis-related ceRNA network implicates a potential endocrine therapy resistance mechanism in ER-positive breast cancer. BMC Med Genomics. 2023;16:96
66. McDonald ES, Clark AS, Tchou J, Zhang P, Freedman GM. Clinical Diagnosis and Management of Breast Cancer. J Nucl Med. 2016;57(Suppl 1):9S-16S
67. Kamal AM, Sakorikar T, Pal UM, Pandya HJ. Engineering Approaches for Breast Cancer Diagnosis: A Review. IEEE Rev Biomed Eng. 2023;16:687-705
68. Wang L. Microwave Imaging and Sensing Techniques for Breast Cancer Detection. Micromachines (Basel). 2023;14:1462
69. Yan S, Wang W, Zhu B, Pan X, Wu X, Tao W. Construction of Nomograms for Predicting Pathological Complete Response and Tumor Shrinkage Size in Breast Cancer. Cancer Manag Res. 2020;12:8313-8323
70. Li J, Wu F, Li C, Sun S, Feng C, Wu H, Chen X, Wang W, Zhang Y, Liu M. et al. The cuproptosis-related signature predicts prognosis and indicates immune microenvironment in breast cancer. Front Genet. 2022;13:977322
71. Shi B, Zhang W, Wang T, Cui Z. The therapeutic and prognostic role of cuproptosis-related genes in triple negative breast cancer. BMC Bioinformatics. 2023;24(1):223
72. Li W, Zhang X, Chen Y, Pang D. Identification of cuproptosis-related patterns and construction of a scoring system for predicting prognosis, tumor microenvironment-infiltration characteristics, and immunotherapy efficacy in breast cancer. Front Oncol. 2022;12:966511
73. Li Z, Zhang H, Wang X, Wang Q, Xue J, Shi Y, Wang M, Wang G, Zhang J. Identification of cuproptosis-related subtypes, characterization of tumor microenvironment infiltration, and development of a prognosis model in breast cancer. Front Immunol. 2022;13:996836
74. Sha S, Si L, Wu X, Chen Y, Xiong H, Xu Y, Liu W, Mei H, Wang T, Li M. Prognostic analysis of cuproptosis-related gene in triple-negative breast cancer. Front Immunol. 2022;13:922780
75. Chen H, Li H, Wang L, Li Y, Yang CY. A 5-gene DNA methylation signature is a promising prognostic biomarker for early-stage cervical cancer. J Obstet Gynaecol. 2022;42:327-332
76. Dang X, Xiong G, Fan C, He Y, Sun G, Wang S, Liu Y, Zhang L, Bao Y, Xu J. et al. Systematic external evaluation of four preoperative risk prediction models for severe postpartum hemorrhage in patients with placenta previa: A multicenter retrospective study. J Gynecol Obstet Hum Reprod. 2022;51(4):102333
77. Pan S, Song C, Meng H, Li N, Li D, Hao B, Lu Z, Geng Q. Identification of cuproptosis-related subtypes in lung adenocarcinoma and its potential significance. Front Pharmacol. 2022;13:934722
78. Mi J, Luo J, Zeng H, Zhang H, Jamil M, Abdel-Maksoud MA, Zakri AM, Alfuraydi AA, Zhang N, Xiao M. Elucidating cuproptosis-related gene SLC31A1 diagnostic and prognostic values in cancer. Am J Transl Res. 2023;15:6026-6041
79. An S, Yao Y, Hu H, Wu J, Li J, Li L, Wu J, Sun M, Deng Z, Zhang Y. et al. PDHA1 hyperacetylation-mediated lactate overproduction promotes sepsis-induced acute kidney injury via Fis1 lactylation. Cell Death Dis. 2023;14:457
80. Deng L, Jiang A, Zeng H, Peng X, Song L. Comprehensive analyses of PDHA1 that serves as a predictive biomarker for immunotherapy response in cancer. Front Pharmacol. 2022;13:947372
81. Huang T, Liu Y, Li J, Shi B, Shan Z, Shi Z, Yang Z. Insights into prognosis and immune infiltration of cuproptosis-related genes in breast cancer. Front Immunol. 2022;13:350
82. Bao JH, Lu WC, Duan H, Ye YQ, Li JB, Liao WT, Li YC, Sun YP. Identification of a novel cuproptosis-related gene signature and integrative analyses in patients with lower-grade gliomas. Front Immunol. 2022;13:933973
83. Shen Y, Li D, Liang Q, Yang M, Pan Y, Li H. Cross-talk between cuproptosis and ferroptosis regulators defines the tumor microenvironment for the prediction of prognosis and therapies in lung adenocarcinoma. Front Immunol. 2023;13:1029092
84. Xia Q, Shen J, Wang Q, Chen R, Zheng X, Yan Q, Du L, Li H, Duan S. Cuproptosis-associated ncRNAs predict breast cancer subtypes. PLoS One. 2024;19(2):e0299138
85. Zhu B, Wang S, Wang R, Wang X. Identification of molecular subtypes and a six-gene risk model related to cuproptosis for triple negative breast cancer. Front Genet. 2022;13:1022236
86. Zheng Q, Shi S, Zhang N, Chen H. A novel cuproptosis-related genes model in breast cancer prognosis. Medicine. 2023;102:e34507
87. Aouad P, Zhang Y, De Martino F, Stibolt C, Ali S, Ambrosini G, Mani SA, Maggs K, Quinn HM, Sflomos G. et al. Epithelial-mesenchymal plasticity determines estrogen receptor positive breast cancer dormancy and epithelial reconversion drives recurrence. Nat Commun. 2022;13(1):4975
88. Hanker AB, Sudhan DR, Arteaga CL. Overcoming Endocrine Resistance in Breast Cancer. Cancer Cell. 2020;37:496-513
89. Fan Y, Luo C, Wang Y, Wang Z, Wang C, Zhong X, Hu K, Wang Y, Lu D, Zheng H. A nomogram based on cuproptosis-related genes predicts 7-year relapse-free survival in patients with estrogen receptor-positive early breast cancer. Front Oncol. 2023;13:1111480
90. Ding D, Wang L, Zhang Y, Shi K, Shen Y. Machine learning developed a programmed cell death signature for predicting prognosis and immunotherapy benefits in lung adenocarcinoma. Transl Oncol. 2023;38:101784
91. Chen L, Yang H, Li H, He C, Yang L, Lv G. Insights into modifiable risk factors of cholelithiasis: A Mendelian randomization study. Hepatology. 2022;75:785-796
92. Wu X, Zhang Y, Liang G, Ye H. Cuproptosis-related lncRNAs potentially predict prognosis and therapy sensitivity of breast cancer. Front Pharmacol. 2023;14:1199883
93. Li F, Yang Y, Zhang X, Yu J, Yu Y. A novel prognostic model of breast cancer based on cuproptosis-related lncRNAs. Discover oncology. 2024;15(1):35
94. Sun L, Chen X, Li F, Liu S. Construction and significance of a breast cancer prognostic model based on cuproptosis-related genotyping and lncRNAs. J Formos Med Assoc. 2024;20:S0929-6646 (24)00243-2
95. Xu QT, Wang ZW, Cai MY, Wei JF, Ding Q. A novel cuproptosis-related prognostic 2-lncRNAs signature in breast cancer. Front Pharmacol. 2023;13:1115608
96. Li C, Zhang Y. Construction and validation of a cuproptosis-related five-lncRNA signature for predicting prognosis, immune response and drug sensitivity in breast cancer. BMC Med Genomics. 2023;16:158
97. Li Y, Na F, Pei J. Construction and characterization of a cuproptosis- and immune checkpoint-based LncRNAs signature for breast cancer risk stratification. Breast Cancer. 2023;30:393-411
98. Yu H, Liu Y, Zhang W, Peng Z, Yu X, Jin F. A signature of cuproptosis-related lncRNAs predicts prognosis and provides basis for future anti-tumor drug development in breast cancer. Transl Cancer Res. 2023;12:1392-1410
99. Guo Q, Qiu P, Pan K, Lin J. Comprehensive analysis of cuproptosis-related long non-coding RNA signature and personalized therapeutic strategy of breast cancer patients. Front Oncol. 2022;12:1081089
100. Jiang ZR, Yang LH, Jin LZ, Yi LM, Bing PP, Zhou J, Yang JS. Identification of novel cuproptosis-related lncRNA signatures to predict the prognosis and immune microenvironment of breast cancer patients. Front Oncol. 2022;12:988680
101. Pan Y, Zhang Q, Zhang H, Kong F. Prognostic and immune microenvironment analysis of cuproptosis-related LncRNAs in breast cancer. Funct Integr Genomics. 2023;23:38
102. Zhang X, Zhang Q. A novel ferroptosis-related gene signature associated with cuproptosis for predicting overall survival in breast cancer patients. Acta Biochim Pol. 2023;70:875-884
103. Wang Y, Wang J, Jiang J, Zhang W, Sun L, Ge Q, Li C, Li X, Li X, Shi S. Identification of cuproptosis-related miRNAs in triple-negative breast cancer and analysis of the miRNA-mRNA regulatory network. Heliyon. 2024;10(7):e28242
104. Liu X, Xu F, Zhao K, Liu Y, Ye G, Zhang X, Qu Y. Comprehending the cuproptosis and cancer-immunity cycle network: delving into the immune landscape and its predictive role in breast cancer immunotherapy responses and clinical endpoints. Front Immunol. 2024;15:1344023
105. Xie J, Yang Y, Gao Y, He J. Cuproptosis: mechanisms and links with cancers. Mol Cancer. 2023;22:46
106. Tong X, Tang R, Xiao M, Xu J, Wang W, Zhang B, Liu J, Yu X, Shi S. Targeting cell death pathways for cancer therapy: recent developments in necroptosis, pyroptosis, ferroptosis, and cuproptosis research. J Hematol Oncol. 2022;15:174
107. Peng F, Liao M, Qin R, Zhu S, Peng C, Fu L, Chen Y, Han B. Regulated cell death (RCD) in cancer: key pathways and targeted therapies. Signal Transduct Target Ther. 2022;7(1):286
108. Wang W, Mo W, Hang Z, Huang Y, Yi H, Sun Z, Lei A. Cuproptosis: Harnessing Transition Metal for Cancer Therapy. ACS Nano. 2023;17:19581-19599
109. Mangion SE, Holmes AM, Roberts MS. Targeted Delivery of Zinc Pyrithione to Skin Epithelia. Int J Mol Sci. 2021;22:9730
110. Liu J, Bai W, Zhou T, Xie Y, Yang B, Sun J, Wang Y, Li X, Hou X, Liu Z. et al. SDCBP promotes pancreatic cancer progression by preventing YAP1 from β-TrCP-mediated proteasomal degradation. Gut. 2023;72:1722-1737
111. Yang X, Deng L, Diao X, Yang S, Zou L, Yang Q, Li J, Nie J, Zhao L, Jiao B. Targeting cuproptosis by zinc pyrithione in triple-negative breast cancer. iScience. 2023;26:108218
112. Duan X, Chan C, Lin W. Nanoparticle-Mediated Immunogenic Cell Death Enables and Potentiates Cancer Immunotherapy. Angewandte Chemie - International Edition. 2019;58:670-680
113. Ye L, Yao Q, Xu F, He L, Ding J, Xiao R, Ding L, Luo B. Preparation and antitumor activity of triphenylphosphine-based mitochondrial targeting polylactic acid nanoparticles loaded with 7-hydroxyl coumarin. J Biomater Appl. 2022;36:1064-1075
114. Zhang J, Wang Y, Bao C, Liu T, Li S, Huang J, Wan Y, Li J. Curcumin-loaded PEG-PDLLA nanoparticles for attenuating palmitate-induced oxidative stress and cardiomyocyte apoptosis through AMPK pathway. Int J Mol Med. 2019;44:672-682
115. Zhang Y, Chen J, Shi L, Ma F. Polymeric nanoparticle-based nanovaccines for cancer immunotherapy. Mater Horiz. 2022;10:361-392
116. Chang J, Yin W, Zhi H, Chen S, Sun J, Zhao Y, Huang L, Xue L, Zhang X, Zhang T. et al. Copper Deposition in Polydopamine Nanostructure to Promote Cuproptosis by Catalytically Inhibiting Copper Exporters of Tumor Cells for Cancer Immunotherapy. Small. 2024;20(27):e2308565
117. Du C, Guo X, Qiu X, Jiang W, Wang X, An H, Wang J, Luo Y, Du Q, Wang R. et al. Self-Reinforced Bimetallic Mito-Jammer for Ca2+ Overload-Mediated Cascade Mitochondrial Damage for Cancer Cuproptosis Sensitization. Adv Sci (Weinh). 2024;11(15):e2306031
118. Xu BB, Jin N, Liu JC, Liao AQ, Lin HY, Qin XY. Arene-Arene Coupled Disulfamethazines (or Sulfadiazine)-Phenanthroline-Metal(II) Complexes were Synthesized by In Situ Reactions and Inhibited the Growth and Development of Triple-Negative Breast Cancer through the Synergistic Effect of Antiangiogenesis, Anti-Inflammation, Pro-Apoptosis, and Cuproptosis. J Med Chem. 2024;67:7088-7111
119. He T, Tang Q, Ren Q, Liu Y, He G, Pan Y, Wang Z, Huang P, Lin J. Different Valence States of Copper Ion Delivery against Triple-Negative Breast Cancer. ACS Nano. 2024;18(7):5434-5445
120. Zhang N, Ping W, Rao K, Zhang Z, Huang R, Zhu D, Li G, Ning S. Biomimetic copper-doped polypyrrole nanoparticles induce glutamine metabolism inhibition to enhance breast cancer cuproptosis and immunotherapy. J Control Release. 2024;371:204-215
121. Lee SY, Seo J, Kim S, Hwang C, Jeong DI, Park J, Yang M, Huh JW, Cho H. Cuproptosis-Inducible Chemotherapeutic/Cascade Catalytic Reactor System for Combating with Breast Cancer. Small. 2023;19:e2301402
122. Ning S, Lyu M, Zhu D, Lam JWY, Huang Q, Zhang T, Tang BZ. Type-I AIE Photosensitizer Loaded Biomimetic System Boosting Cuproptosis to Inhibit Breast Cancer Metastasis and Rechallenge. ACS Nano. 2023;17:10206-10217
123. Li Y, Liu J, Chen Y, Weichselbaum RR, Lin W. Nanoparticles Synergize Ferroptosis and Cuproptosis to Potentiate Cancer Immunotherapy. Adv Sci (Weinh). 2024;11(23):e2310309
124. Huang H, Guo H, Liu J, Ni C, Xia L, Cao X, Xia J, Shi X, Guo R. Dendrimer/metal-phenolic nanocomplexes encapsulating CuO2 for targeted magnetic resonance imaging and enhanced ferroptosis/cuproptosis/chemodynamic therapy by regulating the tumor microenvironment. Acta Biomater. 2024;15:183 252-263
125. Liu WQ, Lin WR, Yan L, Xu WH, Yang J. Copper homeostasis and cuproptosis in cancer immunity and therapy. Immunol Rev. 2024;321:211-227
126. Blockhuys S, Celauro E, Hildesjö C, Feizi A, Stål O, Fierro-González JC, Wittung-Stafshede P. Defining the human copper proteome and analysis of its expression variation in cancers. Metallomics. 2017;9:112-123
127. Yu Z, Zhou R, Zhao Y, Pan Y, Liang H, Zhang JS, Tai S, Jin L, Teng CB. Blockage of SLC31A1-dependent copper absorption increases pancreatic cancer cell autophagy to resist cell death. Cell Prolif. 2019;52(2):e12568
128. van Rensburg MJ, van Rooy M, Bester MJ, Serem JC, Venter C, Oberholzer HM. Oxidative and haemostatic effects of copper, manganese and mercury, alone and in combination at physiologically relevant levels: An ex vivo study. Hum Exp Toxicol. 2019;38:419-433
129. Kim BE, Nevitt T, Thiele DJ. Mechanisms for copper acquisition, distribution and regulation. Nat Chem Biol. 2008;4:176-185
130. Casareno RLB, Waggoner D, Gitlin JD. The copper chaperone CCS directly interacts with copper/zinc superoxide dismutase. J Biol Chem. 1998;273:23625-23628
131. He F, Chang C, Liu B, Li Z, Li H, Cai N, Wang HH. Copper (II) Ions Activate Ligand-Independent Receptor Tyrosine Kinase (RTK) Signaling Pathway. Biomed Res Int. 2019;2019:4158415
132. Ostrakhovitch EA, Lordnejad MR, Schliess F, Sies H, Klotz LO. Copper ions strongly activate the phosphoinositide-3-kinase/Akt pathway independent of the generation of reactive oxygen species. Arch Biochem Biophys. 2002;397:232-239
133. Turski ML, Brady DC, Kim HJ, Kim B-E, Nose Y, Counter CM, Winge DR, Thiele DJ. A novel role for copper in Ras/mitogen-activated protein kinase signaling. Mol Cell Biol. 2012;32:1284-1295
134. Baldari S, Di Rocco G, Heffern MC, Su TA, Chang CJ, Toietta G. Effects of Copper Chelation on BRAFV600E Positive Colon Carcinoma Cells. Cancers (Basel). 2019;11:1-17
135. Wang W, Wang X, Luo J, Chen X, Ma K, He H, Li W, Cui J. Serum Copper Level and the Copper-to-Zinc Ratio Could Be Useful in the Prediction of Lung Cancer and Its Prognosis: A Case-Control Study in Northeast China. Nutr Cancer. 2021;73:1908-1915
136. Sheftel AD, Stehling O, Pierik AJ, Elsässer HP, Mühlenhoff U, Webert H, Hobler A, Hannemann F, Bernhardt R, Lill R. Humans possess two mitochondrial ferredoxins, Fdx1 and Fdx2, with distinct roles in steroidogenesis, heme, and Fe/S cluster biosynthesis. Proc Natl Acad Sci U S A. 2010;107:11775-11780
137. Tsvetkov P, Detappe A, Cai K, Keys HR, Brune Z, Ying W, Thiru P, Reidy M, Kugener G, Rossen J. et al. Mitochondrial metabolism promotes adaptation to proteotoxic stress. Nat Chem Biol. 2019;15:681
138. Zhang C, Zeng Y, Guo X, Shen H, Zhang J, Wang K, Ji M, Huang S. Pan-cancer analyses confirmed the cuproptosis-related gene FDX1 as an immunotherapy predictor and prognostic biomarker. Front Genet. 2022;13:923737
139. Cai Y, He Q, Liu W, Liang Q, Peng B, Li J, Zhang W, Kang F, Hong Q, Yan Y. et al. Comprehensive analysis of the potential cuproptosis-related biomarker LIAS that regulates prognosis and immunotherapy of pan-cancers. Front Oncol. 2022;12:952129
140. Liu H. Pan-cancer profiles of the cuproptosis gene set. Am J Cancer Res. 2022;12:4074
141. Porporato PE, Filigheddu N, Pedro JMBS, Kroemer G, Galluzzi L. Mitochondrial metabolism and cancer. Cell Res. 2018;28:265-280
142. Sriskanthadevan S, Jeyaraju D V, Chung TE, Prabha S, Xu W, Skrtic M, Jhas B, Hurren R, Gronda M, Wang X. et al. AML cells have low spare reserve capacity in their respiratory chain that renders them susceptible to oxidative metabolic stress. Blood. 2015;125:2120-2130
143. Liu SQ, Gao ZJ, Wu J, Zheng HM, Li B, Sun S, Meng XY, Wu Q. Single-cell and spatially resolved analysis uncovers cell heterogeneity of breast cancer. J Hematol Oncol. 2022;15(1):19
144. Taylor BC, Sun X, Gonzalez-Ericsson PI, Sanchez V, Sanders ME, Wescott EC, Opalenik SR, Hanna A, Chou ST, Van Kaer L. et al. NKG2A Is a Therapeutic Vulnerability in Immunotherapy Resistant MHC-I Heterogeneous Triple-Negative Breast Cancer. Cancer Discov. 2024;14:290-307
145. Fan L, Wang ZH, Ma LX, Wu SY, Wu J, Yu K Da, Sui XY, Xu Y, Liu XY, Chen L. et al. Optimising first-line subtyping-based therapy in triple-negative breast cancer (FUTURE-SUPER): a multi-cohort, randomised, phase 2 trial. Lancet Oncol. 2024;25:184-197
146. Amgad M, Hodge JM, Elsebaie MAT, Bodelon C, Puvanesarajah S, Gutman DA, Siziopikou KP, Goldstein JA, Gaudet MM, Teras LR. et al. A population-level digital histologic biomarker for enhanced prognosis of invasive breast cancer. Nat Med. 2024;30:85-97
147. Sirhan Z, Thyagarajan A, Sahu RP. The efficacy of tucatinib-based therapeutic approaches for HER2-positive breast cancer. Mil Med Res. 2022;9(1):39
148. Martínez-Saéz O, Chic N, Pascual T, Adamo B, Vidal M, González-Farré B, Sanfeliu E, Schettini F, Conte B, Brasó-Maristany F. et al. Frequency and spectrum of PIK3CA somatic mutations in breast cancer. Breast Cancer Res. 2020;22(1):45
149. Mosele F, Stefanovska B, Lusque A, Tran Dien A, Garberis I, Droin N, Le Tourneau C, Sablin MP, Lacroix L, Enrico D. et al. Outcome and molecular landscape of patients with PIK3CA-mutated metastatic breast cancer. Ann Oncol. 2020;31:377-386
150. Martelotto LG, Ng CKY, Piscuoglio S, Weigelt B, Reis-Filho JS. Breast cancer intra-tumor heterogeneity. Breast Cancer Res. 2014;16(3):210
151. Wang J, Li S, Guo Y, Zhao C, Chen Y, Ning W, Yang J, Zhang H. Cuproptosis-related gene SLC31A1 expression correlates with the prognosis and tumor immune microenvironment in glioma. Funct Integr Genomics. 2023;23(3):279
152. Tang D, Kroemer G, Kang R. Targeting cuproplasia and cuproptosis in cancer. Nat Rev Clin Oncol. 2024;21:370-388
153. Liang D, Feng Y, Zandkarimi F, Wang H, Zhang Z, Kim J, Cai Y, Gu W, Stockwell BR, Jiang X. Ferroptosis surveillance independent of GPX4 and differentially regulated by sex hormones. Cell. 2023;186:2748-2764.e22
154. Liao M, Qin R, Huang W, Zhu HP, Peng F, Han B, Liu B. Targeting regulated cell death (RCD) with small-molecule compounds in triple-negative breast cancer: a revisited perspective from molecular mechanisms to targeted therapies. J Hematol Oncol. 2022;15(1):44
155. Li J, Li L, Dong Y, Zhong B, Yin W. Comprehensive Analysis of Cuproptosis Genes and Identification of Cuproptosis Subtypes in Breast Cancer. Comb Chem High Throughput Screen. 2023;26:1578-1593
156. Zhang Z, Hu Y, Chen Y, Chen Z, Zhu Y, Chen M, Xia J, Sun Y, Xu W. Immunometabolism in the tumor microenvironment and its related research progress. Front Oncol. 2022;12:1024789
157. Wang X, Zhou M, Liu Y, Si Z. Cope with copper: From copper linked mechanisms to copper-based clinical cancer therapies. Cancer Lett. 2023;561:216157
158. Li N, Sun Q, Yu Z, Gao X, Pan W, Wan X, Tang B. Nuclear-Targeted Photothermal Therapy Prevents Cancer Recurrence with Near-Infrared Triggered Copper Sulfide Nanoparticles. ACS Nano. 2018;12:5197-5206
159. Tang R, Xu J, Zhang B, Liu J, Liang C, Hua J, Meng Q, Yu X, Shi S. Ferroptosis, necroptosis, and pyroptosis in anticancer immunity. J Hematol Oncol. 2020;13(1):110
160. Li J, Liu J, Zhou Z, Wu R, Chen X, Yu C, Stockwell B, Kroemer G, Kang R, Tang D. Tumor-specific GPX4 degradation enhances ferroptosis-initiated antitumor immune response in mouse models of pancreatic cancer. Sci Transl Med. 2023;15(720):eadg3049
Author contact
![]() Corresponding authors: Yunhua Zhou, Ph.D., Department of Wound Repair Surgery, Liyuan Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, 430062, Hubei Province, China. E-mail address: 2042256954com. Qi Zhang, Ph.D., Department of Plastic and Cosmetic Surgery, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, 430030, Hubei, China. E-mail address: zhangqi06172tjmu.edu.cn.
Corresponding authors: Yunhua Zhou, Ph.D., Department of Wound Repair Surgery, Liyuan Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, 430062, Hubei Province, China. E-mail address: 2042256954com. Qi Zhang, Ph.D., Department of Plastic and Cosmetic Surgery, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, 430030, Hubei, China. E-mail address: zhangqi06172tjmu.edu.cn.

 Global reach, higher impact
Global reach, higher impact