Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2025; 21(8):3791-3824. doi:10.7150/ijbs.115242 This issue Cite
Review
Exosomes: a promising microenvironment modulator for spinal cord injury treatment
Department of Spine Surgery, Honghui Hospital, Xi'an Jiaotong University, Xi'an, Youyidong Road, Shaanxi, 710054, China.
# Contribute equally.
Received 2025-4-7; Accepted 2025-5-8; Published 2025-6-5
Abstract
Spinal cord injury (SCI) remains a severely disabling disorder that impacts millions globally by causing irreversible damage to the nervous system. Although cell - based therapies have shown notable progress, the post - injury microenvironment presents significant obstacles that hinder the survival and effectiveness of implanted cells, ultimately limiting sustained functional restoration. Exosomes have emerged as a promising cell - free therapeutic alternative due to their stability, low immunogenicity, and ability to carry bioactive molecules such as proteins, microRNAs, and lipids. These vesicles can modulate the injured microenvironment, support neuroprotection, and facilitate repair. This review begins by discussing the pathological alterations that disrupt the microenvironment following SCI. The review then outlines the process of exosome formation and highlights their structural features. Furthermore, the review delves into the diverse cellular sources of exosomes and evaluates their therapeutic relevance in the context of SCI. Special attention is given to the multifaceted roles exosomes play in neuroprotection, such as reinforcing the blood - spinal cord barrier, stimulating axonal regeneration, promoting new blood vessel formation, suppressing programmed cell death in neurons, and modulating inflammatory responses. The synergistic use of exosomes in combination with biomaterials is also explored, with the aim of optimizing their therapeutic potential. Lastly, the review addresses the key obstacles that must be overcome to bring exosome - based treatments into clinical application and offers perspectives on future advancements in this evolving field. In summary, exosomes offer a novel and promising avenue for SCI intervention, holding considerable promise as an alternative to traditional therapeutic approaches.
Keywords: Spinal Cord Injury, Exosomes, Microenvironment Modulator, Mechanism
Introduction
Spinal cord injury (SCI) is a catastrophic condition of the central nervous system, ranking among the foremost causes of long - term disability and mortality. It involves damage to the spinal cord that leads to the loss of sensory, motor, or autonomic function [1]. SCI can cause partial or complete deficits below the site of injury, resulting in varying levels of impairment [2]. According to estimates by the World Health Organization, between 250,000 and 500,000 new SCI cases occur globally each year [3]. Data from the Global Burden of Disease Study in 2019 reported that approximately 20.6 million individuals were living with SCI. Notably, around 90% of these injuries are caused by external trauma, including traffic collisions, falls, and violent encounters [4]. Initial mechanical insults, also named as primary injury, result in immediate neuronal damage, vascular disruption, and breakdown of the blood - spinal cord barrier (BSCB), which includes contusion, compression, and severance. This is rapidly followed by secondary injury processes, which amplify the damage through glial activation, neuronal and oligodendrocyte apoptosis, and further inflammatory responses. Since primary injury is irreversible, understanding and mitigating the cascade of secondary injury remains the cornerstone of SCI treatment. Nevertheless, there remains no clinically validated method capable of reversing the chronic neurological deficits caused by SCI. The intricacy of post - injury pathophysiology creates a highly detrimental environment that significantly impairs axonal regeneration and limits recovery of function [5]. Consequently, advancing our understanding of SCI mechanisms and developing innovative treatment modalities remains a pressing need.
Over the past decade, cell transplantation has emerged as a promising avenue to facilitate neural repair in SCI. Both neural and mesenchymal cell types have been explored, with mounting evidence indicating their potential to preserve axons, stimulate remyelination, and enhance motor recovery by modifying the pathological microenvironment [6-10]. Despite substantial progress in transplantation techniques, the unfavorable milieu within the damaged spinal cord continues to challenge the survival and integration of grafted cells [11]. To improve outcomes, researchers have investigated the mechanisms by which transplanted cells confer therapeutic benefits. For example, mesenchymal stem cells (MSCs) secrete a range of bioactive molecules, including cytokines and exosomes, that help regulate inflammation and support tissue repair [12-14]. Olfactory ensheathing cells (OECs) are also under investigation due to their capacity to promote axonal regrowth, reduce neuropathic pain, and interact with astrocytes through immunomodulatory and neurotrophic mechanisms. [15, 16].
Mounting evidence indicates that the regenerative effects of transplanted cells may be largely attributed to the exosomes they release. Exosomes are nanoscale vesicles produced by a variety of cells and serve as essential mediators of intercellular signaling [17, 18]. Typically ranging from 30 to 150 nanometers in size, these vesicles are formed within multivesicular endosomes and released into the extracellular space [19]. Enclosed by a lipid bilayer, exosomes safeguard their cargo from enzymatic degradation, which may include proteins, lipids, and nucleic acids. This structural advantage allows them to influence target cells and modulate biological processes effectively [20]. Owing to their inherent biocompatibility, stability, and ability to traverse biological barriers, exosomes are gaining traction as potential therapeutic agents in SCI [21, 22]. Their low immunogenicity makes them suitable for clinical applications, and they can be engineered to deliver therapeutic molecules with high specificity [23, 24]. Consequently, exosomes represent a promising alternative to traditional cell therapy, offering a novel platform for drug delivery and targeted regenerative interventions [25].
In this review, we begin by summarizing the alterations in the spinal cord microenvironment after SCI. Subsequently, we elucidate the origin and biological functions of exosomes. Additionally, we provide a comprehensive overview of the therapeutic applications of exosomes in SCI, highlighting current limitations and discussing future directions for research and therapeutic advancement.
The microenvironment imbalance of SCI
After SCI, a disrupted equilibrium emerges across tissue, cellular, and molecular domains of the microenvironment [26]. At the tissue level, this dysregulation manifests through events such as hemorrhagic damage, reduced blood supply, degradation and partial restoration of myelin, and the development of fibrotic or glial scars [27]. On the cellular scale, pathological responses include the infiltration of immune cells, activation of resident microglia, loss of neurons, and the proliferation of reactive astrocytes [28-30]. At the molecular level, the imbalance is marked by altered expression profiles of neurotrophic factors and their precursors, along with shifts in cytokine and chemokine signaling [31, 32]. Collectively, these changes demonstrate a shift toward the downregulation of protective factors and the upregulation of detrimental factors following SCI.
Tissue imbalance
Tissue imbalance after SCI denotes the disruption of spinal cord homeostasis following injury, primarily involving hemorrhage and ischemia, demyelination and remyelination, and scar formation. (Figure 1).
Hemorrhage and Ischemia
BSCB provides a specialized microenvironment essential for maintaining the homeostasis of the spinal cord parenchyma. When the BSCB is compromised, this environment is disrupted, leading to hemorrhagic and ischemic imbalances [27]. Local microvascular rupture directly exposes spinal cord tissue to blood, which contains various metal ions - particularly iron - that can induce neuronal ferroptosis [33]. Venous stasis and distension can result in the buildup of protein - rich fluid within the tissue, leading to edema. Moreover, neural tissue edema elevates interstitial pressure, compressing adjacent blood vessels and further contributing to ischemia [34]. In addition, rupture of supply vessels leads to cellular hypoxia, which in turn causes cellular edema, thereby exacerbating overall tissue swelling.
Tissue imbalance of microenvironment after SCI, including demyelination and re - myelination, hemorrhage and ischemia, and scar formation. Created with BioRender.com.
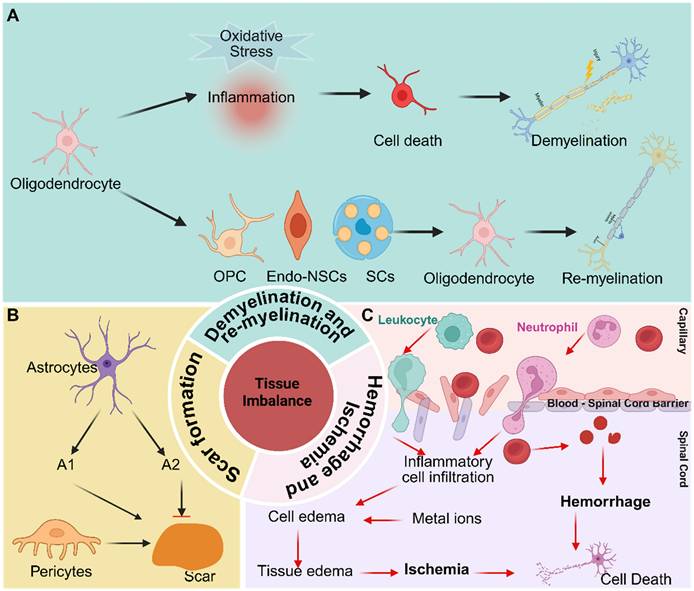
Demyelination and re - myelination
Myelin is essential for maintaining axonal integrity, which in turn facilitates the conduction of axonal signals. Following SCI, oligodendrocyte death results from both direct mechanical damage and an imbalance in the local microenvironment, ultimately leading to demyelination. Potential causes of oligodendrocyte death include mechanical injury, ischemia, pro - inflammatory cytokines, oxidative stress, and excitotoxicity mediated by glutamate and ATP [35, 36]. Mounting evidence suggests that oligodendrocyte death during the acute phase of SCI is primarily mediated through apoptosis and necrosis. Apoptosis can occur as early as 6 hours after SCI in rats and may persist for up to 3 weeks in monkeys, contributing significantly to axonal demyelination [37]. Necrosis, which typically occurs within the first 24 hours post - injury, triggers inflammatory responses [38]. Additionally, several studies have shown that autophagy is induced in oligodendrocytes after SCI [39, 40]. However, autophagy does not appear to be a significant contributor to oligodendrocyte loss in this context.
Conversely, remyelination naturally occurs after SCI, primarily through the replacement of oligodendrocytes. These newly formed oligodendrocytes originate from three principal sources: oligodendrocyte progenitor cells (OPCs), Schwann cells (SCs), and endogenous neural stem cells (Endo - NSCs). Studies have shown that OPCs in the injury core decrease within the first 24 hours post - injury; however, they are subsequently activated, rapidly proliferate, migrate, and differentiate into mature oligodendrocytes [26, 30, 41-43]. Among them, OPCs are the primary source of oligodendrocytes in the injured spinal cord site. Nevertheless, myelin restoration is often incomplete following SCI. Microenvironmental disturbances which includes myelin debris, activation of the MBP signaling pathway, and the presence of pro - inflammatory factors significantly inhibit the differentiation of OPCs and the maturation of new oligodendrocytes. Numerous studies have reported the presence of SCs after SCI. Some indicate that these SCs are primarily derived from nerve roots, with only a small fraction (< 10%) originating from peripheral nerves. Approximately 70% - 80% of SCs, however, arise from resident OPCs, regardless of recombination efficiency [43]. Despite this, the precise functions and mechanisms of newly generated SCs remain to be fully elucidated. In adult mammals, Endo - NSCs are primarily located in the subventricular zone, the hippocampal subgranular zone, and the ependymal layer of the spinal cord [44, 45]. These NSCs have the capacity for proliferation, self - renewal, and differentiation into various cell types. Although Endo - NSCs remain quiescent under physiological conditions, they become activated in response to pathological stimuli, such as SCI. However, their activation is limited, and they predominantly differentiate into astrocytes, and to a lesser extent, oligodendrocytes, rather than neurons [46]. Notably, Llorens - Bobadilla et al. identified a latent lineage of Endo - NSCs capable of contributing to oligodendrocyte replacement [44].
Scar formation
Scar formation is a crucial aspect of the pathology of SCI, persisting throughout the entire pathophysiological process. During the acute phase, astrocytes polarize into distinct A1 and A2 phenotypes, each associated with specific roles in the injury response [47]. Concurrently, pericytes derived from blood vessels migrate toward the injury epicenter [48, 49]. As the injury progresses into the subacute phase, a scar begins to form, comprising astrocytes derived from resident astrocytes, OPCs, and NSCs. Fibroblast - derived pericytes are essential for scar consolidation during this stage [50]. In the chronic phase, the scar stabilizes, effectively limiting inflammation but concurrently inhibiting axonal regeneration [51, 52]. The scar consists of both fibrous and glial components. The fibrous portion contains stromal cells at the scar's core, derived from vascular - associated type A pericytes, while the glial portion comprises astrocytes originating from self - replicating astrocytes and endogenous NSCs. Astrocytes derived from endogenous NSCs contribute to strengthening the glial scar. Additionally, the scar includes microglia, macrophages, and an extracellular matrix, with the latter primarily consisting of chondroitin sulfate proteoglycans (CSPGs), which are known to inhibit axonal regeneration [53]. While the scar functions as a physical and molecular barrier to restrict the spread of inflammation, its excessive formation significantly impedes axonal outgrowth and functional recovery.
Cellular imbalance
Cellular imbalance after SCI denotes the disturbance of the spinal cord's cellular homeostasis following injury, characterized by the activation of macrophages and microglia, reactive astrogliosis and proliferation, and neuronal loss (Figure 2).
Cellular imbalance of microenvironment after SCI, including neural death, activation of macrophages and microglia, and astrocyte reaction and proliferation. Created with BioRender.com.
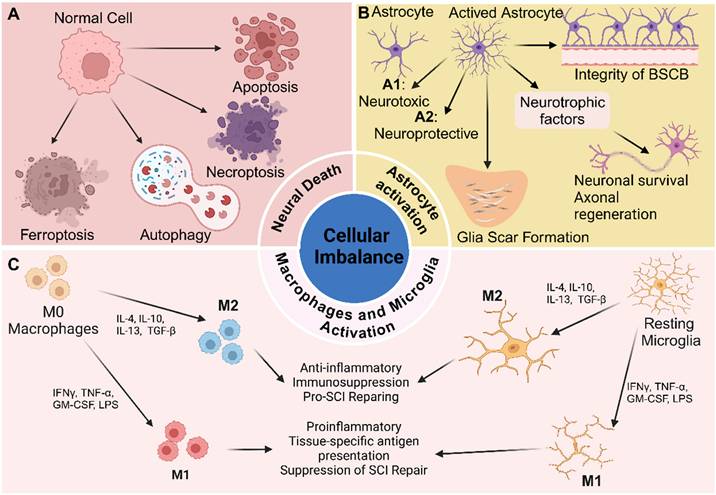
Activation of macrophages and microglia
The infiltration of inflammatory cells following SCI is a key contributor to secondary injury mechanisms, intensifying the initial trauma and impeding recovery. This inflammatory response is characterized by the recruitment of various immune cells, including neutrophils, macrophages, lymphocytes, and microglia, each with distinct roles in mediating both tissue damage and repair [54]. Among these cells, macrophages and microglia have garnered particular attention due to their prominent roles in orchestrating both injury progression and regenerative processes (Figure 2).
As previously mentioned, disruption of the BSCB following SCI permits peripheral circulating blood to enter the lesion site. Macrophages subsequently infiltrate the area in response to this breach [55]. Stimulated by microglia, macrophages from the peripheral circulation begin to accumulate in the injured region approximately 2 - 3 days post - injury. Their numbers peak between 7 and 10 days after SCI and can persist in the lesion for up to 42 days. These cells play a critical role in clearing cellular debris and promoting tissue repair by releasing growth factors and cytokines that support regenerative processes [56]. However, dysregulation of macrophage activity, particularly prolonged pro - inflammatory responses, can exacerbate tissue damage and impair recovery, highlighting their dual role in both repair and secondary injury following SCI. [57].
Microglia are the resident immune cells of the CNS, acting as primary immune sentinels in the brain and spinal cord. Under normal physiological conditions, microglia remain relatively quiescent, but are rapidly activated and play central roles in the immune response following SCI [58]. Stimulated by cytokines and other signaling molecules, such as IL - 1β, TNF - α, and growth factors secreted by astrocytes and other injured cells, microglia become activated, resulting in enhanced microglial proliferation. The number of activated microglia rises sharply within the first day post - injury, continues to increase over the subsequent week, and plateaus between two to four weeks [59]. This sustained activation is essential for clearing cellular debris and damaged cells, thereby contributing to tissue repair. Additionally, microglial activation plays a protective role by limiting the expansion of the lesion site. However, excessive or chronic activation can exacerbate inflammation and secondary injury, underscoring their dual role in mediating both tissue damage and repair.
The polarization of macrophages and microglia is currently an area of intense research interest in the context of SCI. These immune cells can adopt two major polarization states: M1 and M2. The balance between M1 and M2 macrophages/microglia is essential for regulating immune homeostasis [60, 61]. To some extent, the ratio of M1 to M2 subtypes influences the balance of the local microenvironment [62]. M1 macrophages/microglia are the dominant subtype during the acute phase of SCI. They are recruited to the injury site within the first few days and are activated by signals from damaged tissue and pro - inflammatory cytokines, thereby exacerbating neuroinflammation and neuronal injury. Prolonged dominance of M1 macrophages/microglia ultimately impedes SCI recovery [59]. In contrast, M2 macrophages/microglia typically emerge later in the healing process and are associated with tissue repair and the resolution of inflammation. M2 macrophages are anti - inflammatory in nature and contribute to tissue regeneration by producing high levels of IL - 10 and TGF - β, showing impaired activation of the NF - κB pathway, upregulating arginase 1, and downregulating pro - inflammatory cytokines [63-66]. Similarly, M2 - polarized microglia promote neuroprotection and recovery following CNS injury by releasing anti - inflammatory cytokines and neurotrophic factors that support neuronal survival, remyelination, and synaptic plasticity [67].
In summary, developing therapeutic strategies to promote tissue repair and mitigate the adverse effects of chronic inflammation requires a thorough understanding of the mechanisms underlying macrophage/microglial polarization and the dynamic balance between M1 and M2 states. Harnessing the beneficial effects of M2 polarization may enhance recovery and improve outcomes across various pathological conditions.
Neuronal death
Neurons are essential components of the spinal cord, and neuronal loss is a primary contributor to the poor functional recovery observed after SCI. Accumulating evidence indicates that the mechanisms underlying neuronal loss include apoptosis, necroptosis, autophagy, and ferroptosis [68]. Necrosis, in contrast to these programmed forms of cell death, is a passive and uncontrolled process that occurs in a non - programmed manner. During the primary injury phase, direct mechanical trauma causes neurons at the injury site to undergo necrosis [69]. In the subsequent secondary injury phase, necrotic cells induce several pathogenic changes, including swelling and deformation of surrounding neurons, altered membrane permeability, and ultimately rupture - a process consistent with necrosis [70]. The release of intracellular contents following necrosis triggers inflammatory responses, resulting in widespread neuronal necrosis during the subacute phase [71]. As necrosis exacerbates pathological changes, it perpetuates the cycle of neuronal damage. Furthermore, substances released from necrotic cells, such as cytokines and damage - associated molecular patterns (DAMPs), may serve as signals that initiate additional forms of cell death [72].
Apoptosis is a coordinated, energy - dependent, and genetically regulated form of cell death in which caspases serve as the primary executioners. It is a prominent feature of neural tissue following SCI, occurring in nearly all major neural cell types, including neurons, astrocytes, oligodendrocytes, and microglia [73]. During the weeks - long period of neuronal apoptosis post - injury, glial cells undergo apoptosis to a greater extent than neurons. The mechanisms underlying neuronal apoptosis involve three major pathways: the extrinsic pathway, activated by the binding of ligands to death receptors in the tumor necrosis factor (TNF) receptor family (including TNF receptor 1, Fas, Fas ligand, p75, and DR3); the intrinsic, or mitochondrial, pathway; and the endoplasmic reticulum (ER) stress pathway [74-76].
Autophagy is a cellular process responsible for degrading and recycling damaged organelles, misfolded proteins, and other intracellular components. This process involves initiation, autophagosome formation, fusion with lysosomes, and subsequent degradation [77, 78]. Following SCI, the ratio of LC3 - II to LC3 - I, a commonly used marker for autophagy, rises significantly at 3 days post - injury, peaks at 7 days, and then declines sharply by day 21. However, the role of autophagy in traumatic SCI remains controversial [73]. It is widely acknowledged that autophagy plays a dual role: it can promote cell survival by clearing damaged cellular components, yet excessive autophagy may contribute to cell death. Modulating autophagy represents a promising therapeutic strategy for reducing secondary damage and enhancing functional recovery in SCI [79].
Ferroptosis, first identified in 2012 as an iron - dependent, non - apoptotic form of cell death, plays a critical role in neuronal loss following SCI [80]. It is characterized by iron accumulation and lipid peroxidation, and is distinctly dependent on ROS [81]. After SCI, ferroptosis emerges as a key pathological process, marked by elevated iron levels, ROS buildup, and excessive lipid peroxidation at the injury site. Studies using adult mouse models have demonstrated that conditional ablation of GPX4 in neurons can induce motor neuron degeneration, underscoring the pivotal role of ferroptosis in neuronal cell death [82]. Notably, excitotoxicity often precedes the onset of ferroptosis, triggered by glutamate accumulation and the failure of astrocyte - mediated glutamate reuptake. Together, these processes contribute significantly to secondary injury after SCI [83]. Despite these insights, the precise mechanisms and broader implications of ferroptosis in SCI remain incompletely understood. Therefore, further investigation is required to elucidate its role in neuronal degeneration and identify potential therapeutic targets.
Astrocyte reaction and proliferation
Astrocytes, star - shaped glial cells in the CNS, perform a range of essential functions. These include maintaining the integrity of the blood - brain barrier, regulating neurotransmitter levels, providing metabolic support to neurons, and responding to injury [84, 85]. Astrocytes play a pivotal role in maintaining CNS homeostasis and are vital for neuronal function and survival. They supply neurons with energy and neurotransmitters and serve as structural barriers between synapses of adjacent neurons [86].
Following SCI, astrocytes undergo a process termed reactive astrogliosis, characterized by both morphological and functional changes [87]. Multiple factors can activate astrocytes, including the release of DAMPs from neurons and glial cells, pro - inflammatory cytokines from activated microglia and infiltrating immune cells, extracellular matrix remodeling, and ionic imbalances. Activated astrocytes can exert neuroprotective effects by secreting neurotrophic factors such as brain - derived neurotrophic factor (BDNF) and nerve growth factor (NGF), which support neuronal survival and promote axonal regeneration. They also contribute to restoring the integrity of the blood - spinal cord barrier after injury. Moreover, by regulating ion and neurotransmitter levels, astrocytes help reestablish homeostasis in the injured spinal cord - an essential aspect of recovery [88, 89]. However, while reactive astrogliosis is part of the reparative process, it leads to the formation of a glial scar. This scar can inhibit axonal regeneration by forming a physical barrier and secreting inhibitory extracellular matrix components, such as chondroitin sulfate proteoglycans, which restrict neuronal growth and limit functional recovery [90]. In the context of inflammation, astrocytes exhibit a dual role: they can modulate the immune response by releasing anti - inflammatory cytokines, yet excessive activation may foster a pro - inflammatory environment that exacerbates secondary injury.
In summary, astrocytes undergo substantial changes in response to SCI, which may exert both protective and detrimental effects. To develop effective therapeutic strategies that enhance recovery and mitigate the adverse consequences of reactive astrogliosis, a comprehensive understanding of astrocyte function in SCI is essential. To improve functional outcomes, it is crucial to balance astrocyte - mediated neuroprotection with the inhibitory effects of glial scar formation.
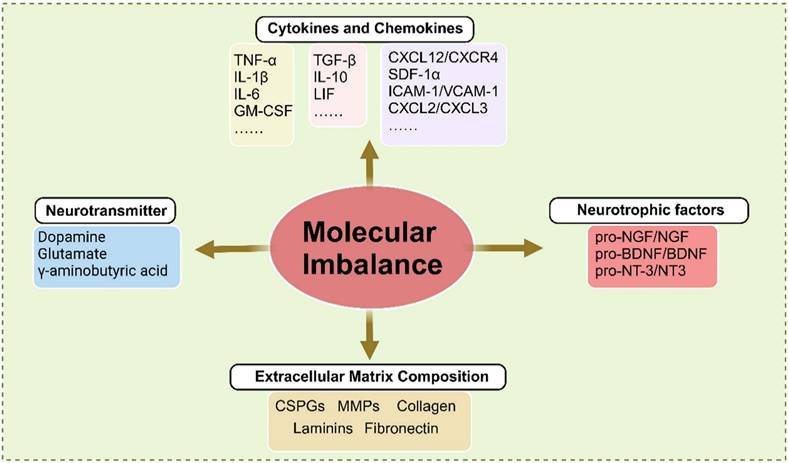
Molecular imbalance
Molecular imbalance plays a critical role in the pathophysiology of SCI, influencing inflammation, cell death, and regeneration. Following SCI, key molecular alterations include an imbalance in inflammatory mediators and chemokines, altered neurotransmitter levels, dysregulation of neurotrophic factors, and remodeling of ECM components (Figure 3).
Inflammatory mediators and chemokines imbalance
After SCI, activated microglia, macrophages, and astrocytes secrete inflammatory mediators such as TNF - α, IL - 1β, and IL - 6, which increase rapidly. Inflammation functions as a double - edged sword: while appropriate inflammatory responses protect neural tissue and help contain damage, excessive and prolonged release of pro - inflammatory cytokines can exacerbate neuronal injury. These cytokines promote neuronal apoptosis and sustain inflammation, thereby impeding recovery [91]. In contrast, anti - inflammatory cytokines such as IL - 10 and TGF - β are essential for resolving inflammation and facilitating tissue repair. Specifically, IL - 10 inhibits the production of pro - inflammatory cytokines, creating a more favorable environment for recovery [92]. Research has shown that increasing the expression of anti - inflammatory cytokines may enhance functional outcomes following SCI.
Concurrently, chemokines such as C - C motif ligand 2 (CCL2) and C - X - C motif ligand 1 (CXCL1) are upregulated following SCI. This upregulation attracts immune cells, including neutrophils and macrophages, to the injury site, which subsequently infiltrate the disrupted BSCB [93]. Research has indicated that different chemokines exhibit distinct spatial and temporal expression profiles after SCI. For instance, CXCL2 and CXCL3 are rapidly upregulated within hours of injury, promoting the recruitment of monocytes and T cells to the damaged area [93, 94]. Initially, the influx of immune cells is beneficial, as it facilitates debris clearance and the secretion of neurotrophic factors. However, the prolonged presence of inflammatory cells can lead to secondary damage, underscoring the dual role of chemokines in SCI pathophysiology.
Molecular imbalance of microenvironment after SCI, including cytokines and chemokine, neurotrophic factor, neurotransmitter, and extracellular matrix composition. TNF - α, Tumor necrosis factor - α. IL, Interleukin. GM - CSF, Granulocyte - macrophage colony stimulating factor. ILF, Leukocyte inhibitory factor. CXCL, C - X - C motif ligand. CXCR, C - X - C chemokine receptor. SDF - 1α, stromal cell - derived factor 1α. ICAM - 1, Intercellular adhesion molecule - 1. VCAM - 1, Vascular cell adhesion protein - 1. NGF, Nerve growth factor. Brain - derived neurotrophic factor. NT - 3, Neurotrophin - 3. CSPGs, Chondroitin sulfate proteoglycans. MMPs, Matrix metalloproteinases. Created with BioRender.com.
Furthermore, specific chemokines such as CXCL12 are involved in promoting neuronal survival and regeneration. CXCL12 has been shown to enhance the migration of neural precursor cells, indicating its therapeutic potential for facilitating repair [95]. In contrast, excessive expression of pro - inflammatory chemokines can exacerbate tissue damage and hinder recovery. Consequently, maintaining a balance between the protective and detrimental effects of chemokines is critical in determining the overall outcome of SCI. Understanding the roles of chemokines and cytokines in SCI offers promising avenues for therapeutic intervention. By targeting specific cytokines or chemokines, or their associated signaling pathways, it is possible to modulate the inflammatory response, reduce secondary damage, and promote functional recovery. Therapeutic strategies such as neutralizing antibodies and small - molecule inhibitors have demonstrated potential in preclinical studies, showing promise in enhancing functional recovery in individuals with SCI.
Neurotransmitter levels change
Neurotransmitters are chemical messengers that facilitate signal transmission across synapses, enabling communication between neurons or from neurons to other target cells, such as muscles and glands. These molecules play a critical role in spinal cord function by mediating interactions between neural circuits and peripheral tissues, including muscles [96]. When an action potential reaches the presynaptic terminal of a neuron, it triggers the release of neurotransmitters into the synaptic cleft. These neurotransmitters then bind to specific receptors on the postsynaptic membrane, inducing excitatory or inhibitory responses that regulate postsynaptic cellular activity. This precise regulation of synaptic transmission is essential for coordinating motor and sensory functions and for maintaining homeostasis within the nervous system [97, 98].
Following SCI, neurotransmitter levels undergo significant changes, influencing both the immediate response to injury and long - term recovery. Glutamate, a major excitatory neurotransmitter essential for synaptic plasticity and cognitive processes such as learning and memory, is excessively released after SCI [96]. Elevated glutamate levels can induce excitotoxicity, whereby overstimulation of glutamate receptors, particularly NMDA receptors, leads to neuronal injury and death, contributing to secondary damage and worsening injury to surrounding tissues. GABA, the primary inhibitory neurotransmitter, may also be dysregulated due to the disrupted balance between excitatory and inhibitory signaling following SCI [99]. This imbalance, often manifested as reduced GABAergic signaling, increases spinal neuron excitability and contributes to neuropathic pain and hyperreflexia. Ultimately, this disruption complicates recovery and may lead to chronic pain syndromes.
In conclusion, neurotransmitters are essential chemical messengers that regulate numerous physiological processes and play a central role in neural communication. Maintaining their proper balance and function is critical for preserving synaptic integrity and neural network stability. Therefore, understanding neurotransmitter alterations following spinal cord injury is crucial for developing targeted therapeutic strategies aimed at mitigating their detrimental effects and facilitating functional recovery.
Alteration of neurotrophic factors
Neurotrophic factors, also known as neurotrophins, constitute a family of proteins that play critical roles in the growth, survival, development, and maintenance of neurons within the nervous system. Members include NGF, BDNF, GDNF, neurotrophin - 3 (NT - 3), and neurotrophin - 4/5 (NT - 4/5). These proteins bind to tropomyosin - related kinase (Trk) receptors and the low - affinity neurotrophin receptor p75. By promoting synaptic plasticity, regeneration, and repair, they support overall neuronal health [100, 101]. During development, neurotrophic factors are essential for guiding neural cells and ensuring the proper formation of neural circuits.
Neurotrophic factors (NFs) play a crucial role in promoting the survival and proliferation of various neural cell types, as well as supporting axonal regeneration following SCI. Enhancing neurotrophic factor signaling can facilitate neuronal differentiation, survival, axonal growth, and synaptic plasticity in the context of SCI treatment [100-103]. However, NFs are initially synthesized as precursors, some of which function as distinct ligands that induce cell death. Multiple studies have shown that the pro - neurotrophin forms of NGF, BDNF, and NT - 3 are present after SCI and contribute to neural cell death. This results in a relative deficiency of mature NFs, thereby disrupting the balance of the neurotrophic microenvironment. For example, pro - NGF has been shown to induce oligodendrocyte death by activating the p75 receptor, which compromises myelin sheath integrity [104]. Additionally, pro - BDNF increases within 1 to 3 days after SCI and suppresses macrophage migration and infiltration [105]. Several studies have demonstrated that reducing pro - neurotrophin levels improves outcomes in SCI models, providing strong evidence of their detrimental role in the injury response.
Extracellular Matrix Changes
ECM of the spinal cord is a complex macromolecular network composed of proteins, glycoproteins, and polysaccharides. It provides structural support and regulates various cellular activities [106]. Among its components, collagens are the most abundant structural proteins, forming the backbone of the ECM framework and conferring tensile strength. CSPGs, the primary proteoglycans in spinal cord tissue, modulate cell adhesion, migration, and axonal growth and are key inhibitors of regeneration after injury [107]. As representative glycoproteins, laminin and fibronectin support cell attachment and migration. Additionally, elastin and matrix metalloproteinases (MMPs) are critical ECM components. Elastin contributes to maintaining tissue elasticity and flexibility, while MMPs facilitate the degradation and remodeling of ECM constituents [108]. The ECM serves multiple essential functions in the spinal cord, including providing mechanical support, regulating cell - cell and cell - matrix interactions, guiding neural development, and maintaining tissue homeostasis. It acts as a scaffold for cellular organization and influences axon guidance, synaptic plasticity, and neuronal survival.
Following SCI, significant alterations occur in the composition of the ECM, influencing both the acute damage response and long - term recovery. Reactive astrocytes become the primary source of CSPGs, whose levels increase substantially, particularly in the perilesional region. In adult mammals, CSPGs signal through two major receptor protein tyrosine phosphatases (RPTPs): protein tyrosine phosphatase sigma (PTPσ) and the leukocyte antigen - related (LAR) subfamily [109]. Through these receptors, CSPGs inhibit axonal regeneration by activating the RhoA/ROCK and PKC signaling pathways [110]. Moreover, CSPGs interfere with autophagy by suppressing autophagosome - lysosome fusion, thereby impairing autophagic regulation at the axonal growth cone. Inhibition of CSPG - PTPσ signaling has been shown to restore autophagic flux, promote axonal and synaptic reorganization, preserve remaining motor neurons, and improve functional recovery after SCI [111]. CSPGs also disrupt immune modulation by blocking the Toll - like receptor 4 (TLR4) - dependent transition of immune cells from a pro - inflammatory to a pro - repair phenotype, highlighting another mechanism of secondary injury [112]. In parallel, collagen levels also undergo dynamic changes following SCI. In the early phase, increased activity of MMPs accelerates collagen degradation, contributing to tissue breakdown and exacerbating inflammation. This degradation compromises spinal cord structural integrity and impairs the ability of axons to regenerate. During the chronic phase, collagen accumulates as part of the glial scar, forming a physical barrier that inhibits axonal regrowth.
Molecular imbalance following SCI arises from the complex interplay among inflammatory mediators, altered neurotransmitter levels, and changes in growth factors and ECM components. Understanding these molecular changes provides critical insights into the mechanisms underlying secondary injury and recovery. Targeting these molecular pathways may provide novel therapeutic opportunities to enhance recovery, promote neuronal regeneration, and improve functional outcomes after SCI.
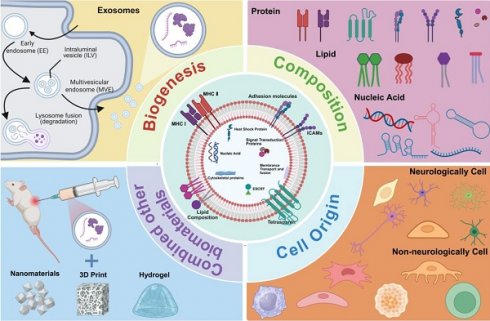
History, biogenesis and structure of exosomes
Exosomes are small extracellular vesicles secreted by numerous cell types, playing a key role in mediating intercellular communication. These vesicles typically range from 30 - 150 nanometers in diameter. They are released into the extracellular space and can be detected in various biological fluids, such as blood, urine, and saliva [19, 113].
History of exosomes - based SCI therapies
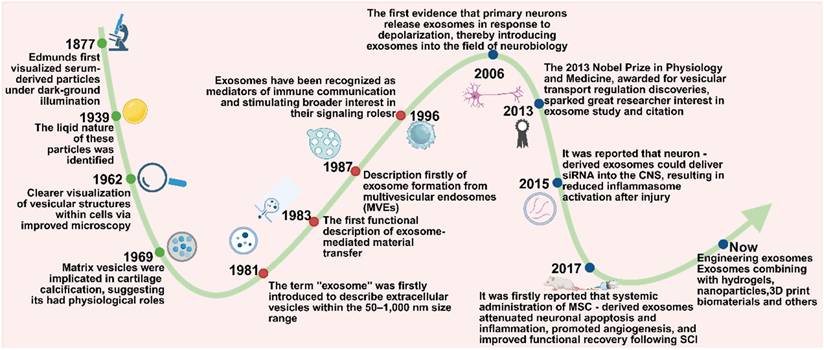
Exosomes, a subtype of extracellular vesicles (EVs), have emerged as key mediators of intercellular communication. However, their recognition and characterization have evolved gradually over time. The earliest observations of extracellular particles date back to 1877, when Edmunds first identified serum-derived particles using dark-field illumination. These particles were later identified as lipid - rich structures in 1939, yet were initially dismissed as “blood dust” due to the lack of functional understanding. By 1962, advancements in microscopy enabled clearer visualization of vesicular structures within cells, though their physiological significance remained speculative [114].
A pivotal shift occurred in 1969 when matrix vesicles were implicated in cartilage calcification, indicating that extracellular vesicles might play active physiological roles [115]. The term exosome was introduced in 1981 to describe vesicles measuring 50 - 1,000 nm in diameter [116]. In 1983, independent studies by Stahl and Johnstone demonstrated that vesicles released by maturing reticulocytes could fuse with the plasma membrane and discharge their cargo via exocytosis, marking the first functional insight into exosome - mediated molecular transfer [117]. This finding was further supported by electron microscopy in 1985, and in 1987, the biogenesis of exosomes via inward budding of multivesicular endosomes (MVEs) was formally described [118]. Nevertheless, their biological functions remained largely undefined until 1996, when Raposo et al. showed that B cell - derived exosomes enriched in MHC class II molecules could activate T cells, thereby establishing exosomes as mediators of immune communication and stimulating broader interest in their signaling roles [119]. In subsequent years, a variety of mammalian cells were found to secrete exosomes into the extracellular milieu [120]. A notable breakthrough came in 2007, when Valadi et al. revealed that exosomes derived from human and murine mast cells carry abundant mRNAs and microRNAs, which can be transferred to recipient cells and translated into functional proteins - further solidifying the role of exosomes in horizontal gene regulation and intercellular signaling [121].
Since the early 21st century, exosomes have garnered increasing attention as promising therapeutic tools for neurological diseases. This interest has been fueled by growing evidence of their roles in immunoregulation and tumor biology, leading researchers to investigate their relevance in the nervous system. In 2006, Fauré et al. provided the first evidence that primary neurons release exosomes in response to depolarization, thereby introducing exosomes into the field of neurobiology [122]. The 2013 Nobel Prize in Physiology and Medicine, awarded for discoveries in vesicular transport regulation, further underscored the foundational relevance of vesicle biology to exosome research, which has generated great interest among researchers in the study and citation of exosomes [123]. Subsequent studies demonstrated that exosomes released from activated glutamatergic synapses preferentially bind to neurons, supporting the concept of exosome-mediated interneuronal communication [124]. In 2015, it was reported that neuron - derived exosomes could deliver small interfering RNA (siRNA) into the CNS, resulting in reduced inflammasome activation after injury [125]. In 2017, Huang et al. demonstrated for the first time that systemic administration of MSC - derived exosomes attenuated neuronal apoptosis and inflammation, promoted angiogenesis, and improved functional recovery following SCI [126].
Since then, the application of exosomes in SCI has progressed considerably. Recent research has focused on engineering exosomes and combining them with biomaterials - such as hydrogels, nanoparticles, and 3D print biomaterials - to enhance targeted delivery, stability, and therapeutic efficacy in spinal cord repair. Over the past decade, exosome - based therapy has rapidly gained momentum. Exosomes are now recognized for their multifaceted roles in reinforcing the BSCB, promoting axonal regeneration, facilitating angiogenesis, mitigating neuronal apoptosis, and modulating immune responses. Owing to their intrinsic cargo of nucleic acids and regulatory proteins, exosomes have become promising candidates for clinical translation, serving as bioactive vehicles for therapeutic interventions in SCI (Figure 4).
Biogenesis of exosomes
Exosomes biogenesis is tightly regulated at the cellular level and generally consists of three key steps: the formation of early endosomes, maturation into multivesicular bodies (MVBs), and the eventual release of exosomes [127]. In the initial step, the plasma membrane invaginates to form a cup - shaped structure that encloses soluble extracellular proteins and membrane - bound proteins, resulting in the formation of an early - sorting endosome (ESE) [128]. The endoplasmic reticulum and trans - Golgi network may also contribute to the development and molecular composition of the ESE. Subsequently, ESEs mature into late - sorting endosomes (LSEs), which then develop into MVBs, also referred to as multivesicular endosomes. MVBs arise through the inward budding of the endosomal limiting membrane, resulting in the formation of intraluminal vesicles (ILVs), the precursors of exosomes. MVBs may either fuse with the plasma membrane to release ILVs as exosomes or fuse with lysosomes or autophagosomes for degradation [129]. This tightly controlled process is regulated by a variety of molecules, including endosomal sorting complexes required for transport ESCRT), ALG - 2 - interacting protein X (Alix), tumor susceptibility gene 101 (TSG101), Rab GTPases, tetraspanins, and heat shock proteins (HSPs), among others [130].
Upon release, exosomes can spread to adjacent tissues and organs or enter the systemic circulation, thereby reaching distant sites within the body. Recipient cells primarily internalize exosomes via three mechanisms: endocytosis, direct fusion with the plasma membrane, and ligand - receptor interactions [131]. It is hypothesized that once internalized, exosomes undergo back - fusion with the membranes of MVBs, releasing their functional cargo, such as RNAs, DNAs, and proteins, into the cytoplasm of recipient cells [20]. This cargo can subsequently regulate cellular processes, enabling exosomes to play a critical role in modulating physiological and pathological activities throughout the body (Figure 5).
A timeline of landmark studies on exosome-based SCI therapies. Created with BioRender.com. Created with BioRender.com.
The biogenesis, structure and composition of exosomes. Created with BioRender.com.
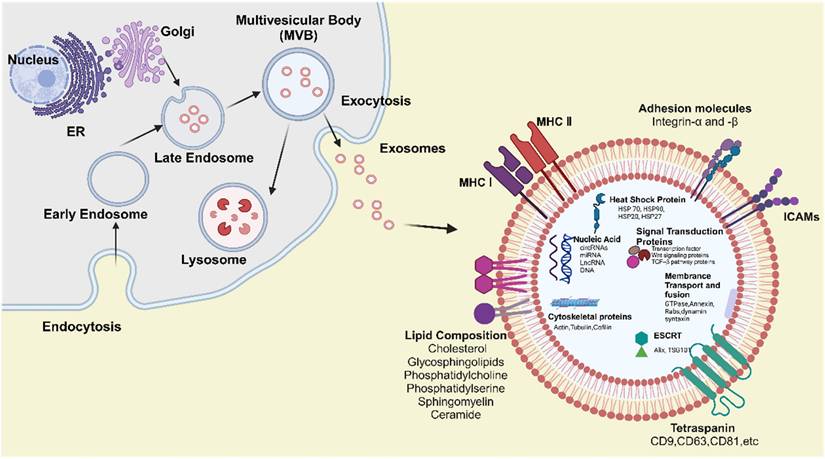
Structure and composition of exosomes
Exosomes are small extracellular vesicles enclosed by a lipid bilayer membrane and are derived from the endosomal membrane during their formation. They are enriched with specific proteins, lipids, enzymes, and nucleic acids. Notably, the composition and biological function of exosomes are highly dynamic and influenced by the cellular origin, physiological status, and external stimuli such as hypoxia or oxidative stress, leading to significant heterogeneity among exosomal populations [19, 132]. These components enable exosomes to contribute to intercellular communication by mediating the transfer of signaling molecules to both local and distant target cells (Figure 5).
Lipid composition
The lipid composition of exosomes differs from that of the plasma membrane of the parent cell, partly due to their enrichment with lipids originating from Golgi and endosomal membranes. These include cholesterol, glycosphingolipids, phosphatidylcholine, phosphatidylserine, sphingomyelin, and ceramide [133, 134]. These lipids contribute to the distinct rigidity of exosomes and play crucial roles in their biogenesis, structural integrity, and biological function. Phosphatidylserine is typically located on the inner leaflet of the exosome membrane; however, it may become externalized during exosome release. This externalization facilitates recognition and binding to recipient cells via specific receptors [135]. Phosphatidylcholine, a major constituent of the exosomal membrane, contributes to maintaining membrane fluidity and bilayer stability [136]. Ceramide is essential for exosome biogenesis, particularly in the formation of ILVs within MVBs, by promoting membrane curvature and vesicle budding through ESCRT - independent mechanisms [137]. Sphingomyelin and glycosphingolipids function as bioactive signaling molecules, capable of modulating cell survival, apoptosis, and immune responses, thereby influencing the behavior of both donor and recipient cells [138].
Proteins composition
Exosomes contain a variety of proteins that are essential for their structure, biogenesis, and functions - including intercellular communication. These proteins are generally classified into membrane - associated proteins and internal (cytosolic) proteins. Membrane - associated proteins can be further categorized into four major groups: tetraspanins, transport and fusion proteins, adhesion molecules, and heat shock proteins (HSPs) [139-141]. Tetraspanins, such as CD9, CD63, and CD81, are highly expressed on exosomes derived from most cell types. They are involved in exosome formation, membrane organization, and cell targeting [142]. Interestingly, emerging evidence indicates that external conditions, such as hypoxia, can selectively enrich specific sets of tetraspanins and adhesion molecules in exosomes, thereby enhancing their targeting efficiency and altering their uptake by recipient cells [132, 143]. Moreover, tetraspanins promote exosome-cell interactions and play essential roles in the binding and uptake of exosomes by recipient cells via ESCRT - independent pathways. These proteins are commonly used as exosomal markers due to their enrichment in exosomes and low abundance in other vesicle types. Alix and TSG101, key transport and fusion proteins, are integral to the formation of ILVs within MVBs, as components of the ESCRT complex responsible for cargo sorting and exosome release [144]. Integrins and intercellular adhesion molecules (ICAMs) facilitate the binding of exosomes to specific receptors on recipient cells, thereby mediating intercellular communication. Notably, integrins also contribute to tissue - specific targeting of exosomes [145]. Within exosomes, HSPs such as HSP70 and HSP90 protect cargo proteins during transit and participate in immune modulation by interacting with antigen - presenting cells.
Internal proteins primarily include enzymes, signal transduction proteins, and cytoskeletal proteins [146]. These proteins originate from the cytoplasm of the parent cell and are selectively packaged into exosomes through specific sorting mechanisms during the formation of MVBs. The sorting of these proteins is tightly regulated by cellular signaling pathways, including those activated under stress conditions, which ensure the functional specificity of exosomal cargo [19]. Exosomes carry various enzymes involved in metabolic processes, which can modulate the metabolic activity of recipient cells and consequently influence their cellular behavior. Some exosomes also contain proteases capable of degrading extracellular matrix components, contributing to physiological and pathological processes such as tissue remodeling and cancer metastasis. Additionally, exosomes are enriched with signal transduction proteins, including kinases, GTPases, transcription factors, receptor tyrosine kinases, adhesion - and migration - related proteins, Wnt signaling components, and proteins involved in the TGF - β signaling pathway [147]. These molecules can activate or suppress diverse signaling cascades, thereby modulating intercellular communication and influencing the functional responses of recipient cells.
Nucleic acid
In addition to lipids and proteins, nucleic acids represent another major component of exosomal cargo. In 2007, Valadi and colleagues first reported that exosomes derived from mast cells contained both mRNA and microRNA (miRNA), demonstrating that exosome - mediated transfer of these nucleic acids constitutes a novel mechanism of genetic exchange between cells. This discovery opened a new avenue of research and potential applications for exosomes. Subsequent studies confirmed the presence of various mRNAs and small non - coding RNAs, including miRNAs, within exosomes [121]. Moreover, RNA sequencing technologies have revealed that exosomes transport diverse types of non - coding RNAs, such as circular RNAs (circRNAs), miRNAs, and long non - coding RNAs (lncRNAs) [148]. Recent findings suggest that the RNA cargo of exosomes is selectively packaged through interactions with RNA - binding proteins, and that stress - related conditions such as hypoxia or inflammation can dramatically reshape the exosomal RNA landscape, influencing gene expression patterns in recipient cells [149-151]. The mRNA encapsulated within exosomes can be delivered to recipient cells and translated into functional proteins, thereby influencing cellular function and behavior. This process plays a pivotal role in biological contexts such as tissue repair, immune regulation, and tumor progression. miRNAs are short, single - stranded non - coding RNAs (ncRNAs) that typically bind to recognition motifs in the 3′ untranslated region (3′ UTR) of target mRNAs, promoting mRNA degradation and inhibiting gene expression. lncRNAs contribute to the regulation of cell differentiation and the cell cycle. CircRNAs have been proposed as miRNA sponges, competing with miRNAs to regulate gene expression [152].
Functions and mechanisms of exosomes on SCI
In SCI, exosomes support multiple neuroprotective and neuroregenerative processes by modulating the injured microenvironment. These mechanisms include suppressing inflammatory responses, enhancing the expression of neurotrophic factors, restoring ECM homeostasis, and modulating astrocyte polarization to limit glial scar formation. In addition, exosomes offer several advantages, including efficient cargo encapsulation and the ability to penetrate the BSCB to reach the site of injury. Due to their therapeutic bioactivity, inherent biocompatibility, and capacity for targeted delivery, exosomes are considered key components of the cellular secretome and hold significant promise as candidates for cell - free therapies. These therapies offer the potential to circumvent challenges associated with cell transplantation, such as immune rejection and uncontrolled cell proliferation or differentiation. This section focuses on the cellular sources of exosomes, their functional roles in SCI, and the underlying mechanisms through which exosome - mediated therapies exert their effects (Figure 6).
Cell source of exosomes and its function on SCI
Various cell types can secrete exosomes with distinct functions, depending on their cellular origin. These functions include regulation of apoptosis, promotion of angiogenesis, facilitation of nerve regeneration, and support of tissue repair. Exosomes are commonly classified as neurologically or non - neurologically derived based on their cellular source. Neurologically derived exosomes include those secreted by neural stem cells, neurons, microglia, astrocytes, Schwann cells, and olfactory ensheathing cells (Table 1). Non - neurologically derived exosomes include exosomes secreted by mesenchymal stromal cells, macrophages, platelet - rich plasma, pericytes, endothelial cells, induced pluripotent stem cells, and regulatory T cells (Table 2).
Cell source of exosomes and its mechanism for modulating microenvironment after SCI. Created with BioRender.com.
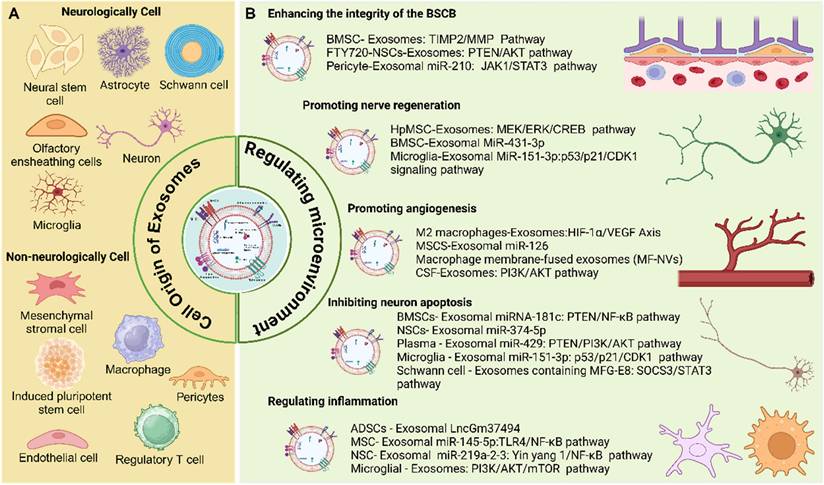
The mechanism of neurologically cells - derived exosomes for regulating microenvironment after SCI.
| Cell source | Highlighted Exosomes-associated cargo | Pathway | Mechanism of regulating microenvironment after SCI | Ref |
|---|---|---|---|---|
| Nerve Stem Cells | FTY720 | PTEN/AKT pathway | Ameliorating the morphology of neurons | [153] |
| Reducing inflammatory response | ||||
| Inhibiting cell apoptosis | ||||
| Neural stem cell | miR-219a-2-3p | miR-219a-2-3p/YY1 pathway | Ameliorating inflammatory response | [154] |
| Inhibiting cell apoptosis | ||||
| Neural stem cell | miR-374-5p | miR-374-5p/STK-4 axis | Inhibiting neural cell apoptosis | [155] |
| Activating autophagy | ||||
| Neural stem cell | VEGF-A | Attenuating cell apoptosis | [156] | |
| Attenuating neuroinflammation | ||||
| Activating of autophagy | ||||
| Promoting angiogensis | ||||
| Neuron | miR-124-3p | PI3K/AKT/NF-κB pathway | Suppressing the activation of M1 microglia | [157] |
| Suppressing neuroinfammation | ||||
| Inhibiting A1 astrocytes. | ||||
| Neuron | miR-21-5p | Regulating microglia polarization | [158] | |
| Suppressing neuroinfammation | ||||
| M2 Microglia | NF-κB pathway | Enhancing neuronal survival and axon preservation | [159] | |
| Alleviating A1 astrocyte activation | ||||
| M2 Microglia | miR-672-5p | AIM2/ASC/Caspase-1 pathway | Promoting axon regeneration | [160] |
| Reducing pyroptosis | ||||
| Microglia | miR‑615‑5p | miR‑615‑5p/MYRF | Promoting remyelination | [161] |
| Microglia | miR-151-3p | p53/p21/CDK1 pathway | Inhibiting neuronal apoptosis | [162] |
| Promoting axonal regrowth | ||||
| Microglia | keap1/Nrf2/HO-1 signaling pathway | Modulating vascular regeneration | [163] | |
| Astrocyte | miR-34c | NF-κB/MAPK | Inhibiting apoptosis | [164] |
| Astrocyte | miR-873a-5p | NF-κB pathway | Inhibiting microglial M1 phenotype | [165] |
| Attenuating neuroinflammation | ||||
| Astrocyte | miR-148a-3p | Regulating the microglial phenotype | [166] | |
| Suppressing neuroinflammation | ||||
| Astrocyte | Nrf2/HO-1 pathway | Inhibiting neuronal apoptosis | [167] | |
| Schwann cells | Rho/ROCK pathway | Reducing glial scar deposition | [168] | |
| Inducing neuronal axon growth | ||||
| Schwann cells | TLR4/NF-κB pathway MAPK pathway Akt/mTOR pathways | Increasing M2-type macrophages | [24] | |
| Reducing neuronal apoptosis | ||||
| Schwann cells | AMPK pathway | Reducing inflammatory response | [169] | |
| Suppressing necroptosis | ||||
| Schwann cells | MFG-E8 | SOCS3/STAT3 pathway | Regulating macrophage/microglial polarization | [170] |
| Schwann cells | NF-κB/PI3K pathway | Inhibiting CSPGs deposition | [171] | |
| Schwann cells | EGFR/Akt/mTOR pathway | Increasing autophagy | [172] | |
| Decreasing apoptosis | ||||
| Olfactory ensheathing cells | NF-κB and c-Jun signaling pathways | Switching the phenotype of macrophages/microglia | [173] | |
| Olfactory ensheathing cells | BDNF | Preventing neuronal cells from apoptosis | [174] |
Neurologically derived exosomes
Neural stem cell‑derived exosomes
NSCs are multipotent stem cells residing in the CNS, with the ability to self - renew and differentiate into neurons, oligodendrocytes, and astrocytes. After SCI, quiescent NSCs in the spinal cord become activated in response to injury - associated signals such as cytokines and growth factors, particularly in regions near the injury site, such as the ependymal zone surrounding the central canal. However, most activated NSCs preferentially differentiate into astrocytes rather than neurons [175], largely due to the pro - inflammatory microenvironment, which promotes astrocytic differentiation and contributes to glial scar formation, a major physical and chemical barrier to axonal regeneration. Therefore, strategies aimed at promoting neuronal differentiation of NSCs represent a promising approach for SCI therapy. Studies have shown that NSC transplantation, combined with modulation of cell fate, can enhance nerve regeneration [176-178]. After transplantation, NSCs not only have the potential to replace damaged neurons but also secrete neuroprotective and immunomodulatory factors, such as BDNF and GDNF [179, 180]. However, NSC transplantation faces several significant limitations, including low survival rates, poor BSCB penetration due to their large size, tumorigenic potential, and ethical concerns [181, 182]. Increasing evidence supports the therapeutic potential of exosomes derived from NSCs. For instance, exosomes from NSCs undergoing necroptosis have been shown to mediate cellular communication after SCI by upregulating TSC2 in recipient cells [183]. NSC - derived exosomes (NSC - Exos) have demonstrated efficacy in SCI repair, including reducing pathological changes, improving hindlimb motor function, alleviating hypoxia, and regulating key molecular pathways such as PTEN/AKT [153, 184, 185]. These exosomes improve neuronal morphology, reduce inflammation and edema, downregulate pro - apoptotic markers such as Bax and AQP4, upregulate tight junction proteins like claudin - 5 and anti - apoptotic Bcl - 2, and inhibit neuronal apoptosis. Ma et al. found that NSC - Exos preconditioned with IGF - 1 could promote neuronal regeneration, inhibit apoptosis, and reduce neuroinflammation via miR - 219a - 2 - 3p [154]. Zhang et al. reported that NSC - Exos enhanced SCI recovery by activating autophagy through the miR - 374 - 5p/STK4 axis [155]. Other studies have demonstrated that these exosomes deliver VEGF - A to spinal cord microvascular endothelial cells (SCMECs), promoting angiogenesis and facilitating neurological recovery [156]. Collectively, these findings indicate that NSC - Exos are a novel and promising therapeutic candidate for SCI repair.
Neuron - derived exosomes
Neurons are fundamental cellular components of the CNS and play a vital role in its diverse functions. As the primary conduits for electrical and chemical signals, neurons are crucial for processing and transmitting information. Together, they form a complex network that underpins all aspects of brain and spinal cord activity [186]. Moreover, neurons contribute to CNS homeostasis by facilitating crosstalk with adjacent astrocytes and microglia [187]. Following SCI, neurons suffer significant loss of structural integrity due to mechanical trauma, leading to cell death and functional deficits at the injury site [6, 188]. Consequently, numerous studies have aimed to promote neuronal survival or increase neuronal populations as part of SCI treatment strategies. Among these, neuron - derived exosomes (Neuron - Exos) have have garnered increasing interest for their therapeutic potential. Xu et al. reported that Neuron - Exos can reverse microglial and astrocyte activation, promote the maturation of OPCs both in vivo and in vitro, and enhance neurite outgrowth and neuronal differentiation from neural stem cells [189]. Jiang et al. demonstrated that Neuron - Exos are enriched with miR - 124 - 3p, which inhibits the activation of M1 microglia and A1 astrocytes by suppressing myosin heavy chain 9 (MYH9) activity. This, in turn, modulates the PI3K/AKT/NF - κB signaling pathway, thereby promoting functional recovery in murine SCI models [157]. Wang, et al. developed an injectable decellularized extracellular matrix hydrogel embedded with cortical Neuron - Exos, which enhanced the local microenvironment, reduced early - stage neuronal apoptosis, stimulated the activation and differentiation of endogenous NSCs, partially suppressed glial scar formation, and synergistically promoted myelinated axon regeneration and locomotor recovery [190].
Microglia - derived exosomes
Microglia are specialized immune cells of the central nervous system (CNS), comprising approximately 10-15% of all CNS cells. Unlike other CNS - resident cells derived from neural progenitors, microglia originate from the myeloid lineage and are closely related to peripheral macrophages. However, they remain functionally confined within the CNS throughout their lifespan. As such, microglia play a vital role in maintaining CNS homeostasis. Their functions include immune surveillance, clearance of apoptotic cells, misfolded proteins, and cellular debris, neuroprotection, injury repair, and regulation of inflammatory responses [191, 192].
Following SCI, microglia are among the earliest responders at the lesion site. Upon activation, they release pro - inflammatory cytokines that contribute to the initiation of the inflammatory cascade. While this inflammation facilitates debris clearance, it can also exacerbate secondary tissue damage, leading to an unfavorable microenvironment for neural repair [193]. Depending on the nature of external stimuli, activated microglia may exhibit either neuroprotective or neurotoxic phenotypes. They support wound healing by facilitating the formation of glial repair tissue and promoting astrocyte clustering through cytokine signaling, which is essential for astrocyte proliferation and scar formation [194]. Furthermore, microglia phagocytose axonal debris and release excessive levels of pro - inflammatory cytokines, which can negatively affect neuronal regeneration. Prolonged microglial activation following SCI has been linked to ongoing neurodegeneration and persistent neurological deficits [195].
The mechanism of non-neurologically cells - derived exosomes for regulating microenvironment after SCI.
| Cell source | Highlighted Exosomes-associated cargo | Pathway | Mechanism of regulating microenvironment after SCI | Reference |
|---|---|---|---|---|
| BMSCs | miR-219-5p | UBE2Z/NRF2 pathway | Inhibiting neuronal cells ferroptosis | [247] |
| BMSCs | miR‑17‑92 | Alleviating apoptosis and inflammation | [223] | |
| hUC-MSCs | miR-540-3p | SIX4/Yap1 pathway | Inhibiting the activation astrocytes Alleviating inflammation | [248] |
| ADSCs | circ-WDfy3 | circ-WDfy3/miR-423-3p/GPX4 signaling pathway | Inhibiting ferroptosis and inflammatory response | [224] |
| BMSCs | Zinc finger and BTB domain-containing protein 4 (ZBTB4) | ZBTB4/ITIH3 | Reducing neuronal apoptosis | [249] |
| Repressing astrocyte activation | ||||
| BMSCs | IL-17 pathway | Inhibiting ferroptosis | [250] | |
| UC-MSCs | NF-κB/MAPK pathways | Inhibiting inflammatory response | [225] | |
| BMSCs | miR-26a-5p | BDNF-TrkB-CREB-KCC2 pathway | Inhibiting the inflammatory response and cell apoptosis | [251] |
| BMSCs | miR-21a-5p | miR-21a-5p/PELI1 axis | Inhibit macrophage/microglia pyroptosis | [252] |
| Increasing antophagy | ||||
| BMSCs | Circ_0006640 | circ_0006640/miR-382-5p/ IGF-1 | Inhibiting microglial apoptosis | [253] |
| Inhibiting inflammatory response | ||||
| CD271+ CD56+ BMSCs | miR-431-3p | miR-431-3p/RGMA axis | Promoting xon regeneration | [254] |
| ADSCs | LRRC75A-AS1 | LRRC75A-AS1/FDFT1 | Suppressing inflammatory response and apoptosis | [255] |
| hUCMSCs | ET-1 | Maintaining BSCB's structural integrity | [256] | |
| ADMSCs | Nrf2/HO-1 pathway | Ameliorating inflammatory response | [227] | |
| Regulating microglial polarization | ||||
| BMSCs | MiR-216a-5p | TLR4/NF-κB pathway | Suppressing inflammatory response | [257] |
| Regulating microglia polarization | ||||
| RGD-CD146+CD271+ UCMSCs | miR-501-5p | miRNA-501-5p/ MLCK | Reducing BSCB destruction | [258] |
| BMSCs | miR-199a-5p | GSK-3β/β-catenin pathway | Promoting the proliferation of NSCs | [259] |
| BMSCs | MiR-137 | Diminishing neuronal apoptosis | [260] | |
| Ameliorating inflammatory response | ||||
| HucMSCs | TLR2/MyD88/NF-κB/Rsad2 | Restraining the activation of microglia | [261] | |
| Inhibiting inflammatory response | ||||
| BMSCs | MiR-146a | Diminishing neuronal apoptosis | [262] | |
| Ameliorating inflammatory response | ||||
| BMSCs | circZFHX3 | mir-16-5p/IGF-1 axis | Repressing apoptosis and inflammatory response | [263] |
| MSCs | lncGm36569 | Gm36569/ miR-5627-5p/FSP1 | Suppressing neuronal cell ferroptosis | [264] |
| Ameliorating inflammatory response | ||||
| BMSCs | miR-9-5p | HDAC5/FGF2 pathway | Alleviating apoptosis, inflammation and endoplasmic reticulum stress | [265] |
| ADSCs | miR-499a-5p | JNK3/c-jun-apoptotic signaling pathway | Alleviating apoptosis | [266] |
| BMSCs | miR-338-5p | Cnr1/Rap1/Akt pathway | Repressing cell apoptosis | [267] |
| Promoting neuronal survival | ||||
| MSCs | miR-145-5p | TLR4/NF-κB pathway | Inhibiting inflammatory response | [268] |
| BMSCs | miR-26a | PTEN-AKT-mTOR pathway | Promoting axonal regeneration | [269] |
| Improve neurogenesis | ||||
| Attenuating glial scarring | ||||
| Pericyte | PTEN/PI3K/AKT pathway | Inhibiting cell apoptosis | [234] | |
| Promoting cell survival | ||||
| Protecting blood-spinal cord barrier | ||||
| Pericyte | miR-210-5p | JAK1/STAT3 pathway | Improving BBB integrity | [235] |
| Promoting angiogenesis | ||||
| M2 Macrophage | Enhancing anti-inflammatory response | [270] | ||
| Promoting neuronal survival | ||||
| Reducing the glial scar | ||||
| M2 Macrophage | HIF-1α/VEGF axis | Improving angiogenesis and neurogenesis | [241] | |
| M2 Macrophage | OTULIN | Wnt/ β-catenin pathway | Promoting vascular regeneration | [200] |
| M1 Macrophages | miR-155 | NF-κB/SOCS6/p65 pathway | Regulating the M1-polarized macrophages and microglia | [271] |
| Peripheral Macrophage | PI3K/AKT/mTOR pathway | Increasing autophagy | [240] | |
| Enhancing the polarization of anti-inflammatory type microglia | ||||
| M2 macrophage | miR-23a-3p | PTEN/PI3K/AKT pathway | Promoting M2 macrophage polarization | [272] |
| M2 macrophage | IKVAV peptides | Inhibiting inflammatory response | [242] | |
| Reducing infiltration of macrophages | ||||
| Microvascular endothelial cell | USP13 | NF-κB pathway | Regulating microglia/macrophages polarization | [246] |
| Endothelial cell | miR199-5p | PI3K/AKT/PTEN pathway | Promoting nerve regeneration | [273] |
| Maintaining repair-related phenotypes of Schwann cells | ||||
| iPSC-NSCs | let-7b-5p | let-7b-5p/LRIG3 pathway | Reducing inflammatory response | [274] |
| Modulating microglial/macrophage pyroptosis | ||||
| iPSC | miR-199b-5p | miR-199b-5p/Hgf/PI3K pathway | Improving neural regeneration | [275] |
| Regulating the polarization of macrophage | ||||
| iPSC | miR-23b, miR-21-5p, miR-199b-5p | Reducing inflammatory response | [276] | |
| Regulatory T cell | miR-709 | miR-709/ NKAP | Attenuating microglia pyroptosis | [277] |
| Reducing inflammatory response | ||||
| Regulatory T cell | miR-2861 | miR-2861/IRAK1 | Promoting blood-spinal cord barrier repair | [278] |
Recent studies have highlighted the significant role of exosomes in modulating microglial function and promoting neurorepair following neurotrauma [23, 196-198]. For example, Li et al. reported that microglia - derived exosomes (Microglia - Exos) enhance neurological recovery in SCI mice by delivering miR - 151 - 3p to neurons. This microRNA exerts neuroprotective effects by reducing neuronal apoptosis and promoting axonal growth through the p53/p21/CDK1 signaling pathway [162]. Similarly, Peng and his colleague demonstrated that Microglia - Exos exert antioxidant effects and positively regulate vascular regeneration and neurological recovery after SCI via activation of the Keap1/Nrf2/HO - 1 signaling pathway [163]. Huang et al. reported that elevated levels of miR - 124 - 3p in Microglia - Exos following TBI suppress neuronal inflammation and enhance neurite outgrowth by targeting neurons [199]. In the context of TSCI, exosomes derived from M2 - polarized microglia have shown notable therapeutic potential. These exosomes reduce pyroptosis in spinal neurons, support axonal regeneration, and improve functional outcomes by inhibiting the AIM2/ASC/Caspase - 1 signaling pathway [160]. Additionally, they attenuate the activation of neurotoxic A1 astrocytes by suppressing NF - κB signaling, thereby preserving spinal tissue and enhancing motor function recovery [159]. Furthermore, M2 Microglia - Exos have been shown to facilitate vascular regeneration and neurological repair through OTULIN - mediated activation of the Wnt/β - catenin pathwa [200].
In summary, Microglia - Exos represent promising therapeutic agents for modulating the post - injury microenvironment and supporting functional recovery in SCI.
Astrocyte - derived exosomes
Astrocytes, star - shaped glial cells in the CNS, originate from NSCs during development. They play essential roles in maintaining the BBB, regulating cerebral blood flow, and supplying metabolic support to neurons. In addition, astrocytes help maintain neurotransmitter homeostasis by clearing excess neurotransmitters, particularly glutamate, from the synaptic cleft to prevent excitotoxicity. In response to CNS injury, astrocytes undergo a process known as reactive astrogliosis, which can have both protective and detrimental effects on surrounding tissue [201, 202]. Reactive astrocytes are further categorized into A1 and A2 phenotypes. A1 astrocytes, typically induced by inflammatory stimuli, exhibit neurotoxic properties and can exacerbate neuronal damage. In contrast, A2 astrocytes are considered neuroprotective, supporting cell survival and promoting tissue repair following injury [203, 204]. Representing a double - edged sword, reactive astrocytes also contribute to glial scar formation. Depending on their activation state, this scar can serve to contain the injury and limit damage spread, but it may simultaneously act as a barrier to axonal regeneration.
Recent studies have demonstrated that astrocyte - derived exosomes (Astrocyte - Exos) play an important role in CNS injury and have become a growing focus in neuroregenerative research. These exosomes, enriched with various biologically active macromolecules, act as carriers that deliver functional cargo to both neighboring and distant recipient cells [205]. This transfer can induce diverse functional changes, including neuroprotective effects through the regulation of neuronal uptake, differentiation, and activity [203, 206]. Wu et al. reported that Astrocyte - Exos can target Toll - like receptor 7 (TLR7) to transport miR - 34c, thereby attenuating ischemia/reperfusion - induced brain injury by inhibiting the NF - κB/MAPK signaling axis [164]. Long claimed that Astrocyte - Exos enriched with miR - 873a - 5p alleviated neurological deficits after TBI by suppressing ERK and NF - κB p65 phosphorylation and promoting the polarization of microglia toward the anti - inflammatory M2 phenotype [165]. In both rat and mouse models, Zhang et al. showed that Astrocyte - Exos mitigated TBI - induced mitochondrial oxidative stress and neuronal apoptosis through activation of the Nrf2/HO - 1 signaling pathway [167]. Despite these encouraging findings in TBI, to date, only one study has reported the application of Astrocyte - Exos in SCI, showing that they reduced fibrosis and improved functional outcomes [207]. Given the shared injury mechanisms and therapeutic targets between TBI and SCI, it is likely that future research will increasingly explore the role of Astrocyte - Exos in SCI repair. Collectively, these findings suggest that Astrocyte - Exos hold significant promise as novel therapeutic agents for promoting functional recovery after SCI.
Schwann cells - derived exosomes
Schwann cells (SCs), a type of glial cell in the peripheral nervous system (PNS), play a critical role in the maintenance and regeneration of nerve fibers and have been extensively studied for their contributions to nerve repair [208, 209]. Although they originate in the PNS, SCs are among the most widely investigated cell types due to their pivotal role in facilitating axonal regeneration and myelination. Notably, when transplanted into the spinal cord, SCs exhibit regenerative and myelination - supportive properties comparable to their activity in peripheral nerves [8, 210]. Despite these advantages, the therapeutic efficacy of Schwann cell transplantation in promoting functional recovery after SCI remains limited. This is attributed to several key challenges, including the low survival rate of transplanted cells, the inability of SCs to traverse BBB, their poor migratory capacity within astrocytic tissue, and restricted axonal outgrowth beyond regions with high SC density [211]. Proteomic analyses have identified several signaling pathways enriched and functionally relevant to the CNS microenvironment, including the neurotrophin, PI3K - Akt, and cAMP signaling pathways, all of which are involved in CNS repair processes [212]. Furthermore, Schwann cell - derived exosomes (SCs - Exos) have been shown to promote axonal regeneration and attenuate inflammatory responses, offering promising therapeutic benefits for nerve tissue repair and inflammation reduction following neural injury.
Ren et al. demonstrated that SCs - Exos possess anti - inflammatory properties, suppressing M1 polarization while promoting M2 polarization of macrophages and microglia through the regulation of the SOCS3/STAT3 signaling pathway. These effects contribute to reduced neuronal apoptosis and improved motor function recovery following SCI [170]. Pan et al. reported that SCs - Exos enhance functional recovery in SCI mice by reducing CSPG deposition and upregulating TLR2 expression on astrocytes via the NF - κB/PI3K signaling pathway [171]. Furthermore, these exosomes inhibit PTP - σ activation by modulating the Rho/ROCK pathway, thereby limiting glial scar formation, promoting axonal regeneration, improving neuronal integrity, and facilitating locomotor recovery [168]. Huang demonstrated that SCs - Exos could promote angiogenesis through the delivery of integrin - β1 [213]. Given that cell death represents a key feature of the post - injury microenvironment, SCs - Exos have also been shown to protect axons by enhancing autophagy and reducing apoptosis, potentially through the EGFR/Akt/mTOR signaling pathway [172]. In addition, SCs - Exos help alleviate mitochondrial dysfunction and necroptosis via AMPK signaling pathway - driven mitophagy following SCI [169].
Overall, SCs - Exos have the comprehensive ability to restore the balance in the microenvironment after SCI. Thus, they hold significant potential as an innovative therapeutic approach for improving neurological functional recovery after neurotrauma.
Olfactory ensheathing cells - derived exosomes
Olfactory ensheathing cells (OECs), specialized glial cells within the olfactory system, play a vital role in supporting the regeneration of sensory neurons in the olfactory bulb. This is achieved through promoting axon growth and myelination. OECs are unique in their ability to facilitate nerve regeneration in both the central and peripheral nervous systems [15, 214]. Due to these regenerative properties, OECs have been widely explored as a potential therapy for SCI, with demonstrated effects in promoting axonal regrowth and functional recovery. In recent years, OECs - derived exosomes (OECs - Exos) have garnered increasing interest as cell - free therapeutic agents for SCI. Tu et al. demonstrated that exosome - like vesicles derived from human OECs (hOECs - EV) promote NPC growth and alleviate t - BHP - induced oxidative toxicity. While the study was limited to in vitro experiments, the findings suggest that OECs - Exos enhance the proliferation and differentiation of NPCs, supporting their potential utility in regenerative applications [215]. In a rat model of SCI, Fan et al. reported that OECs - Exos conferred neuroprotection by modulating the phenotype of macrophages and microglia via inhibition of the NF - κB and c - Jun signaling pathways, thereby reducing neuronal apoptosis [173]. Additionally, OECs - Exos have been shown to deliver BDNF, which activates its receptor TrkB on neurons and counteracts TNF - α - induced apoptosis, further supporting neuronal survival and repair [174].
Non - neurologically derived exosomes
Mesenchymal stromal cell‑derived exosomes
Mesenchymal stromal cells (MSCs), which are multipotent stem cells derived from various tissues such as bone marrow, adipose tissue, and umbilical cord, have the capacity to differentiate into multiple cell types. This makes them valuable for tissue repair and regeneration [216-218]. Moreover, MSCs possess immunomodulatory properties. These properties enable them to modulate immune responses, reduce inflammation, and support tissue healing. Due to these characteristics, MSCs have been widely investigated for their therapeutic potential in the treatment of SCI [6, 219]. Emerging evidence indicates that the therapeutic benefits of MSCs primarily arise from their paracrine activity, rather than direct transdifferentiation or long - term engraftment. As such, MSC - derived exosomes (MSCs - Exos), which carry diverse paracrine mediators, have emerged as a promising acellular therapeutic strategy [220]. Recent studies have highlighted the ability of MSCs - Exos to promote tissue regeneration, stimulate axonal growth, and inhibit glial scar formation, thereby enhancing neurological recovery following SCI [221].
MSCs - Exos are more readily available than those from many other cell types. Furthermore, their significantly smaller size compared to MSCs allows them to evade pulmonary and hepatic sequestration, enabling penetration of the BSCB. As a result, MSCs - Exos have gained considerable attention in the field of SCI therapy. Inflammation is a major pathological component of SCI, and reducing it is a key therapeutic strategy [222]. Wang et al. reported that the miR - 17 - 92 cluster in bone marrow MSC (BMSCs) - Exos improves neurological function after SCI by mitigating inflammation and modulating neuronal apoptosis [223]. Shao and colleagues showed that adipose - derived stem cell (ADSCs) - Exos more effectively reduce ROS levels and inflammatory cytokine expression following SCI [224]. Luan et al. demonstrated that exosomes derived from umbilical cord - derived MSCs (UC - MSCs) protect against SCI by inhibiting inflammation through the NF - κB/MAPK signaling pathway, suggesting potential therapeutic targets [225]. In addition, MSCs - Exos can modulate macrophage polarization to further suppress inflammation and support tissue repair. Chang et al. reported that microRNA - 125a in BMSCs - Exos promotes M2 macrophage polarization via IRF5 suppression [226]. Moreover, inhibiting M1 microglia and promoting M2 microglia are also effective strategies for fostering functional recovery after SCI. ADSC - Exos inhibit M1 microglia, promote M2 microglial phenotypes, and activate the Nrf2/HO - 1 pathway, enhancing anti - inflammatory responses [227]. Exosomes derived from human umbilical cord MSCs (hUC - MSCs) have also been shown to preserve BSCB integrity and improve functional recovery by upregulating junctional proteins and downregulating inflammatory mediators such as endothelin - 1 (ET - 1). Zhou et al. demonstrated that BMSCs reduce BSCB permeability, promote axonal regeneration, and accelerate motor function recovery by decreasing caspase - 1 expression and inhibiting IL - 1β release [228]. Suppressing the activation of A1 neurotoxic reactive astrocytes can promote the function recovery after SCI. And the administration of BMSCs - Exos efficiently suppressed inflammation after traumatic SCI and suppressed the activation of A1 neurotoxic reactive astrocytes [229].
In summary, MSCs - derived exosomes contribute to SCI repair through multiple mechanisms, including promoting angiogenesis and axonal growth, regulating immune responses, inhibiting apoptosis, and preserving BSCB integrity. As a cell - free and immunologically safer alternative to MSCs transplantation, exosomes are unlikely to elicit strong immune reactions. Their therapeutic potential can be further enhanced through engineering approaches, such as the loading of specific microRNAs or therapeutic agents targeting SCI pathologies. However, further research is needed to clarify their long - term safety, stability, and off - target effects. Continued investigation into the detailed mechanisms and clinical translation of MSCs - Exos will be essential for advancing this promising field.
Pericytes - derived exosomes
Pericytes are specialized mural cells that reside within the walls of capillaries and microvessels, where they contribute to vascular stability, BBB integrity, and various repair mechanisms [230]. Functionally, pericytes are essential for maintaining vascular homeostasis, supporting angiogenesis, and preserving BBB structure. They enhance endothelial cell function, promote vessel stabilization, and regulate cerebral blood flow through the secretion of numerous growth factors and regulatory molecules [231, 232]. In the context of the CNS, pericytes play a particularly important role in maintaining BBB integrity and mitigating neuroinflammation, especially following traumatic injuries such as TBI and SCI [49, 233]. Upon injury, pericytes respond by modulating inflammation and promoting tissue repair, making them a promising target for regenerative therapies in neurotrauma. In line with these roles, pericytes - derived exosomes (Pericytes - Exos) have also garnered increasing attention for their potential therapeutic applications.
Pericytes - Exos are produced and released under both physiological and pathological conditions, playing a critical role in mediating intercellular communication. Through the transfer of bioactive molecules, these exosomes enable pericytes to influence adjacent cells and regulate various biological processes, particularly those associated with injury response and tissue repair. In CNS injuries, Pericytes - Exos have been shown to reduce inflammation and oxidative stress, promote vascular regeneration, and facilitate neuronal recovery. Yuan et al. demonstrated that Pericytes - Exos exert therapeutic effects in SCI by mitigating pathological changes, improving motor function, and enhancing blood flow and oxygen delivery post - injury [234]. These exosomes support endothelial regulation of circulation, preserve the integrity of the BSCB, and reduce edema. Mechanistically, they downregulate the expression of HIF - α, Bax, Aquaporin - 4, and MMP2, while upregulating Claudin - 5 and Bcl - 2, thereby inhibiting apoptosis. Under hypoxic conditions, Pericytes - Exos also protect spinal cord microvascular endothelial cells through activation of the PTEN/AKT signaling pathway. Additionally, Gao et al. reported that Pericytes - derived exosomes enriched with miR - 210 mitigate lipid peroxidation and preserve mitochondrial function, primarily by enhancing BBB integrity through the JAK1/STAT3 signaling pathway [235]. By modulating this pathway, miR - 210 reduces inflammatory responses and reinforces structural components of the BBB, contributing to vascular stability and neural tissue repair. Collectively, these findings further elucidate the complex crosstalk between pericytes and endothelial cells in SCI and highlight Pericytes - Exos as a promising therapeutic target.
Macrophage‑derived exosomes
Macrophages are innate immune cells that play a pivotal role in maintaining tissue homeostasis, defending against pathogens, and facilitating wound repair. In CNS, macrophages are primarily present as microglia (resident macrophages) and infiltrating macrophages (recruited during injury or disease). These cells contribute to immune surveillance by detecting pathogens and cellular debris and are essential mediators of the inflammatory response. Following SCI, macrophages participate in clearing myelin debris, promoting angiogenesis, and supporting neural repair. However, their function is context - dependent and highly dynamic. Depending on environmental cues, macrophages may polarize into either pro - inflammatory (M1) or anti - inflammatory (M2) phenotypes. This plasticity underlies their dual roles in neuroinflammation and regeneration [59]. Mounting researchers have suggested that M2 macrophages secrete anti - inflammatory cytokines and growth factors such as BDNF and vascular endothelial growth factor (VEGF). These mediators contribute to tissue repair by promoting axonal regeneration, stimulating angiogenesis, and modulating glial scar formation [236]. M2 macrophages also support the resolution of inflammation and enhance remyelination [237]. Furthermore, studies have shown that exosomes released by M2 macrophages during CNS injuries can promote vascular regeneration, cellular proliferation, and tissue repair [238].
Peng et al. reported that exosomes derived from M2 macrophages (Macrophages - Exos) increase the M2/M1 macrophage ratio via the miR - 23a - 3p/PTEN/PI3K/AKT signaling pathway. This modulation of the immune microenvironment facilitates SCI repair by promoting an anti - inflammatory state [239]. Similarly, Zhang et al. demonstrated that peripheral Macrophages - Exos exert beneficial effects in SCI recovery by promoting microglial autophagy through suppression of the PI3K/AKT/mTOR pathway. This regulation enhances microglial polarization toward an anti - inflammatory phenotype, contributing to inflammation resolution [240]. Huang et al. reported that M2 Macrophages - Exos enhance neurological recovery and angiogenesis following SCI, in part through activation of the HIF - 1/VEGF signaling pathway [241].
Moreover, studies have demonstrated that engineered Macrophages - Exos loaded with therapeutic agents can modulate the SCI microenvironment, offering promising treatment options. For SCI therapy, a novel nanomagnetic platform has been developed using click chemistry to functionalize the surface of M2 Macrophages - Exos with bioactive Ile - Lys - Val - Ala - Val (IKVAV) peptides. This engineered system reprograms macrophages toward an anti - inflammatory phenotype and promotes the neuronal differentiation of NSCs. In a murine SCI model, intravenous administration of these modified exosomes enabled targeted delivery to infiltrating macrophages at the injury site, leading to reduced pro - inflammatory cytokine expression and enhanced regeneration of neural tissue, ultimately improving motor function recovery [242]. In another application, berberine - loaded M2 Macrophages - Exos have been employed as a targeted drug delivery system to transport berberine to the injured spinal cord. These exosomes facilitate the polarization of microglia from a pro - inflammatory M1 state to an anti - inflammatory M2 phenotype and suppress the production of inflammatory mediators. Additionally, they help inhibit neuronal apoptosis and support the restoration of motor function following SCI [243]. Collectively, these studies highlight the potential of Macrophages - Exos as a promising strategy for targeted and effective SCI treatment.
Endothelial cell‑derived exosomes
Endothelial cells (ECs) in CNS are specialized components of BBB and BSCB, both of which are essential for maintaining CNS homeostasis. These cells are characterized by tight junctions, low transcytosis rates, and selective permeability, allowing them to protect neural tissue from toxins, pathogens, and immune cell infiltration while facilitating the exchange of nutrients and metabolic waste. Importantly, CNS ECs interact closely with neurons, astrocytes, pericytes, and immune cells to form the neurovascular unit (NVU), a critical structure that supports neural function and orchestrates the response to injury [244].
Following SCI, the integrity of the BSCB is compromised, leading to inflammation, oxidative stress, and neuronal damage. Studies have shown that spinal cord microvascular endothelial cells can promote axonal growth by downregulating miR - 323 - 5p expression. The reduction of this microRNA leads to the upregulation of genes associated with axon development, thereby facilitating axonal elongation and contributing to functional recovery after SCI [245]. A recent study demonstrated that exosomes derived from vascular endothelial cells play an important role in promoting functional recovery after SCI. This effect is achieved by regulating communication between endothelial cells and microglia/macrophages through the delivery of USP13, a deubiquitinating enzyme with key roles in inflammation control. USP13 inhibits the ubiquitination and degradation of IκBα, a critical inhibitor of the NF - κB signaling pathway. By stabilizing IκBα, these exosomes suppress pro - inflammatory responses and promote the polarization of microglia and macrophages toward the anti - inflammatory M2 phenotype for SCI repair and neural regeneration [246]. Despite these encouraging findings, limited research has been conducted on the application of endothelial cell - derived exosomes in SCI treatment, highlighting the need for further investigation to fully understand their therapeutic potential.
Induced pluripotent stem cell‑derived exosomes
Induced pluripotent stem cells (iPSCs) hold significant potential for treating SCI. Their capacity to differentiate into various neural cell types, such as neurons, astrocytes, and oligodendrocytes, enables the restoration of neural circuitry, promotion of axonal regeneration, and facilitation of remyelination in SCI models [279]. Additionally, iPSCs can be derived from a patient's own somatic cells, thereby minimizing the risk of immune rejection. Nevertheless, several challenges hinder the clinical application of iPSC - based therapies. Due to their pluripotent nature, iPSC transplantation carries the risk of teratoma formation [280]. Moreover, ensuring the precise differentiation and functional integration of iPSCs into damaged spinal cord tissue remains technically complex. Large - scale production of clinical - grade iPSCs and their derivatives also presents considerable logistical and regulatory challenges.
To address the limitations associated with iPSCs - based cell therapy, increasing attention has been directed toward the therapeutic use of iPSC - derived exosomes (iPSCs - Exos) in SCI. Azar Abbas and colleagues engineered exosomes derived from iPSCs that were enriched with miR - 23b, miR - 21 - 5p, and miR - 199b - 5p. These exosomes exhibited significant neuroprotective properties in SCI models by reducing inflammation and enhancing functional recovery. Notably, they exerted anti - inflammatory effects on neurons stimulated with LPS and IFN - γ, underscoring their potential as a cell - free therapeutic strategy for promoting spinal cord repair. [276]. Moreover, iPSCs - Exos have been shown to enhance motor function in SCI mouse models. These exosomes promoted the polarization of macrophages from the pro - inflammatory M1 phenotype to the anti - inflammatory M2 phenotype, regulated inflammatory factor expression, and facilitated spinal cord recovery through the action of miR - 199b - 5p. This microRNA targetedHgf and modulated the PI3K signaling pathway to mediate therapeutic effects [275].
Regulatory T cell‑derived exosomes
Regulatory T cells (Tregs) play a crucial role in maintaining immune homeostasis by regulating inflammation and promoting tissue repair. Following SCI, Tregs have demonstrated therapeutic potential by modulating the inflammatory microenvironment, limiting secondary tissue damage, and supporting functional recovery [281]. Nevertheless, the clinical application of Treg - based therapies in SCI faces several challenges. A major limitation lies in the difficulty of expanding and sustaining functional Tregs ex vivo while preserving their immunosuppressive activity. Additionally, systemic administration of Tregs may pose risks of broad immune suppression, potentially leading to increased susceptibility to infections or cancer if immune balance is disrupted. As a result, Treg - derived exosomes (Treg - Exos) have garnered increasing interest as a safer and more targeted alternative for SCI treatment.
Treg - Exos miR - 709 has been shown to attenuate microglial pyroptosis and promote motor function recovery following SCI [277]. Moreover, Kong et al. demonstrated that exosomes secreted by Treg cells contribute to the repair of BSCB by reducing its permeability. This effect alleviates excitotoxic injury caused by inflammatory infiltration and ultimately supports motor function recovery. These exosomes exert their effects, in part, through enrichment of miR - 2861, which negatively regulates interleukin - 1 receptor - associated kinase 1 (IRAK1), a key mediator of the inflammatory response [282].
Collectively, these findings underscore the therapeutic potential of Treg - Exos in SCI. However, the underlying mechanisms by which these exosomes contribute to tissue repair and functional recovery remain incompletely understood, warranting further investigation.
Mechanisms of exosomes - mediated treatment for SCI
As previously discussed, SCI results in a dysregulated microenvironment that exacerbates tissue damage and impedes neural regeneration. Due to their multifaceted roles in modulating the injury microenvironment, exosomes have demonstrated notable neuroprotective potential and have been extensively investigated in various preclinical SCI models for their ability to promote recovery. This section focuses on the underlying mechanisms through which exosomes contribute to SCI repair, with particular attention to their roles in regulating inflammation, promoting neuroprotection, and enhancing tissue regeneration. These findings underscore the therapeutic value of exosomes in restoring homeostasis and facilitating functional recovery after SCI (Figure 6).
Enhance the integrity of the BSCB
The BSCB is crucial for maintaining the microenvironment of the spinal cord. First, it restricts the entry of peripheral immune cells, thereby minimizing excessive inflammatory responses and protecting the spinal cord from immune - mediated injury. Second, as a neuroprotective barrier, the BSCB shields spinal neurons from potentially neurotoxic molecules present in the bloodstream. Third, it maintains ionic balance and molecular homeostasis, which are critical for proper neural signal transmission. After SCI, restoring the integrity of the BSCB is vital for minimizing secondary damage, optimizing the repair environment, and promoting tissue regeneration. Despite its protective role, however, the BSCB also poses a significant obstacle for therapeutic delivery. As such, innovative strategies, including nanotechnology and exosome - based delivery systems, are increasingly being explored to effectively transport therapeutic agents to the injured spinal cord. Maintaining BSCB integrity not only mitigates ongoing damage but also facilitates neural repair and enhances functional recovery following SCI. [283-285].
The BSCB primarily consists of endothelial cells, pericytes, astrocytes, and the basement membrane. Enhancing the function and interactions among these components can support BSCB integrity and contribute to SCI repair. BMSCs - Exos have been shown to preserve BSCB integrity and promote motor recovery after SCI by modulating the TIMP2/MMP signaling pathway. Specifically, tissue inhibitor of TIMP2 within these exosomes inhibits MMP activity, thereby increasing the expression of key tight junction proteins, including claudin - 5, occludin, zonula occludens - 1 (ZO - 1), and β - catenin, ultimately reducing BSCB damage [286]. Similarly, exosomes from MSCs upregulate M2 macrophage markers, enhance TGF - β signaling, and increase tight junction protein expression, collectively reducing BSCB permeability [287]. Additionally, maintaining the endothelial barrier in SCMECs under hypoxic conditions can aid in hindlimb functional recovery. NSCs - Exos loaded with FTY720, an immunomodulatory agent, preserve endothelial integrity via the PTEN/PI3K/AKT signaling pathway [153]. Pericytes, as integral components of the neurovascular unit, are critical for maintaining BSCB structure. Enhancing pericytes function or increasing their number may improve SCI outcomes by strengthening the barrier. Treatment with BMSCs - derived exosomes has been shown to inhibit pericytes migration and reinforce BSCB integrity through the NF - κB/p65 signaling pathway [288]. Moreover, exosomes can reduce edema and preserve BSCB function by suppressing pericytes pyroptosis via inhibition of the Nod1 inflammasome, thereby improving pericyte coverage and supporting recovery [155]. Pericytes - Exos enriched with miR - 210 - 5p further stabilize the BSCB by attenuating lipid peroxidation and enhancing mitochondrial function through suppression of the JAK1/STAT3 signaling pathway [235].
Promote nerve regeneration
Neurons are the fundamental structural and functional units of the nervous system. Neural regeneration following SCI is a highly complex process involving neuronal outgrowth, axonal regeneration, and restoration of the local microenvironment.
In adults, the regenerative capacity of neurons following SCI is inherently limited. However, in preclinical models, various exosomes have demonstrated the ability to promote neuronal proliferation and regeneration. For instance, NSCs - Exos loaded with FTY720 enhance neuronal morphology through activation of the PTEN/AKT signaling pathway [153]. Neurotrophic factor - enriched exosomes obtained from bone marrow BMSCs promote the differentiation of NSCs into neurons [155]. Additionally, HpMSCs - Exos stimulate NSC proliferation via the MEK/ERK/CREB signaling cascade, increasing phosphorylation levels of MEK, ERK, and CREB [269]. Axon extension represents a crucial step in neural regeneration. CD271+CD56+ BMSCs - Exos deliver MiR - 431 - 3p, which significantly enhances axonal elongation and branching in DRG neurons by targeting repulsive guidance molecule A (RGMa) [254]. Furthermore, Microglia - Exos enriched with miR - 151 - 3p promote axonal regrowth and reduce neuronal apoptosis by inhibiting the p53/p21/CDK1 pathway [162]. In summary, promoting axonal regeneration through exosome - mediated delivery of specific molecular signals represents a key strategy for SCI repair.
Promote angiogenesis
Neurogenesis and angiogenesis must work synergistically to accelerate regeneration following SCI. Restoring neurological function requires not only the regeneration of neural cells but also structural and vascular support from the extracellular matrix and surrounding blood vessels. Notably, the formation of new blood vessels is essential for tissue repair, as it ensures adequate oxygen and nutrient delivery to regenerating tissues. Exosomes play a critical role in promoting angiogenesis after SCI by stimulating endothelial cell proliferation and migration, as well as by regulating key angiogenesis - related signaling pathways.
After SCI, the preservation of endothelial cells helps limit secondary vascular damage, thereby ensuring the continued delivery of oxygen and nutrients necessary for restoring the microenvironment and neuronal circuitry. Vascular endothelial cells are particularly receptive to hypoxia - treated MSCs - Exos. Treatment with SCs - Exos has been shown to stimulate endothelial cell proliferation, migration, and tube formation. These effects collectively promote angiogenesis, reduce tissue damage, and enhance neurological recovery after SCI. Elevated HIF - 1α expression in hypoxia - stimulated MSCs - Exos enhances VEGF levels, suggesting a promising potential to enhance angiogenesis in SCI [289]. Similarly, NSCs - Exos can significantly upregulate VEGF expression, thereby promoting SCMEC migration, proliferation, and tube formation processes in angiogenesis and tissue repair [156]. M2 Macrophages - Exos also improve vascular regeneration and motor function recovery via activation of the HIF - 1α/VEGF signaling axis [241]. Furthermore, miR - 126 - modified MSCs - Exos enhance microvascular regeneration and facilitate the migration of HUVECs by downregulating Sprouty - related EVH1 domain - containing protein 1 and phosphoinositide - 3 - kinase regulatory subunit 2 (PIK3R2) [290]. In addition, macrophage membrane - fused exosome - mimetic nanovesicles have shown the potential to stimulate angiogenesis and selectively target ischemic endothelium, providing an innovative platform for targeted vascular repair following SCI [291].
Vascular regeneration is a complex process involving multiple signaling pathways that act synergistically to promote angiogenesis. These include the Wnt/β - catenin, PI3K/Akt, JNK/c - Jun, and PTEN/mTOR signaling cascades. Exosomes derived from M2 macrophages containing the ubiquitin isopeptidase OTULIN can activate the Wnt/β - catenin pathway by upregulating β - catenin expression. This stimulation, in turn, promotes the expression of angiogenesis - related genes in SCMECs [200]. CSF - derived exosomes have also been shown to facilitate vascular regeneration and motor function recovery by activating the PI3K/Akt signaling pathway [292]. Additionally, MSCs - Exos carrying siRNA targeting PTEN can suppress PTEN expression while promoting both axonal growth and neovascularization [293]. Exosome - mimetic nanovesicles loaded with iron oxide nanoparticles (NV - IONPs) have been demonstrated to activate the JNK/c - Jun signaling axis, and their accumulation significantly contributes to the formation of new blood vessels following SCI [294].
Inhibit neuron apoptosis
Apoptosis, a form of programmed cell death, plays a critical role in the pathophysiology of SCI. It significantly impedes neurological recovery, primarily by inducing neuronal loss. Consequently, suppressing neuronal apoptosis is essential for enhancing therapeutic outcomes following SCI.
Various exosomes exhibit anti - apoptotic effects following SCI. BMSCs - Exos can protect against SCI by delivering miR - 181c, which reduces inflammation in microglia and spinal cord tissue via suppression of the PTEN/NF - κB signaling pathway. This results in decreased inflammation and neuronal apoptosis, ultimately improving recovery outcomes [295]. Additionally, BMSCs - Exos promote motor function recovery by inhibiting neuronal apoptosis through the activation of the Wnt/β - catenin signaling pathway [296]. NSCs - Exos attenuate SCI by initiating autophagic flux and suppressing apoptosis. miR - 374 - 5p, abundantly expressed in these exosomes, contributes significantly to neuroprotection by promoting autophagy and reducing apoptosis of injured neural cells [155]. Furthermore, p53 has been identified as a target of miR - 151 - 3p in Microglia - Exos, implicating the p53/p21/CDK1 axis in the regulation of neuronal apoptosis and axonal regeneration [162]. EPCs - Exos loaded with miR - 210 attenuate hypoxia/reoxygenation (H/R) - induced neuronal apoptosis and oxidative stress by modulating BDNF/TrkB and Nox2/Nox4 pathways [297]. Overexpression of miR - 137 in EPCs - derived exosomes enhances neuroprotection by inhibiting apoptosis and mitochondrial dysfunction via the COX2/PGE2 signaling pathway [298]. SCs - Exos containing MFG - E8 suppress neuronal apoptosis through activation of the SOCS3/STAT3 pathway [170].
Overall, exosomes have been shown to exhibit anti - apoptotic effects in various pathological conditions. These findings highlight the potential of exosome - based therapies as a promising strategy for reducing neuronal apoptosis and enhancing functional recovery following SCI.
Regulate inflammation
Excessive neuroinflammation impairs neuronal regeneration, thereby worsening the prognosis of patients with SCI. At the site of injury, persistent neuroinflammation disrupts axonal regeneration, hindering the re - establishment of neural connections and resulting in prolonged neurological deficits. Importantly, the inhibitory effects of inflammation and glial scarring following SCI are influenced, in part, by exosomes released from activated immune and glial cells.
According to previous studies, exosomes with anti - inflammatory effects can be classified based on their cellular origin or molecular content. During the initial stages of secondary damage in spinal cord tissue, exosomes contribute to neuroprotection by transporting bioactive molecules that reduce levels of ROS and inflammatory cytokines at the lesion site. For example, ADSCs - Exos carry LncGm37494, which significantly promotes SCI repair by downregulating inflammatory factor expression and improving functional outcomes [299]. NSCs - Exos can deliver IGF - 1, which suppresses neuroinflammation by modulating the NF - κB signaling pathway [154]. MiR - 544 - modified BMSCs - Exos can reduce the expression of pro - inflammatory cytokines such as IL - 1α, TNF - α, IL - 17β, and IL - 36β at the injury site following SCI [300]. MicroRNAs delivered via exosomes can regulate inflammation through multiple signaling cascades. For instance, MSCs - Exos overexpressing miR - 145 - 5p inhibit the TLR4/NF - κB pathway, thereby attenuating the inflammatory response [268]. Similarly, hypoxia - conditioned MSCs - Exos enriched with miR - 216a - 5p exert anti - inflammatory effects by targeting the TLR4/NF - κB/PI3K/AKT pathway and promoting microglial polarization [301].
Macrophage and microglial activity plays a pivotal role in regulating the inflammatory response following SCI. Various types of exosomes modulate this activity through multiple mechanisms, including inhibition of microglial and macrophage activation, suppression of M1 polarization, promotion of M2 polarization, and induction of autophagy in microglia. hUC - MSCs - Exos exhibit both anti - apoptotic and anti - inflammatory effects by suppressing the activation of microglia and macrophages through modulation of the BCL2/Bax and Wnt/β - catenin signaling pathways [302]. Likewise, SCs - Exos containing milk fat globule - EGF factor 8 (MFG - E8) suppress M1 polarization and promote M2 polarization through the SOCS3/STAT3 pathway, thereby exerting anti - inflammatory effects [170]. DPSCs - Exos have also been shown to reduce M1 macrophage polarization by modulating the ROS - MAPK - NFκB/p65 signaling pathway, contributing to inflammation resolution in SCI models [303]. Furthermore, peripheral Macrophages - Exos enhance microglial autophagy by inhibiting the PI3K/AKT/mTOR pathway, thereby promoting microglial polarization toward an anti - inflammatory phenotype, which likely contributes significantly to the overall anti - inflammatory response [240].
Exosomes exert anti - inflammatory effects through multiple mechanisms. They can reduce ROS levels and suppress the production of inflammatory cytokines by delivering bioactive molecules to the injury site. Additionally, exosomes inhibit the activation of microglia and macrophages, suppress M1 polarization while promoting M2 polarization, and activate autophagy in microglia. Overall, exosomes and their cargo modulate several key molecular signaling pathways, contributing to the mitigation of the pathological microenvironment following SCI.
Exosomes combined with biomaterials to repair SCI
Exosomes have demonstrated promising therapeutic effects in the treatment of SCI. However, studies have identified several limitations associated with their intravenous administration, including low bioavailability, short circulation half - life, and poor targeting efficiency. Consequently, there is an urgent need to explore alternative strategies, particularly the integration of medical biomaterials, to enhance the therapeutic efficacy of exosome - based treatments. Biomaterials, primarily derived from natural or synthetic polymers, are widely utilized in tissue engineering and regenerative medicine. These materials can generally be categorized into three major types: hydrogels, 3D - printed scaffolds, and nanomaterials (Figure 7).
Exosomes combined with hydrogels
Hydrogels are three - dimensional, hydrophilic polymers capable of retaining large amounts of water while closely mimicking the extracellular matrix (ECM). Their key properties, including biodegradability, adhesiveness, bioactivity, and tunable mechanical characteristics, make hydrogels ideal candidates for biomedical applications, particularly in tissue engineering and SCI repair [304]. Acting as structural scaffolds, hydrogels support cellular growth, migration, and differentiation while providing a favorable environment for the sustained release of therapeutic agents.
In the context of SCI therapy, combining exosomes with hydrogels offers several advantages. Hydrogels serve as delivery vehicles, encapsulating exosomes and enabling controlled, localized release at the injury site, thus extending their therapeutic effect. Their high-water content supports cellular infiltration, which is an essential process for tissue regeneration. Moreover, hydrogels can be engineered to degrade gradually, allowing the time - dependent release of exosomes and other bioactive molecules necessary for neuronal repair.
Recent studies have explored exosome - loaded hydrogels in SCI models. For instance, electroconductive hydrogels loaded with M2 Microglia - Exos promote neural stem cell and axon growth in the dorsal root ganglion, regulate M2 microglial polarization, reduce acute inflammation, and enhance neuronal and axonal regeneration, significantly improving functional recovery in SCI rats [23]. Similarly, BMSCs - Exos embedded in electroconductive hydrogels modulate M2 polarization via the NF - κB pathway, promote the neuronal and oligodendrocyte differentiation of NSCs, suppress astrocyte differentiation, and facilitate axon outgrowth through the PTEN/PI3K/AKT/mTOR pathway [305]. Liu et al. developed a biomimetic magneto-electric hydrogel that integrates Fe3O4@BaTiO3 core-shell nanoparticles and HUMSC-Exos to enable remote, noninvasive electrical stimulation for the combined treatment of SCI. The Fe3O4@BaTiO3 nanoparticles are activated by an external magnetic field, generating electrical stimulation. This stimulation, coupled with the beneficial effects of HUMSC-Exos, effectively reduces early inflammatory responses following SCI and promotes the regeneration of new neurons and axons, thereby facilitating functional recovery after SCI [306]. Additionally, injectable decellularized extracellular matrix hydrogels loaded with cortical Neuron - Exos have been shown to induce early M2 macrophage polarization, reduce neuronal apoptosis, and establish a pro - regenerative microenvironment in rodent SCI models [190].
Hydrogels also possess tunable physical properties, such as elasticity and stiffness, which can be optimized to match the mechanical characteristics of native spinal cord tissue, thereby enhancing tissue integration. Taken together, the incorporation of exosomes into hydrogel platforms represents a promising strategy to improve the efficacy of exosome - based therapies for SCI repair.
Exosomes combined with 3D print
3D printing technologies have revolutionized tissue engineering by enabling the precise fabrication of scaffolds that replicate the complex architecture of biological tissues. The ability to create custom - designed scaffolds with tailored mechanical properties and geometries makes 3D printing a powerful tool for SCI repair [307]. These scaffolds can be used to deliver exosomes in a spatially and temporally controlled manner, providing a supportive microenvironment for tissue regeneration.
The integration of exosomes with 3D printing represents a novel strategy for SCI therapy. Also known as additive manufacturing, 3D printing enables the construction of complex, biomimetic scaffolds that closely resemble the architecture and mechanical characteristics of native spinal cord tissue. Exosomes can be incorporated into these scaffolds and released gradually to facilitate cell migration, differentiation, and neuroprotection at the injury site. For instance, plant - derived exosomes loaded with isoliquiritigenin, when embedded within 3D - printed bionic scaffolds, have been shown to promote functional recovery and repair of spinal cord injury [308].
Furthermore, 3D printing allows for the creation of anatomically precise scaffolds, ensuring that the implanted construct fits the injury site accurately. This minimizes the risk of tissue rejection and enhances therapeutic efficacy. Overall, the combination of exosomes with 3D - printed scaffolds represents an exciting advancement in SCI repair, offering a promising platform for personalized regenerative medicine.
Exosomes combined with nanomaterials
Nanomaterials are ideal for combination therapies with exosomes in SCI repair. This is because of their unique properties such as small size, high surface area, and the ability to interact with biological systems at the molecular level.
Nanomaterials can enhance the stability, bioavailability, and controlled release of exosomes, which in turn improves their effectiveness in promoting tissue regeneration and reducing inflammation. Recent studies have explored the application of exosome - loaded nanomaterials in SCI models. For instance, biocompatible macrophage - derived exosome - enclosed biomimetic manganese (Mn) - iron prussian blue analogues (MPBs) have been used for immunotherapy in SCI. These exosome - coated MPBs (E - MPBs) can enhance microglia accumulation, relieve the H2O2 - induced microenvironment, and inhibit apoptosis and inflammation in vitro [270]. Moreover, a nanofiber scaffold combined with a hyaluronic acid hydrogel patch has been reported to release both exosomes and methylprednisolone. This dual - release system promotes macrophage polarization from the M1 to M2 phenotype, thus reducing inflammation, and inhibits apoptosis to enhance neuronal survival after SCI. The underlying mechanisms are related to the TLR4/NF - κB, MAPK, and Akt/mTOR signaling pathways [24].
In conclusion, the integration of nanomaterials with exosomes has great potential for enhancing the precision and efficacy of SCI therapies, which may lead to better patient outcomes.
Engineering strategies for exosomes in SCI repair
Exosome engineering has emerged as a pivotal approach to enhance the therapeutic efficacy and specificity of exosome-based interventions for SCI. Various strategies have been developed to optimize the cargo content, targeting ability, and functional outcomes of exosomes.
Schematic diagram of exosomes combined with biomaterials for the treatment of SCI. Exosomes can be combined with hydrogel, 3D print biomaterials, and nanomaterials respectively to treat spinal cord injury. Created with BioRender.com.
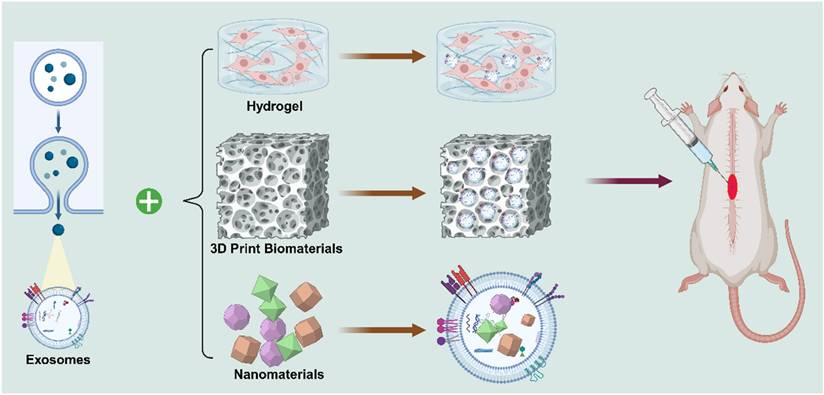
Cargo loading techniques are commonly categorized into endogenous and exogenous methods. Endogenous loading involves modifying donor cells to naturally package desired therapeutic molecules into exosomes during biogenesis, such as through overexpression of specific RNAs or proteins [309]. This method ensures stability and functional integration of the cargo. In contrast, exogenous loading directly introduces therapeutic agents into isolated exosomes using physical or chemical methods, including electroporation, sonication, extrusion, and chemical transfection [310, 311]. These techniques allow for a wider range of cargos, such as siRNAs, miRNAs, mRNAs, proteins, or small-molecule drugs, to be efficiently incorporated into exosomes. Recent advancements have improved the efficiency and preservation of exosomal integrity during loading, making them promising carriers for neuroprotective and anti-inflammatory agents in SCI models [312].
Surface modification of exosomes has also been employed to enhance their targeting capability toward injured spinal cord tissue. Genetic engineering of donor cells to express targeting ligands, peptides, or antibodies on exosome membranes has shown promising results in improving lesion site accumulation. Alternatively, post-isolation chemical conjugation strategies, such as click chemistry and lipid insertion, have been utilized to functionalize exosome surfaces without significantly affecting their biophysical properties [258, 313].
Overall, the rational design and engineering of exosomes provide a promising avenue for overcoming the current limitations of natural exosome therapies, including poor targeting, low cargo loading capacity, and suboptimal therapeutic potency. Future work should focus on optimizing loading efficiency, maintaining exosome integrity, and ensuring scalable, reproducible manufacturing processes for clinical translation.
Challenges in the application of exosomes to repair SCI
The application of exosomes in SCI repair faces several significant challenges. First, the isolation and purification of exosomes are complex processes. Yield, quality, and functional properties can vary depending on the cellular source and the isolation technique used. Therefore, standardizing exosome preparation protocols is essential to ensure consistency and reproducibility in therapeutic outcomes. Second, achieving targeted delivery to the injured spinal cord remains a major obstacle. Exosomes must traverse biological barriers and accumulate efficiently at the lesion site. Additionally, the potential immunogenicity and off - target effects of exosomes must be carefully assessed to avoid adverse immune responses or unintended tissue interactions.
Moreover, although exosomes exhibit generally low immunogenicity compared to cell therapies, recent studies suggest that their immunological safety should not be overlooked. Exosome membranes may retain immunogenic molecules from donor cells, and under pathological conditions or repeated administration, they could potentially elicit undesired immune responses. Therefore, careful characterization of exosomal surface markers and rigorous purification procedures are necessary to minimize immunogenicity risks. Long-term toxicity is another critical concern. Most current studies primarily assess short-term therapeutic effects, while data regarding the chronic biodistribution, clearance kinetics, and long-term safety of exosomes remain limited. Accumulation in non-target organs and potential off-target biological effects require thorough evaluation in future preclinical and clinical studies. Furthermore, exosome heterogeneity remains a major translational challenge. Exosomes derived even from the same cell type can exhibit substantial variability in size, cargo composition, and biological activity, depending on culture conditions, donor cell status, and isolation methods. Such heterogeneity may compromise the reproducibility and efficacy of exosome-based therapies. Therefore, standardized protocols for exosome production, isolation, and quality control are urgently needed to ensure consistent therapeutic outcomes.
Although exosomes have demonstrated neuroprotective and anti - inflammatory effects, their ability to fully regenerate damaged spinal cord tissue remains limited. Concerns regarding their stability, long - term therapeutic efficacy, and scalability for clinical - grade production continue to hinder widespread clinical translation. Addressing these challenges is critical to optimizing exosome - based therapies for SCI and realizing their full potential in regenerative medicine.
Conclusions and future orientation
Exosome - based therapies hold great promise for modulating the pathological microenvironment following SCI. They have demonstrated the ability to promote the integrity of BSCB and angiogenesis, inhibit neuronal apoptosis, provide neuroprotection, facilitate axonal regeneration, exert anti - inflammatory effects, and support overall tissue repair. Nevertheless, the clinical translation of exosome - based approaches remains limited by challenges related to isolation, purification, targeting specificity, and delivery efficiency. Despite encouraging results in preclinical studies, the regenerative capacity and long - term efficacy of exosomes are still constrained. Moreover, although preclinical studies have demonstrated encouraging results, the clinical translation of exosome-based therapies for SCI remains in its early stages. A recent phase I clinical trial evaluated safety and potential effects of intrathecal injection of HUC - MSCs - Exos in complete subacute SCI, which demonstrated that intrathecal administration of allogeneic HUC - MSCs - Exos is safe in patients with subacute SCI. However, challenges such as scalable exosome production, standardization of isolation protocols, and regulatory compliance remain critical hurdles to overcome.
Moving forward, future research should prioritize the optimization of exosome isolation and purification methods to ensure reproducibility and high - quality production. Additionally, the development of efficient and precise delivery strategies is crucial to enhance exosome accumulation at the injury site. Innovations in exosome engineering, such as surface modification to improve cellular uptake and minimize immunogenicity, may further advance their clinical utility. Moreover, combining exosome - based therapies with gene editing technologies or stem cell interventions could synergistically enhance their regenerative potential.
To fully realize the therapeutic potential of exosomes in SCI and other neurodegenerative conditions, it is imperative to overcome existing technical barriers and deepen our understanding of exosome biology, biodistribution, and mechanisms of action.
Acknowledgements
Funding
This study was funded by Natural Science Basic Research Program of Shaanxi (Program No. 2025JC-YBQN-249).
Author Contributions
Yanming Ma: Writing - original draft, Investigation, Conceptualization. Xiaojun Yu: Writing - original draft, Methodology, Investigation. Jinxing Pan: Writing - original draft, Investigation. Yingguang Wang: Investigation. Ruoyu Li: Writing - review & editing, Supervision. Huimin Hu: Writing - review & editing. Xiaodong Wang: Writing - review & editing, Supervision, Methodology. Dingjun Hao: Writing - review & editing, Supervision, Methodology.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Skinnider MA. et al. Reverse engineering spinal-cord injury. Nature. 2024;613:150-163
2. Christopher S. et al. Traumatic spinal cord injury. Nature Reviews Disease Primers. 2017;3:17019
3. Fehlings MG, Tetreault LA, Wilson JR, Kwon BK, Burns AS, Martin AR. et al. A Clinical Practice Guideline for the Management of Acute Spinal Cord Injury: Introduction, Rationale, and Scope. Global Spine J. 2017;7:84s-94s
4. Global regional, national burden of spinal cord injury 1990-2019. a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2023;22:1026-47
5. Mahar M, Cavalli V. Intrinsic mechanisms of neuronal axon regeneration. Nat Rev Neurosci. 2018;19:323-37
6. Liu Y, Zhao C, Zhang R, Pang Y, Li L, Feng S. Progression of mesenchymal stem cell regulation on imbalanced microenvironment after spinal cord injury. Stem Cell Res Ther. 2024;15:343
7. Jeon J, Park SH, Choi J, Han SM, Kim HW, Shim SR. et al. Association between neural stem/progenitor cells and biomaterials in spinal cord injury therapies: A systematic review and network meta-analysis. Acta Biomater. 2024;183:50-60
8. Ribeiro BF, da Cruz BC, de Sousa BM, Correia PD, David N, Rocha C. et al. Cell therapies for spinal cord injury: a review of the clinical trials and cell-type therapeutic potential. Brain. 2023;146:2672-93
9. Gao W, Kim MW, Dykstra T, Du S, Boskovic P, Lichti CF. et al. Engineered T cell therapy for central nervous system injury. Nature. 2024
10. Hosseini SM, Borys B, Karimi-Abdolrezaee S. Neural stem cell therapies for spinal cord injury repair: an update on recent preclinical and clinical advances. Brain. 2024;147:766-93
11. Ahuja CS, Mothe A, Khazaei M, Badhiwala JH, Gilbert EA, van der Kooy D. et al. The leading edge: Emerging neuroprotective and neuroregenerative cell-based therapies for spinal cord injury. Stem Cells Transl Med. 2020;9:1509-30
12. Yang L, Cao J, Du Y, Zhang X, Hong W, Peng B. et al. Initial IL-10 production dominates the therapy of mesenchymal stem cell scaffold in spinal cord injury. Theranostics. 2024;14:879-91
13. Li K, Liu Z, Wu P, Chen S, Wang M, Liu W. et al. Micro electrical fields induced MSC-sEVs attenuate neuronal cell apoptosis by activating autophagy via lncRNA MALAT1/miR-22-3p/SIRT1/AMPK axis in spinal cord injury. J Nanobiotechnology. 2023;21:451
14. Giovannelli L, Bari E, Jommi C, Tartara F, Armocida D, Garbossa D. et al. Mesenchymal stem cell secretome and extracellular vesicles for neurodegenerative diseases: Risk-benefit profile and next steps for the market access. Bioact Mater. 2023;29:16-35
15. Chen X, Liu Y, Stavrinou P, Stavrinou L, Hu W, Goldbrunner R. et al. Spinal cord injury: Olfactory ensheathing cell-based therapeutic strategies. J Neurosci Res. 2024;102:e25283
16. Nakhjavan-Shahraki B, Yousefifard M, Rahimi-Movaghar V, Baikpour M, Nasirinezhad F, Safari S. et al. Transplantation of olfactory ensheathing cells on functional recovery and neuropathic pain after spinal cord injury; systematic review and meta-analysis. Sci Rep. 2018;8:325
17. Iorio R, Petricca S, Di Emidio G, Falone S, Tatone C. Mitochondrial Extracellular Vesicles (mitoEVs): emerging mediators of cell-to-cell communication in health, aging and age-related diseases. Ageing Res Rev. 2024: 102522.
18. Garcia-Martin R, Wang G, Brandão BB, Zanotto TM, Shah S, Kumar Patel S. et al. MicroRNA sequence codes for small extracellular vesicle release and cellular retention. Nature. 2022;601:446-51
19. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020 367
20. Zhao J, Zhu W, Mao Y, Li X, Ling G, Luo C. et al. Unignored intracellular journey and biomedical applications of extracellular vesicles. Adv Drug Deliv Rev. 2024;212:115388
21. Nie X, Liu Y, Yuan T, Yu T, Yun Z, Xue W. et al. Platelet-rich plasma-derived exosomes promote blood-spinal cord barrier repair and attenuate neuroinflammation after spinal cord injury. J Nanobiotechnology. 2024;22:456
22. Miron RJ, Estrin NE, Sculean A, Zhang Y. Understanding exosomes: Part 2-Emerging leaders in regenerative medicine. Periodontol 2000. 2024;94:257-414
23. Guan P, Fan L, Zhu Z, Yang Q, Kang X, Li J. et al. M2 microglia-derived exosome-loaded electroconductive hydrogel for enhancing neurological recovery after spinal cord injury. J Nanobiotechnology. 2024;22:8
24. Zhu B, Gu G, Ren J, Song X, Li J, Wang C. et al. Schwann Cell-Derived Exosomes and Methylprednisolone Composite Patch for Spinal Cord Injury Repair. ACS Nano. 2023;17:22928-43
25. Li Y, Luo W, Meng C, Shi K, Gu R, Cui S. Exosomes as promising bioactive materials in the treatment of spinal cord injury. Stem Cell Res Ther. 2024;15:335
26. Fan B, Wei Z, Feng S. Progression in translational research on spinal cord injury based on microenvironment imbalance. Bone Res. 2022;10:35
27. Fan B, Wei Z, Yao X, Shi G, Cheng X, Zhou X. et al. Microenvironment Imbalance of Spinal Cord Injury. Cell Transplant. 2018;27:853-66
28. Zhou HY, Wang X, Li Y, Wang D, Zhou XZ, Xiao N. et al. Dynamic development of microglia and macrophages after spinal cord injury. Neural Regen Res. 2024
29. Yue WWS, Touhara KK, Toma K, Duan X, Julius D. Endogenous opioid signalling regulates spinal ependymal cell proliferation. Nature. 2024;634:407-14
30. Marangon D, Castro ESJH, Cerrato V, Boda E, Lecca D. Oligodendrocyte Progenitors in Glial Scar: A Bet on Remyelination. Cells. 2024 13
31. Sanati M, Manavi MA, Noruzi M, Behmadi H, Akbari T, Jalali S. et al. Carbohydrates and neurotrophic factors: A promising partnership for spinal cord injury rehabilitation. Biomater Adv. 2024;166:214054
32. Talifu Z, Zhang C, Xu X, Pan Y, Ke H, Li Z. et al. Neuronal repair after spinal cord injury by in vivo astrocyte reprogramming mediated by the overexpression of NeuroD1 and Neurogenin-2. Biol Res. 2024;57:53
33. Chaudhari LR, Kawale AA, Desai SS, Kashte SB, Joshi MG. Pathophysiology of Spinal Cord Injury and Tissue Engineering Approach for Its Neuronal Regeneration: Current Status and Future Prospects. Adv Exp Med Biol. 2023;1409:51-81
34. Gater DR Jr, Farkas GJ, Tiozzo E. Pathophysiology of Neurogenic Obesity After Spinal Cord Injury. Top Spinal Cord Inj Rehabil. 2021;27:1-10
35. Al Mamun A, Quan Z, Geng P, Wang S, Shao C, Xiao J. Targeting Remyelination in Spinal Cord Injury: Insights and Emerging Therapeutic Strategies. CNS Neurosci Ther. 2024;30:1-15
36. Zhang Y, Peng Z, Guo M, Wang Y, Liu J, Liu Y. et al. TET3-facilitated differentiation of human umbilical cord mesenchymal stem cells into oligodendrocyte precursor cells for spinal cord injury recovery. J Transl Med. 2024;22:1118
37. Li GL, Farooque M, Holtz A, Olsson Y. Apoptosis of oligodendrocytes occurs for long distances away from the primary injury after compression trauma to rat spinal cord. Acta Neuropathol. 1999;98:473-80
38. Fan H, Tang HB, Shan LQ, Liu SC, Huang DG, Chen X. et al. Quercetin prevents necroptosis of oligodendrocytes by inhibiting macrophages/microglia polarization to M1 phenotype after spinal cord injury in rats. J Neuroinflammation. 2019;16:206
39. Kanno H, Ozawa H, Sekiguchi A, Yamaya S, Itoi E. Induction of autophagy and autophagic cell death in damaged neural tissue after acute spinal cord injury in mice. Spine (Phila Pa 1976). 2011;36:E1427-34
40. Xu C, Mao L, Tian H, Lin S, Zhao X, Lin J. et al. MICAL1 (molecule interacting with CasL 1) protects oligodendrocyte cells from oxidative injury through regulating apoptosis, autophagy in spinal cord injury. Neurosci Lett. 2021;750:135712
41. Harlow DE, Macklin WB. Inhibitors of myelination: ECM changes, CSPGs and PTPs. Exp Neurol. 2014;251:39-46
42. Rabchevsky AG, Sullivan PG, Scheff SW. Temporal-spatial dynamics in oligodendrocyte and glial progenitor cell numbers throughout ventrolateral white matter following contusion spinal cord injury. Glia. 2007;55:831-43
43. Assinck P, Duncan GJ, Plemel JR, Lee MJ, Stratton JA, Manesh SB. et al. Myelinogenic Plasticity of Oligodendrocyte Precursor Cells following Spinal Cord Contusion Injury. J Neurosci. 2017;37:8635-54
44. Llorens-Bobadilla E, Chell JM, Le Merre P, Wu Y, Zamboni M, Bergenstråhle J. et al. A latent lineage potential in resident neural stem cells enables spinal cord repair. Science. 2020 370
45. Xing C, Liu S, Wang L, Ma H, Zhou M, Zhong H. et al. Metformin enhances endogenous neural stem cells proliferation, neuronal differentiation, and inhibits ferroptosis through activating AMPK pathway after spinal cord injury. J Transl Med. 2024;22:723
46. Benner EJ, Luciano D, Jo R, Abdi K, Paez-Gonzalez P, Sheng H. et al. Protective astrogenesis from the SVZ niche after injury is controlled by Notch modulator Thbs4. Nature. 2013;497:369-73
47. Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L. et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541:481-7
48. Dias DO, Kim H, Holl D, Werne Solnestam B, Lundeberg J, Carlén M. et al. Reducing Pericyte-Derived Scarring Promotes Recovery after Spinal Cord Injury. Cell. 2018;173:153-65.e22
49. Göritz C, Dias DO, Tomilin N, Barbacid M, Shupliakov O, Frisén J. A pericyte origin of spinal cord scar tissue. Science. 2011;333:238-42
50. Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146-56
51. Anderson MA, Burda JE, Ren Y, Ao Y, O'Shea TM, Kawaguchi R. et al. Astrocyte scar formation aids central nervous system axon regeneration. Nature. 2016;532:195-200
52. Wanner IB, Anderson MA, Song B, Levine J, Fernandez A, Gray-Thompson Z. et al. Glial scar borders are formed by newly proliferated, elongated astrocytes that interact to corral inflammatory and fibrotic cells via STAT3-dependent mechanisms after spinal cord injury. J Neurosci. 2013;33:12870-86
53. Verstappen K, Bieler L, Barroca N, Bronkhorst EM, Couillard-Després S, Leeuwenburgh SCG. et al. Application of Adipose Extracellular Matrix and Reduced Graphene Oxide Nanocomposites for Spinal Cord Injury Repair. Adv Healthc Mater. 2025;14:e2402775
54. Li J, Wang H, Li Y, Wang C, Feng H, Pang Y. et al. Novel carbon dots with dual Modulatory effects on the bone marrow and spleen as a potential therapeutic candidate for treating spinal cord injury. Bioact Mater. 2025;45:534-50
55. Liu X, Du J, Sun J, Wang H, An J, Li Y. et al. Borneol-Functionalized Macrophage Membrane-Encapsulated Mesoporous Selenium Nanoparticles Loaded with Resveratrol for the Treatment of Spinal Cord Injury. ACS Appl Mater Interfaces. 2024;16:63170-85
56. Ahuja CS, Nori S, Tetreault L, Wilson J, Kwon B, Harrop J. et al. Traumatic Spinal Cord Injury-Repair and Regeneration. Neurosurgery. 2017;80:S9-s22
57. Perrin FE, Lacroix S, Avilés-Trigueros M, David S. Involvement of monocyte chemoattractant protein-1, macrophage inflammatory protein-1alpha and interleukin-1beta in Wallerian degeneration. Brain. 2005;128:854-66
58. Liu Z, Xiang C, Zhao X, Aizawa T, Niu R, Zhao J. et al. Regulation of dynamic spatiotemporal inflammation by nanomaterials in spinal cord injury. J Nanobiotechnology. 2024;22:767
59. David S, Kroner A. Repertoire of microglial and macrophage responses after spinal cord injury. Nat Rev Neurosci. 2011;12:388-99
60. Nakajima H, Honjoh K, Watanabe S, Kubota A, Matsumine A. Distribution and polarization of microglia and macrophages at injured sites and the lumbar enlargement after spinal cord injury. Neurosci Lett. 2020;737:135152
61. Milich LM, Ryan CB, Lee JK. The origin, fate, and contribution of macrophages to spinal cord injury pathology. Acta Neuropathol. 2019;137:785-97
62. Hu X, Leak RK, Shi Y, Suenaga J, Gao Y, Zheng P. et al. Microglial and macrophage polarization—new prospects for brain repair. Nat Rev Neurol. 2015;11:56-64
63. Shahrezaei A, Sohani M, Sohouli M, Taherkhani S, Nasirinezhad F. The involvement and significance of M2 macrophages in neuropathic pain following spinal cord injury: a systematic review. J Physiol Sci. 2024;74:45
64. An N, Yang J, Wang H, Sun S, Wu H, Li L. et al. Mechanism of mesenchymal stem cells in spinal cord injury repair through macrophage polarization. Cell Biosci. 2021;11:41
65. Wang J, Tian F, Cao L, Du R, Tong J, Ding X. et al. Macrophage polarization in spinal cord injury repair and the possible role of microRNAs: A review. Heliyon. 2023;9:e22914
66. Xiao CL, Lai HT, Zhou JJ, Liu WY, Zhao M, Zhao K. Nrf2 Signaling Pathway: Focus on Oxidative Stress in Spinal Cord Injury. Mol Neurobiol. 2025;62:2230-49
67. Liu H, Zhang J, Xu X, Lu S, Yang D, Xie C. et al. SARM1 promotes neuroinflammation and inhibits neural regeneration after spinal cord injury through NF-κB signaling. Theranostics. 2021;11:4187-206
68. Song Q, Cui Q, Sun S, Wang Y, Yuan Y, Zhang L. Crosstalk Between Cell Death and Spinal Cord Injury: Neurology and Therapy. Mol Neurobiol. 2024
69. Hu X, Xu W, Ren Y, Wang Z, He X, Huang R. et al. Spinal cord injury: molecular mechanisms and therapeutic interventions. Signal Transduct Target Ther. 2023;8:245
70. Li C, Wu Z, Zhou L, Shao J, Hu X, Xu W. et al. Temporal and spatial cellular and molecular pathological alterations with single-cell resolution in the adult spinal cord after injury. Signal Transduct Target Ther. 2022;7:65
71. Alizadeh A, Dyck SM, Karimi-Abdolrezaee S. Traumatic Spinal Cord Injury: An Overview of Pathophysiology, Models and Acute Injury Mechanisms. Front Neurol. 2019;10:282
72. Hu X, Xu Y, Zhang H, Li Y, Wang X, Xu C. et al. Role of necroptosis in traumatic brain and spinal cord injuries. J Adv Res. 2022;40:125-34
73. Shi Z, Yuan S, Shi L, Li J, Ning G, Kong X. et al. Programmed cell death in spinal cord injury pathogenesis and therapy. Cell Prolif. 2021;54:e12992
74. Wang Q, Wang X, Shang Z, Zhao L. Mechanism and prospects of mitochondrial transplantation for spinal cord injury treatment. Stem Cell Res Ther. 2024;15:457
75. Zhang HY, Wang ZG, Lu XH, Kong XX, Wu FZ, Lin L. et al. Endoplasmic reticulum stress: relevance and therapeutics in central nervous system diseases. Mol Neurobiol. 2015;51:1343-52
76. Xu X, Lai Y, Hua ZC. Apoptosis and apoptotic body: disease message and therapeutic target potentials. Biosci Rep. 2019 39
77. Shen Y, Wang YP, Cheng X, Yang X, Wang G. Autophagy regulation combined with stem cell therapy for treatment of spinal cord injury. Neural Regen Res. 2023;18:1629-36
78. Griffey CJ, Yamamoto A. Macroautophagy in CNS health and disease. Nat Rev Neurosci. 2022;23:411-27
79. Li Z, Chen T, Cao Y, Jiang X, Lin H, Zhang J. et al. Pros and Cons: Autophagy in Acute Spinal Cord Injury. Neurosci Bull. 2019;35:941-5
80. Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE. et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060-72
81. Tang D, Chen X, Kang R, Kroemer G. Ferroptosis: molecular mechanisms and health implications. Cell Res. 2021;31:107-25
82. Feng Z, Min L, Chen H, Deng W, Tan M, Liu H. et al. Iron overload in the motor cortex induces neuronal ferroptosis following spinal cord injury. Redox Biol. 2021;43:101984
83. Li F, Wang H, Chen H, Guo J, Dang X, Ru Y. et al. Mechanism of Ferroptosis and Its Role in Spinal Cord Injury. Front Neurol. 2022;13:926780
84. Vainchtein ID, Chin G, Cho FS, Kelley KW, Miller JG, Chien EC. et al. Astrocyte-derived interleukin-33 promotes microglial synapse engulfment and neural circuit development. Science. 2018;359:1269-73
85. Lee SY, Chung WS. The roles of astrocytic phagocytosis in maintaining homeostasis of brains. J Pharmacol Sci. 2021;145:223-7
86. Lee JH, Kim JY, Noh S, Lee H, Lee SY, Mun JY. et al. Astrocytes phagocytose adult hippocampal synapses for circuit homeostasis. Nature. 2021;590:612-7
87. Hara M, Kobayakawa K, Ohkawa Y, Kumamaru H, Yokota K, Saito T. et al. Interaction of reactive astrocytes with type I collagen induces astrocytic scar formation through the integrin-N-cadherin pathway after spinal cord injury. Nat Med. 2017;23:818-28
88. Hou J, Bi H, Ge Q, Teng H, Wan G, Yu B. et al. Heterogeneity analysis of astrocytes following spinal cord injury at single-cell resolution. Faseb j. 2022;36:e22442
89. Li Q, Gao S, Qi Y, Shi N, Wang Z, Saiding Q. et al. Regulating Astrocytes via Short Fibers for Spinal Cord Repair. Adv Sci (Weinh). 2024;11:e2406742
90. Karimi-Abdolrezaee S, Billakanti R. Reactive astrogliosis after spinal cord injury-beneficial and detrimental effects. Mol Neurobiol. 2012;46:251-64
91. Orr MB, Gensel JC. Spinal Cord Injury Scarring and Inflammation: Therapies Targeting Glial and Inflammatory Responses. Neurotherapeutics. 2018;15:541-53
92. Zhou X, Wahane S, Friedl M-S, Kluge M, Friedel CC, Avrampou K. et al. Microglia and macrophages promote corralling, wound compaction and recovery after spinal cord injury via Plexin-B2. Nature Neuroscience. 2020;23:337-50
93. Hassanshahi G, Amin M, Shunmugavel A, Vazirinejad R, Vakilian A, Sanji M. et al. Temporal expression profile of CXC chemokines in serum of patients with spinal cord injury. Neurochem Int. 2013;63:363-7
94. Mordillo-Mateos L, Sánchez-Ramos A, Coperchini F, Bustos-Guadamillas I, Alonso-Bonilla C, Vargas-Baquero E. et al. Development of chronic pain in males with traumatic spinal cord injury: role of circulating levels of the chemokines CCL2 and CXCL10 in subacute stage. Spinal Cord. 2019;57:953-9
95. Ma J, Zhang M, Fu P, Yin X, Chen Z. Chemokines play a role in nerve damage and neuroprotection in vascular dementia. IBRO Neurosci Rep. 2024;17:154-60
96. Bertels H, Vicente-Ortiz G, El Kanbi K, Takeoka A. Neurotransmitter phenotype switching by spinal excitatory interneurons regulates locomotor recovery after spinal cord injury. Nat Neurosci. 2022;25:617-29
97. Paulose CS, John PS, Chinthu R, Akhilraj PR, Anju TR. Spinal cord regeneration by modulating bone marrow with neurotransmitters and Citicholine: Analysis at micromolecular level. Biomed J. 2017;40:94-100
98. Zuo Y, Ye J, Cai W, Guo B, Chen X, Lin L. et al. Controlled delivery of a neurotransmitter-agonist conjugate for functional recovery after severe spinal cord injury. Nat Nanotechnol. 2023;18:1230-40
99. Gwak YS, Hulsebosch CE. GABA and central neuropathic pain following spinal cord injury. Neuropharmacology. 2011;60:799-808
100. Shamsnia HS, Peyrovinasab A, Amirlou D, Sirouskabiri S, Rostamian F, Basiri N. et al. BDNF-TrkB Signaling Pathway in Spinal Cord Injury: Insights and Implications. Mol Neurobiol. 2024
101. Gao X, Cheng W, Zhang X, Zhou Z, Ding Z, Zhou X. et al. Nerve Growth Factor-Laden Anisotropic Silk Nanofiber Hydrogels to Regulate Neuronal/Astroglial Differentiation for Scarless Spinal Cord Repair. ACS Appl Mater Interfaces. 2022;14:3701-15
102. Wu Q, Xiang Z, Ying Y, Huang Z, Tu Y, Chen M. et al. Nerve growth factor (NGF) with hypoxia response elements loaded by adeno-associated virus (AAV) combined with neural stem cells improve the spinal cord injury recovery. Cell Death Discov. 2021;7:301
103. Keefe KM, Sheikh IS, Smith GM. Targeting Neurotrophins to Specific Populations of Neurons: NGF, BDNF, and NT-3 and Their Relevance for Treatment of Spinal Cord Injury. Int J Mol Sci. 2017 18
104. Beattie MS, Harrington AW, Lee R, Kim JY, Boyce SL, Longo FM. et al. ProNGF induces p75-mediated death of oligodendrocytes following spinal cord injury. Neuron. 2002;36:375-86
105. Wong I, Liao H, Bai X, Zaknic A, Zhong J, Guan Y. et al. ProBDNF inhibits infiltration of ED1+ macrophages after spinal cord injury. Brain Behav Immun. 2010;24:585-97
106. Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 2014;15:786-801
107. Zheng B, Tuszynski MH. Regulation of axonal regeneration after mammalian spinal cord injury. Nat Rev Mol Cell Biol. 2023;24:396-413
108. Chaves Filho AJM, Mottin M, Lós DB, Andrade CH, Macedo DS. The tetrapartite synapse in neuropsychiatric disorders: Matrix metalloproteinases (MMPs) as promising targets for treatment and rational drug design. Biochimie. 2022;201:79-99
109. Shen Y, Tenney AP, Busch SA, Horn KP, Cuascut FX, Liu K. et al. PTPsigma is a receptor for chondroitin sulfate proteoglycan, an inhibitor of neural regeneration. Science. 2009;326:592-6
110. Roy A, Pathak Z, Kumar H. Strategies to neutralize RhoA/ROCK pathway after spinal cord injury. Exp Neurol. 2021;343:113794
111. Wang H, Feng N, Liu C, Xie Y, Zhou Z, Zhao H. et al. Inhibition of CSPG-PTPσ Activates Autophagy Flux and Lysosome Fusion, Aids Axon and Synaptic Reorganization in Spinal Cord Injury. Mol Neurobiol. 2024
112. Francos-Quijorna I, Sánchez-Petidier M, Burnside ER, Badea SR, Torres-Espin A, Marshall L. et al. Chondroitin sulfate proteoglycans prevent immune cell phenotypic conversion and inflammation resolution via TLR4 in rodent models of spinal cord injury. Nat Commun. 2022;13:2933
113. Tkach M, Théry C. Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell. 2016;164:1226-32
114. Frazer AC, Stewart HC. Ultramicroscopic particles in normal human blood. J Physiol. 1937;90:18-30
115. Anderson HC. Vesicles associated with calcification in the matrix of epiphyseal cartilage. J Cell Biol. 1969;41:59-72
116. Trams EG, Lauter CJ, Salem N Jr, Heine U. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim Biophys Acta. 1981;645:63-70
117. Harding C, Heuser J, Stahl P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol. 1983;97:329-39
118. Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem. 1987;262:9412-20
119. Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ. et al. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183:1161-72
120. Simpson RJ, Lim JW, Moritz RL, Mathivanan S. Exosomes: proteomic insights and diagnostic potential. Expert Rev Proteomics. 2009;6:267-83
121. Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654-9
122. Fauré J, Lachenal G, Court M, Hirrlinger J, Chatellard-Causse C, Blot B. et al. Exosomes are released by cultured cortical neurones. Molecular and Cellular Neuroscience. 2006;31:642-8
123. Zheng X, Chen F, Zhang J, Zhang Q, Lin J. Exosome analysis: a promising biomarker system with special attention to saliva. J Membr Biol. 2014;247:1129-36
124. Chivet M, Javalet C, Laulagnier K, Blot B, Hemming FJ, Sadoul R. Exosomes secreted by cortical neurons upon glutamatergic synapse activation specifically interact with neurons. J Extracell Vesicles. 2014;3:24722
125. de Rivero Vaccari JP, Brand F 3rd, Adamczak S, Lee SW, Perez-Barcena J, Wang MY. et al. Exosome-mediated inflammasome signaling after central nervous system injury. J Neurochem. 2016;136(Suppl 1):39-48
126. Huang JH, Yin XM, Xu Y, Xu CC, Lin X, Ye FB. et al. Systemic Administration of Exosomes Released from Mesenchymal Stromal Cells Attenuates Apoptosis, Inflammation, and Promotes Angiogenesis after Spinal Cord Injury in Rats. J Neurotrauma. 2017;34:3388-96
127. He C, Zheng S, Luo Y, Wang B. Exosome Theranostics: Biology and Translational Medicine. Theranostics. 2018;8:237-55
128. Lin S, Yu Z, Chen D, Wang Z, Miao J, Li Q. et al. Progress in Microfluidics-Based Exosome Separation and Detection Technologies for Diagnostic Applications. Small. 2020;16:e1903916
129. Yang Q, Li S, Ou H, Zhang Y, Zhu G, Li S. et al. Exosome-based delivery strategies for tumor therapy: an update on modification, loading, and clinical application. J Nanobiotechnology. 2024;22:41
130. Ciardiello C, Cavallini L, Spinelli C, Yang J, Reis-Sobreiro M, de Candia P. et al. Focus on Extracellular Vesicles: New Frontiers of Cell-to-Cell Communication in Cancer. Int J Mol Sci. 2016;17:175
131. Li Y, Zhu Z, Li S, Xie X, Qin L, Zhang Q. et al. Exosomes: compositions, biogenesis, and mechanisms in diabetic wound healing. J Nanobiotechnology. 2024;22:398
132. Mathieu M, Martin-Jaular L, Lavieu G, Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21:9-17
133. Choi D-S, Kim D-K, Kim Y-K, Gho YS. Proteomics, transcriptomics and lipidomics of exosomes and ectosomes. PROTEOMICS. 2013;13:1554-71
134. Subra C, Laulagnier K, Perret B, Record M. Exosome lipidomics unravels lipid sorting at the level of multivesicular bodies. Biochimie. 2007;89:205-12
135. Kang M, Li Z, Chang I, Xu C, Chiang M, Kim L. et al. Phosphatidylserine-incorporated exosome mimetics encapsulating CXCR3 antagonist alleviate osteoporosis. Adv Funct Mater. 2024 34
136. Kumar A, Sundaram K, Mu J, Dryden GW, Sriwastva MK, Lei C. et al. High-fat diet-induced upregulation of exosomal phosphatidylcholine contributes to insulin resistance. Nat Commun. 2021;12:213
137. Kong X, Patel NA, Chalfant CE, Cooper DR. Ceramide synthesis regulates biogenesis and packaging of exosomal MALAT1 from adipose derived stem cells, increases dermal fibroblast migration and mitochondrial function. Cell Commun Signal. 2023;21:221
138. Fayyazpour P, Fayyazpour A, Abbasi K, Vaez-Gharamaleki Y, Zangbar MS, Raeisi M. et al. The role of exosomes in cancer biology by shedding light on their lipid contents. Pathol Res Pract. 2023;250:154813
139. Kugeratski FG, Hodge K, Lilla S, McAndrews KM, Zhou X, Hwang RF. et al. Quantitative proteomics identifies the core proteome of exosomes with syntenin-1 as the highest abundant protein and a putative universal biomarker. Nat Cell Biol. 2021;23:631-41
140. Yang Y, Hong Y, Cho E, Kim GB, Kim IS. Extracellular vesicles as a platform for membrane-associated therapeutic protein delivery. J Extracell Vesicles. 2018;7:1440131
141. Hánělová K, Raudenská M, Masařík M, Balvan J. Protein cargo in extracellular vesicles as the key mediator in the progression of cancer. Cell Commun Signal. 2024;22:25
142. Duke LC, Cone AS, Sun L, Dittmer DP, Meckes DG, Tomko RJ Jr. Tetraspanin CD9 alters cellular trafficking and endocytosis of tetraspanin CD63, affecting CD63 packaging into small extracellular vesicles. J Biol Chem. 2025: 108255.
143. Gurunathan S, Kang MH, Jeyaraj M, Qasim M, Kim JH. Review of the Isolation, Characterization, Biological Function, and Multifarious Therapeutic Approaches of Exosomes. Cells. 2019 8
144. Sharma Y, Mohanty S. Targeted knockdown of MSC-sEVs biogenesis regulator proteins to elucidate the mechanisms of their production: a step towards translational applications. Cytotherapy. 2025
145. Bergqvist M, Park KS, Karimi N, Yu L, Lässer C, Lötvall J. Extracellular vesicle surface engineering with integrins (ITGAL & ITGB2) to specifically target ICAM-1-expressing endothelial cells. J Nanobiotechnology. 2025;23:64
146. Budnik V, Ruiz-Cañada C, Wendler F. Extracellular vesicles round off communication in the nervous system. Nature Reviews Neuroscience. 2016;17:160-72
147. Bobrie A, Colombo M, Krumeich S, Raposo G, Théry C. Diverse subpopulations of vesicles secreted by different intracellular mechanisms are present in exosome preparations obtained by differential ultracentrifugation. J Extracell Vesicles. 2012 1
148. Nouri Z, Barfar A, Perseh S, Motasadizadeh H, Maghsoudian S, Fatahi Y. et al. Exosomes as therapeutic and drug delivery vehicle for neurodegenerative diseases. J Nanobiotechnology. 2024;22:463
149. King HW, Michael MZ, Gleadle JM. Hypoxic enhancement of exosome release by breast cancer cells. BMC Cancer. 2012;12:421
150. Li L, Mu J, Zhang Y, Zhang C, Ma T, Chen L. et al. Stimulation by Exosomes from Hypoxia Preconditioned Human Umbilical Vein Endothelial Cells Facilitates Mesenchymal Stem Cells Angiogenic Function for Spinal Cord Repair. ACS Nano. 2022;16:10811-23
151. Guo R, Wu Z, Liu A, Li Q, Han T, Shen C. Hypoxic preconditioning-engineered bone marrow mesenchymal stem cell-derived exosomes promote muscle satellite cell activation and skeletal muscle regeneration via the miR-210-3p/KLF7 mechanism. Int Immunopharmacol. 2024;142:113143
152. Krylova SV, Feng D. The Machinery of Exosomes: Biogenesis, Release, and Uptake. Int J Mol Sci. 2023 24
153. Chen J, Zhang C, Li S, Li Z, Lai X, Xia Q. Exosomes Derived from Nerve Stem Cells Loaded with FTY720 Promote the Recovery after Spinal Cord Injury in Rats by PTEN/AKT Signal Pathway. J Immunol Res. 2021;2021:8100298
154. Ma K, Xu H, Zhang J, Zhao F, Liang H, Sun H. et al. Insulin-like growth factor-1 enhances neuroprotective effects of neural stem cell exosomes after spinal cord injury via an miR-219a-2-3p/YY1 mechanism. Aging (Albany NY). 2019;11:12278-94
155. Zhang L, Han P. Neural stem cell-derived exosomes suppress neuronal cell apoptosis by activating autophagy via miR-374-5p/STK-4 axis in spinal cord injury. J Musculoskelet Neuronal Interact. 2022;22:411-21
156. Zhong D, Cao Y, Li CJ, Li M, Rong ZJ, Jiang L. et al. Neural stem cell-derived exosomes facilitate spinal cord functional recovery after injury by promoting angiogenesis. Exp Biol Med (Maywood). 2020;245:54-65
157. Jiang D, Gong F, Ge X, Lv C, Huang C, Feng S. et al. Neuron-derived exosomes-transmitted miR-124-3p protect traumatically injured spinal cord by suppressing the activation of neurotoxic microglia and astrocytes. J Nanobiotechnology. 2020;18:105
158. Yin Z, Han Z, Hu T, Zhang S, Ge X, Huang S. et al. Neuron-derived exosomes with high miR-21-5p expression promoted polarization of M1 microglia in culture. Brain Behav Immun. 2020;83:270-82
159. Zhang J, Hu D, Li L, Qu D, Shi W, Xie L. et al. M2 Microglia-derived Exosomes Promote Spinal Cord Injury Recovery in Mice by Alleviating A1 Astrocyte Activation. Mol Neurobiol. 2024;61:7009-25
160. Zhou Z, Li C, Bao T, Zhao X, Xiong W, Luo C. et al. Exosome-Shuttled miR-672-5p from Anti-Inflammatory Microglia Repair Traumatic Spinal Cord Injury by Inhibiting AIM2/ASC/Caspase-1 Signaling Pathway Mediated Neuronal Pyroptosis. J Neurotrauma. 2022;39:1057-74
161. Ji XY, Guo YX, Wang LB, Wu WC, Wang JQ, He J. et al. Microglia-derived exosomes modulate myelin regeneration via miR-615-5p/MYRF axis. J Neuroinflammation. 2024;21:29
162. Li C, Qin T, Liu Y, Wen H, Zhao J, Luo Z. et al. Microglia-Derived Exosomal microRNA-151-3p Enhances Functional Healing After Spinal Cord Injury by Attenuating Neuronal Apoptosis via Regulating the p53/p21/CDK1 Signaling Pathway. Front Cell Dev Biol. 2021;9:783017
163. Peng W, Wan L, Luo Z, Xie Y, Liu Y, Huang T. et al. Microglia-Derived Exosomes Improve Spinal Cord Functional Recovery after Injury via Inhibiting Oxidative Stress and Promoting the Survival and Function of Endothelia Cells. Oxid Med Cell Longev. 2021;2021:1695087
164. Wu W, Liu J, Yang C, Xu Z, Huang J, Lin J. Astrocyte-derived exosome-transported microRNA-34c is neuroprotective against cerebral ischemia/reperfusion injury via TLR7 and the NF-κB/MAPK pathways. Brain Res Bull. 2020;163:84-94
165. Long X, Yao X, Jiang Q, Yang Y, He X, Tian W. et al. Astrocyte-derived exosomes enriched with miR-873a-5p inhibit neuroinflammation via microglia phenotype modulation after traumatic brain injury. J Neuroinflammation. 2020;17:89
166. Qian Y, Li X, Li G, Liu H, Li Q, Liu X. et al. Astrocyte-Derived Exosomal miR-148a-3p Suppresses Neuroinflammation and Restores Neurological Function in Traumatic Brain Injury by Regulating the Microglial Phenotype. eNeuro. 2024 11
167. Zhang W, Hong J, Zhang H, Zheng W, Yang Y. Astrocyte-derived exosomes protect hippocampal neurons after traumatic brain injury by suppressing mitochondrial oxidative stress and apoptosis. Aging (Albany NY). 2021;13:21642-58
168. Zhu S, Ma H, Hou M, Li H, Ning G. Schwann Cell-Derived Exosomes Induced Axon Growth after Spinal Cord Injury by Decreasing PTP-σ Activation on CSPGs via the Rho/ROCK Pathway. Neurochem Res. 2024;49:2120-30
169. Xu B, Zhou Z, Fang J, Wang J, Tao K, Liu J. et al. Exosomes derived from schwann cells alleviate mitochondrial dysfunction and necroptosis after spinal cord injury via AMPK signaling pathway-mediated mitophagy. Free Radic Biol Med. 2023;208:319-33
170. Ren J, Zhu B, Gu G, Zhang W, Li J, Wang H. et al. Schwann cell-derived exosomes containing MFG-E8 modify macrophage/microglial polarization for attenuating inflammation via the SOCS3/STAT3 pathway after spinal cord injury. Cell Death Dis. 2023;14:70
171. Pan D, Li Y, Yang F, Lv Z, Zhu S, Shao Y. et al. Increasing toll-like receptor 2 on astrocytes induced by Schwann cell-derived exosomes promotes recovery by inhibiting CSPGs deposition after spinal cord injury. J Neuroinflammation. 2021;18:172
172. Pan D, Zhu S, Zhang W, Wei Z, Yang F, Guo Z. et al. Autophagy induced by Schwann cell-derived exosomes promotes recovery after spinal cord injury in rats. Biotechnol Lett. 2022;44:129-42
173. Fan H, Chen Z, Tang HB, Shan LQ, Chen ZY, Wang XH. et al. Exosomes derived from olfactory ensheathing cells provided neuroprotection for spinal cord injury by switching the phenotype of macrophages/microglia. Bioeng Transl Med. 2022;7:e10287
174. Chen Z, Fan H, Chen ZY, Jiang C, Feng MZ, Guo XY. et al. OECs Prevented Neuronal Cells from Apoptosis Partially Through Exosome-derived BDNF. J Mol Neurosci. 2022;72:2497-506
175. Kumamaru H, Kadoya K, Adler AF, Takashima Y, Graham L, Coppola G. et al. Generation and post-injury integration of human spinal cord neural stem cells. Nat Methods. 2018;15:723-31
176. Li Z, Qi Y, Sun L, Li Z, Chen S, Zhang Y. et al. Three-dimensional nanofibrous sponges with aligned architecture and controlled hierarchy regulate neural stem cell fate for spinal cord regeneration. Theranostics. 2023;13:4762-80
177. Nekanti U, Sakthivel PS, Zahedi A, Creasman DA, Nishi RA, Dumont CM. et al. Multichannel bridges and NSC synergize to enhance axon regeneration, myelination, synaptic reconnection, and recovery after SCI. NPJ Regen Med. 2024;9:12
178. Zhao H, Xiong T, Chu Y, Hao W, Zhao T, Sun X. et al. Biomimetic Dual-Network Collagen Fibers with Porous and Mechanical Cues Reconstruct Neural Stem Cell Niche via AKT/YAP Mechanotransduction after Spinal Cord Injury. Small. 2024;20:e2311456
179. De Gioia R, Biella F, Citterio G, Rizzo F, Abati E, Nizzardo M. et al. Neural Stem Cell Transplantation for Neurodegenerative Diseases. International Journal of Molecular Sciences. 2020;21:3103
180. Svendsen SP, Svendsen CN. Cell therapy for neurological disorders. Nature Medicine. 2024;30:2756-70
181. Yu T, Yang LL, Zhou Y, Wu MF, Jiao JH. Exosome-mediated repair of spinal cord injury: a promising therapeutic strategy. Stem Cell Res Ther. 2024;15:6
182. Assinck P, Duncan GJ, Hilton BJ, Plemel JR, Tetzlaff W. Cell transplantation therapy for spinal cord injury. Nature Neuroscience. 2017;20:637-47
183. Li S, Li J, Chen G, Lin T, Zhang P, Tong K. et al. Exosomes originating from neural stem cells undergoing necroptosis participate in cellular communication by inducing TSC2 upregulation of recipient cells following spinal cord injury. Neural Regen Res. 2024
184. Webb RL, Kaiser EE, Scoville SL, Thompson TA, Fatima S, Pandya C. et al. Human Neural Stem Cell Extracellular Vesicles Improve Tissue and Functional Recovery in the Murine Thromboembolic Stroke Model. Transl Stroke Res. 2018;9:530-9
185. Li X, Zhu Y, Wang Y, Xia X, Zheng JC. Neural stem/progenitor cell-derived extracellular vesicles: A novel therapy for neurological diseases and beyond. MedComm (2020). 2023;4:e214
186. Ma D, Fu C, Li F, Ruan R, Lin Y, Li X. et al. Functional biomaterials for modulating the dysfunctional pathological microenvironment of spinal cord injury. Bioact Mater. 2024;39:521-43
187. Almeida A, Jimenez-Blasco D, Bolaños JP. Cross-talk between energy and redox metabolism in astrocyte-neuron functional cooperation. Essays Biochem. 2023;67:17-26
188. Tomé D, Almeida RD. The injured axon: intrinsic mechanisms driving axonal regeneration. Trends Neurosci. 2024
189. Xu Y, Zhu ZH, Xu X, Sun HT, Zheng HM, Zhang JL. et al. Neuron-Derived Exosomes Promote the Recovery of Spinal Cord Injury by Modulating Nerve Cells in the Cellular Microenvironment of the Lesion Area. Mol Neurobiol. 2023;60:4502-16
190. Wang G, Li Q, Liu S, Li M, Liu B, Zhao T. et al. An injectable decellularized extracellular matrix hydrogel with cortical neuron-derived exosomes enhances tissue repair following traumatic spinal cord injury. Mater Today Bio. 2024;28:101250
191. Nayak D, Roth TL, McGavern DB. Microglia development and function. Annu Rev Immunol. 2014;32:367-402
192. Schreiner TG, Schreiner OD, Ciobanu RC. Spinal Cord Injury Management Based on Microglia-Targeting Therapies. J Clin Med. 2024 13
193. Bellver-Landete V, Bretheau F, Mailhot B, Vallières N, Lessard M, Janelle ME. et al. Microglia are an essential component of the neuroprotective scar that forms after spinal cord injury. Nat Commun. 2019;10:518
194. Sun M, You H, Hu X, Luo Y, Zhang Z, Song Y. et al. Microglia-Astrocyte Interaction in Neural Development and Neural Pathogenesis. Cells. 2023 12
195. Parthasarathy G, Pattison MB, Midkiff CC. The FGF/FGFR system in the microglial neuroinflammation with Borrelia burgdorferi: likely intersectionality with other neurological conditions. J Neuroinflammation. 2023;20:10
196. Hering C, Shetty AK. Extracellular Vesicles Derived From Neural Stem Cells, Astrocytes, and Microglia as Therapeutics for Easing TBI-Induced Brain Dysfunction. Stem Cells Transl Med. 2023;12:140-53
197. Wang Y, Li D, Zhang L, Yin Z, Han Z, Ge X. et al. Exosomes derived from microglia overexpressing miR-124-3p alleviate neuronal endoplasmic reticulum stress damage after repetitive mild traumatic brain injury. Neural Regen Res. 2024;19:2010-8
198. Ceccarelli L, Giacomelli C, Marchetti L, Martini C. Microglia extracellular vesicles: focus on molecular composition and biological function. Biochem Soc Trans. 2021;49:1779-90
199. Huang S, Ge X, Yu J, Han Z, Yin Z, Li Y. et al. Increased miR-124-3p in microglial exosomes following traumatic brain injury inhibits neuronal inflammation and contributes to neurite outgrowth via their transfer into neurons. Faseb j. 2018;32:512-28
200. Luo Z, Peng W, Xu Y, Xie Y, Liu Y, Lu H. et al. Exosomal OTULIN from M2 macrophages promotes the recovery of spinal cord injuries via stimulating Wnt/β-catenin pathway-mediated vascular regeneration. Acta Biomater. 2021;136:519-32
201. Zhao Y, Huang Y, Cao Y, Yang J. Astrocyte-Mediated Neuroinflammation in Neurological Conditions. Biomolecules. 2024 14
202. Giovannoni F, Quintana FJ. The Role of Astrocytes in CNS Inflammation. Trends Immunol. 2020;41:805-19
203. Bilalova A, Tutova O, Mukhamedshina Y, Rizvanov A. Astrocytes in Spinal Cord Injury: Current Opportunities and Prospects for Directional Polarization. Front Biosci (Landmark Ed). 2024;29:94
204. Khodadadei F, Arshad R, Morales DM, Gluski J, Marupudi NI, McAllister JP 2nd. et al. The effect of A1 and A2 reactive astrocyte expression on hydrocephalus shunt failure. Fluids Barriers CNS. 2022;19:78
205. Xu H, Li H, Zhang P, Gao Y, Ma H, Gao T. et al. The functions of exosomes targeting astrocytes and astrocyte-derived exosomes targeting other cell types. Neural Regen Res. 2024;19:1947-53
206. Gayen M, Bhomia M, Balakathiresan N, Knollmann-Ritschel B. Exosomal MicroRNAs Released by Activated Astrocytes as Potential Neuroinflammatory Biomarkers. Int J Mol Sci. 2020 21
207. Lu Y, Chen C, Wang H, Du R, Ji J, Xu T. et al. Astrocyte-derived sEVs alleviate fibrosis and promote functional recovery after spinal cord injury in rats. Int Immunopharmacol. 2022;113:109322
208. Xu J, Ruan X. Schwann cell autotransplantation for the treatment of peripheral nerve injury. Life Sci. 2024;358:123129
209. Liao S, Chen Y, Luo Y, Zhang M, Min J. The phenotypic changes of Schwann cells promote the functional repair of nerve injury. Neuropeptides. 2024;106:102438
210. Fu H, Hu D, Chen J, Wang Q, Zhang Y, Qi C. et al. Repair of the Injured Spinal Cord by Schwann Cell Transplantation. Front Neurosci. 2022;16:800513
211. Guest JD, Santamaria AJ, Solano JP, de Rivero Vaccari JP, Dietrich WD, Pearse DD. et al. Challenges in advancing Schwann cell transplantation for spinal cord injury repair. Cytotherapy. 2024
212. Wei Z, Fan B, Ding H, Liu Y, Tang H, Pan D. et al. Proteomics analysis of Schwann cell-derived exosomes: a novel therapeutic strategy for central nervous system injury. Mol Cell Biochem. 2019;457:51-9
213. Huang JH, Chen YN, He H, Fu CH, Xu ZY, Lin FY. Schwann cells-derived exosomes promote functional recovery after spinal cord injury by promoting angiogenesis. Front Cell Neurosci. 2022;16:1077071
214. Liao JX, Huang QM, Pan ZC, Wu J, Zhang WJ. The anti-inflammatory and immunomodulatory effects of olfactory ensheathing cells transplantation in spinal cord injury and concomitant pathological pain. Eur J Pharmacol. 2024;982:176950
215. Tu YK, Hsueh YH. Extracellular vesicles isolated from human olfactory ensheathing cells enhance the viability of neural progenitor cells. Neurol Res. 2020;42:959-67
216. Skoracka J, Bajewska K, Kulawik M, Suchorska W, Kulcenty K. Advances in cartilage tissue regeneration: a review of stem cell therapies, tissue engineering, biomaterials, and clinical trials. Excli j. 2024;23:1170-82
217. Advani D, Farid N, Tariq MH, Kohli N. A systematic review of mesenchymal stem cell secretome: Functional annotations, gene clusters and proteomics analyses for bone formation. Bone. 2024;190:117269
218. Xu C, Xie Y, Wang B. Genetically modified mesenchymal stromal cells: a cell-based therapy offering more efficient repair after myocardial infarction. Stem Cell Res Ther. 2024;15:323
219. Akhlaghpasand M, Tavanaei R, Hosseinpoor M, Yazdani KO, Soleimani A, Zoshk MY. et al. Safety and potential effects of intrathecal injection of allogeneic human umbilical cord mesenchymal stem cell-derived exosomes in complete subacute spinal cord injury: a first-in-human, single-arm, open-label, phase I clinical trial. Stem Cell Res Ther. 2024;15:264
220. Liu WZ, Ma ZJ, Li JR, Kang XW. Mesenchymal stem cell-derived exosomes: therapeutic opportunities and challenges for spinal cord injury. Stem Cell Res Ther. 2021;12:102
221. An J, Chen B, Zhang R, Tian D, Shi K, Zhang L. et al. Therapeutic Potential of Mesenchymal Stem Cell-Derived Exosomes in Spinal Cord Injury. Mol Neurobiol. 2024
222. Qian D, Xu J, Zhang X, Hu F, Cao S, Dong Y. et al. Microenvironment Self-Adaptive Nanomedicine Promotes Spinal Cord Repair by Suppressing Inflammation Cascade and Neural Apoptosis. Adv Mater. 2024: e2307624.
223. Wang W, Yao F, Xing H, Yang F, Yan L. Exosomal miR-17-92 Cluster from BMSCs Alleviates Apoptosis and Inflammation in Spinal Cord Injury. Biochem Genet. 2024
224. Shao M, Ye S, Chen Y, Yu C, Zhu W. Exosomes from hypoxic ADSCs ameliorate neuronal damage post spinal cord injury through circ-Wdfy3 delivery and inhibition of ferroptosis. Neurochem Int. 2024;177:105759
225. Luan Z, Liu J, Li M, Wang Y, Wang Y. Exosomes derived from umbilical cord-mesenchymal stem cells inhibit the NF-κB/MAPK signaling pathway and reduce the inflammatory response to promote recovery from spinal cord injury. J Orthop Surg Res. 2024;19:184
226. Chang Q, Hao Y, Wang Y, Zhou Y, Zhuo H, Zhao G. Bone marrow mesenchymal stem cell-derived exosomal microRNA-125a promotes M2 macrophage polarization in spinal cord injury by downregulating IRF5. Brain Res Bull. 2021;170:199-210
227. Luo Y, He YZ, Wang YF, Xu YX, Yang L. Adipose-derived mesenchymal stem cell exosomes ameliorate spinal cord injury in rats by activating the Nrf2/HO-1 pathway and regulating microglial polarization. Folia Neuropathol. 2023;61:326-35
228. Zhou Y, Wen LL, Li YF, Wu KM, Duan RR, Yao YB. et al. Exosomes derived from bone marrow mesenchymal stem cells protect the injured spinal cord by inhibiting pericyte pyroptosis. Neural Regen Res. 2022;17:194-202
229. Liu W, Wang Y, Gong F, Rong Y, Luo Y, Tang P. et al. Exosomes Derived from Bone Mesenchymal Stem Cells Repair Traumatic Spinal Cord Injury by Suppressing the Activation of A1 Neurotoxic Reactive Astrocytes. J Neurotrauma. 2019;36:469-84
230. Jin LY, Li J, Wang KF, Xia WW, Zhu ZQ, Wang CR. et al. Blood-Spinal Cord Barrier in Spinal Cord Injury: A Review. J Neurotrauma. 2021;38:1203-24
231. Guo X, Xia S, Ge T, Lin Y, Hu S, Wu H. et al. Atp13a5 Marker Reveals Pericyte Specification in the Mouse Central Nervous System. J Neurosci. 2024 44
232. Zhang Y, Neng L, Sharma K, Hou Z, Johnson A, Song J. et al. Pericytes control vascular stability and auditory spiral ganglion neuron survival. Elife. 2023 12
233. Du M, Li J, Yu S, Chen X, She Y, Lu Y. et al. RAGE mediates hippocampal pericyte responses and neurovascular unit lesions after TBI. Exp Neurol. 2024;380:114912
234. Yuan X, Wu Q, Wang P, Jing Y, Yao H, Tang Y. et al. Exosomes Derived From Pericytes Improve Microcirculation and Protect Blood-Spinal Cord Barrier After Spinal Cord Injury in Mice. Front Neurosci. 2019;13:319
235. Gao P, Yi J, Chen W, Gu J, Miao S, Wang X. et al. Pericyte-derived exosomal miR-210 improves mitochondrial function and inhibits lipid peroxidation in vascular endothelial cells after traumatic spinal cord injury by activating JAK1/STAT3 signaling pathway. J Nanobiotechnology. 2023;21:452
236. Zhang K, Zheng J, Bian G, Liu L, Xue Q, Liu F. et al. Polarized Macrophages Have Distinct Roles in the Differentiation and Migration of Embryonic Spinal-cord-derived Neural Stem Cells After Grafting to Injured Sites of Spinal Cord. Mol Ther. 2015;23:1077-91
237. Fu SP, Chen SY, Pang QM, Zhang M, Wu XC, Wan X. et al. Advances in the research of the role of macrophage/microglia polarization-mediated inflammatory response in spinal cord injury. Front Immunol. 2022;13:1014013
238. Zhu L, Ma L, Du X, Jiang Y, Gao J, Fan Z. et al. M2 Microglia-Derived Exosomes Protect Against Glutamate-Induced HT22 Cell Injury via Exosomal miR-124-3p. Mol Neurobiol. 2024;61:7845-61
239. Peng P, Yu H, Xing C, Tao B, Li C, Huang J. et al. Exosomes-mediated phenotypic switch of macrophages in the immune microenvironment after spinal cord injury. Biomed Pharmacother. 2021;144:112311
240. Zhang B, Lin F, Dong J, Liu J, Ding Z, Xu J. Peripheral Macrophage-derived Exosomes promote repair after Spinal Cord Injury by inducing Local Anti-inflammatory type Microglial Polarization via Increasing Autophagy. Int J Biol Sci. 2021;17:1339-52
241. Huang JH, He H, Chen YN, Liu Z, Romani MD, Xu ZY. et al. Exosomes derived from M2 Macrophages Improve Angiogenesis and Functional Recovery after Spinal Cord Injury through HIF-1α/VEGF Axis. Brain Sci. 2022 12
242. Zeng J, Gu C, Sun Y, Chen X. Engineering of M2 Macrophages-Derived Exosomes via Click Chemistry for Spinal Cord Injury Repair. Adv Healthc Mater. 2023;12:e2203391
243. Gao ZS, Zhang CJ, Xia N, Tian H, Li DY, Lin JQ. et al. Berberine-loaded M2 macrophage-derived exosomes for spinal cord injury therapy. Acta Biomater. 2021;126:211-23
244. Schaeffer S, Iadecola C. Revisiting the neurovascular unit. Nat Neurosci. 2021;24:1198-209
245. Li C, Xiang Z, Hou M, Yu H, Peng P, Lv Y. et al. miR-NPs-RVG promote spinal cord injury repair: implications from spinal cord-derived microvascular endothelial cells. J Nanobiotechnology. 2024;22:590
246. Ge X, Zhou Z, Yang S, Ye W, Wang Z, Wang J. et al. Exosomal USP13 derived from microvascular endothelial cells regulates immune microenvironment and improves functional recovery after spinal cord injury by stabilizing IκBα. Cell Biosci. 2023;13:55
247. Dong J, Gong Z, Bi H, Yang J, Wang B, Du K. et al. BMSC-derived exosomal miR-219-5p alleviates ferroptosis in neuronal cells caused by spinal cord injury via the UBE2Z/NRF2 pathway. Neuroscience. 2024;556:73-85
248. Wang Y, Zhao T, Chen WC, Zheng Y, Xu W, Huang S. miR-540-3p partially recovers the locomotor function of spinal cord injury mice by targeting SIX4/Yap1 and inactivation of astrocytes. Neurol Res. 2024;46:823-34
249. Wu H, Wang Q, Liao Y, Wang S. MSC-derived exosomes deliver ZBTB4 to mediate transcriptional repression of ITIH3 in astrocytes in spinal cord injury. Brain Res Bull. 2024;212:110954
250. Tang W, Zhao K, Li X, Zhou X, Liao P. Bone Marrow Mesenchymal Stem Cell-Derived Exosomes Promote the Recovery of Spinal Cord Injury and Inhibit Ferroptosis by Inactivating IL-17 Pathway. J Mol Neurosci. 2024;74:33
251. Chen M, Lin Y, Guo W, Chen L. BMSC-Derived Exosomes Carrying miR-26a-5p Ameliorate Spinal Cord Injury via Negatively Regulating EZH2 and Activating the BDNF-TrkB-CREB Signaling. Mol Neurobiol. 2024;61:8156-74
252. Gu J, Wu J, Wang C, Xu Z, Jin Z, Yan D. et al. BMSCs-derived exosomes inhibit macrophage/microglia pyroptosis by increasing autophagy through the miR-21a-5p/PELI1 axis in spinal cord injury. Aging (Albany NY). 2024;16:5184-206
253. Yang D, Wei H, Sheng Y, Peng T, Zhao Q, Xie L. et al. Circ_0006640 transferred by bone marrow-mesenchymal stem cell-exosomes suppresses lipopolysaccharide-induced apoptotic, inflammatory and oxidative injury in spinal cord injury. J Orthop Surg Res. 2024;19:50
254. Sun Y, Liu Q, Qin Y, Xu Y, Zhao J, Xie Y. et al. Exosomes derived from CD271(+)CD56(+) bone marrow mesenchymal stem cell subpopoulation identified by single-cell RNA sequencing promote axon regeneration after spinal cord injury. Theranostics. 2024;14:510-27
255. Xing X, Xu P, Xing X, Xu Z, Huang Z, Li Z. et al. Effects of ADSC-Derived Exosome LRRC75A-AS1 on Anti-inflammatory Function After SCI. Appl Biochem Biotechnol. 2024;196:5920-35
256. Xue C, Ma X, Guan X, Feng H, Zheng M, Yang X. Small extracellular vesicles derived from umbilical cord mesenchymal stem cells repair blood-spinal cord barrier disruption after spinal cord injury through down-regulation of Endothelin-1 in rats. PeerJ. 2023;11:e16311
257. Xue H, Ran B, Li J, Wang G, Chen B, Mao H. Bone marrow mesenchymal stem cell exosomes-derived microRNA-216a-5p on locomotor performance, neuronal injury, and microglia inflammation in spinal cord injury. Front Cell Dev Biol. 2023;11:1227440
258. Xie Y, Sun Y, Liu Y, Zhao J, Liu Q, Xu J. et al. Targeted Delivery of RGD-CD146(+)CD271(+) Human Umbilical Cord Mesenchymal Stem Cell-Derived Exosomes Promotes Blood-Spinal Cord Barrier Repair after Spinal Cord Injury. ACS Nano. 2023;17:18008-24
259. Yang Y, Li Y, Zhang S, Cao L, Zhang Y, Fang B. miR-199a-5p from bone marrow mesenchymal stem cell exosomes promotes the proliferation of neural stem cells by targeting GSK-3β. Acta Biochim Biophys Sin (Shanghai). 2023;55:783-94
260. Shao Y, Wang Q, Liu L, Wang J, Wu M. Alleviation of Spinal Cord Injury by MicroRNA 137-Overexpressing Bone Marrow Mesenchymal Stem Cell-Derived Exosomes. Tohoku J Exp Med. 2023;259:237-46
261. Gao X, Gao LF, Zhang YN, Kong XQ, Jia S, Meng CY. Huc-MSCs-derived exosomes attenuate neuropathic pain by inhibiting activation of the TLR2/MyD88/NF-κB signaling pathway in the spinal microglia by targeting Rsad2. Int Immunopharmacol. 2023;114:109505
262. Shao Y, Wang Q, Liu L, Wang J, Wu M. Exosomes from microRNA 146a overexpressed bone marrow mesenchymal stem cells protect against spinal cord injury in rats. J Orthop Sci. 2023;28:1149-56
263. Tian F, Yang J, Xia R. Exosomes Secreted from circZFHX3-modified Mesenchymal Stem Cells Repaired Spinal Cord Injury Through mir-16-5p/IGF-1 in Mice. Neurochem Res. 2022;47:2076-89
264. Shao C, Chen Y, Yang T, Zhao H, Li D. Mesenchymal Stem Cell Derived Exosomes Suppress Neuronal Cell Ferroptosis Via lncGm36569/miR-5627-5p/FSP1 Axis in Acute Spinal Cord Injury. Stem Cell Rev Rep. 2022;18:1127-42
265. He X, Zhang J, Guo Y, Yang X, Huang Y, Hao D. Exosomal miR-9-5p derived from BMSCs alleviates apoptosis, inflammation and endoplasmic reticulum stress in spinal cord injury by regulating the HDAC5/FGF2 axis. Mol Immunol. 2022;145:97-108
266. Liang Y, Wu JH, Zhu JH, Yang H. Exosomes Secreted by Hypoxia-Pre-conditioned Adipose-Derived Mesenchymal Stem Cells Reduce Neuronal Apoptosis in Rats with Spinal Cord Injury. J Neurotrauma. 2022;39:701-14
267. Zhang A, Bai Z, Yi W, Hu Z, Hao J. Overexpression of miR-338-5p in exosomes derived from mesenchymal stromal cells provides neuroprotective effects by the Cnr1/Rap1/Akt pathway after spinal cord injury in rats. Neurosci Lett. 2021;761:136124
268. Jiang Z, Zhang J. Mesenchymal stem cell-derived exosomes containing miR-145-5p reduce inflammation in spinal cord injury by regulating the TLR4/NF-κB signaling pathway. Cell Cycle. 2021;20:993-1009
269. Chen Y, Tian Z, He L, Liu C, Wang N, Rong L. et al. Exosomes derived from miR-26a-modified MSCs promote axonal regeneration via the PTEN/AKT/mTOR pathway following spinal cord injury. Stem Cell Res Ther. 2021;12:224
270. Bai L, Gao J, Zhang P, Lin S, Zhang C. Immunotherapy of M2 macrophage derived from exosome-based nanoparticles for spinal cord injury. Int Immunopharmacol. 2024;132:111983
271. Ge X, Tang P, Rong Y, Jiang D, Lu X, Ji C. et al. Exosomal miR-155 from M1-polarized macrophages promotes EndoMT and impairs mitochondrial function via activating NF-κB signaling pathway in vascular endothelial cells after traumatic spinal cord injury. Redox Biol. 2021;41:101932
272. Peng P, Yu H, Xing C, Tao B, Li C, Huang J. et al. Exosomes-mediated phenotypic switch of macrophages in the immune microenvironment after spinal cord injury. Biomedicine & Pharmacotherapy. 2021;144:112311
273. Huang J, Zhang G, Li S, Li J, Wang W, Xue J. et al. Endothelial cell-derived exosomes boost and maintain repair-related phenotypes of Schwann cells via miR199-5p to promote nerve regeneration. J Nanobiotechnology. 2023;21:10
274. Liu J, Kong G, Lu C, Wang J, Li W, Lv Z. et al. IPSC-NSCs-derived exosomal let-7b-5p improves motor function after spinal cord Injury by modulating microglial/macrophage pyroptosis. J Nanobiotechnology. 2024;22:403
275. Li J, Jing Y, Bai F, Wu Y, Wang L, Yan Y. et al. Induced pluripotent stem cells as natural biofactories for exosomes carrying miR-199b-5p in the treatment of spinal cord injury. Front Pharmacol. 2022;13:1078761
276. Abbas A, Huang X, Ullah A, Luo L, Xi W, Qiao Y. et al. Enhanced spinal cord repair using bioengineered induced pluripotent stem cell-derived exosomes loaded with miRNA. Mol Med. 2024;30:168
277. Xiong W, Li C, Kong G, Zeng Q, Wang S, Yin G. et al. Treg cell-derived exosomes miR-709 attenuates microglia pyroptosis and promotes motor function recovery after spinal cord injury. J Nanobiotechnology. 2022;20:529
278. Kong G, Xiong W, Li C, Xiao C, Wang S, Li W. et al. Treg cells-derived exosomes promote blood-spinal cord barrier repair and motor function recovery after spinal cord injury by delivering miR-2861. Journal of Nanobiotechnology. 2023;21:364
279. Inoue M, Yamaguchi R, He CCJ, Ikeda A, Okano H, Kohyama J. Current status and prospects of regenerative medicine for spinal cord injury using human induced pluripotent stem cells: a review. Stem Cell Investig. 2023;10:6
280. Aboul-Soud MAM, Alzahrani AJ, Mahmoud A. Induced Pluripotent Stem Cells (iPSCs)-Roles in Regenerative Therapies, Disease Modelling and Drug Screening. Cells. 2021 10
281. Chen H, Peng H, Wang PC, Zou T, Feng XM, Wan BW. Role of regulatory T cells in spinal cord injury. Eur J Med Res. 2023;28:163
282. Kong G, Xiong W, Li C, Xiao C, Wang S, Li W. et al. Treg cells-derived exosomes promote blood-spinal cord barrier repair and motor function recovery after spinal cord injury by delivering miR-2861. J Nanobiotechnology. 2023;21:364
283. Hu Z, Wu T, Zhou Z, Zhang Y, Chen Q, Yao H. et al. Asiaticoside Attenuates Blood-Spinal Cord Barrier Disruption by Inhibiting Endoplasmic Reticulum Stress in Pericytes After Spinal Cord Injury. Mol Neurobiol. 2024;61:678-92
284. Tail M, Zhang H, Zheng G, Harms AK, Hatami M, Skutella T. et al. Sonic Hedgehog reduces inflammatory response, decreases blood-spinal cord barrier permeability, and improves locomotor function recovery in an acute spinal cord injury rat model. J Inflamm (Lond). 2024;21:34
285. Deng B, Jiang S, Liu G, Li X, Zhao Y, Fan X. et al. Tetramethylpyrazine-loaded electroconductive hydrogels promote tissue repair after spinal cord injury by protecting the blood-spinal cord barrier and neurons. J Mater Chem B. 2024;12:4409-26
286. Xin W, Qiang S, Jianing D, Jiaming L, Fangqi L, Bin C. et al. Human Bone Marrow Mesenchymal Stem Cell-Derived Exosomes Attenuate Blood-Spinal Cord Barrier Disruption via the TIMP2/MMP Pathway After Acute Spinal Cord Injury. Mol Neurobiol. 2021;58:6490-504
287. Nakazaki M, Morita T, Lankford KL, Askenase PW, Kocsis JD. Small extracellular vesicles released by infused mesenchymal stromal cells target M2 macrophages and promote TGF-β upregulation, microvascular stabilization and functional recovery in a rodent model of severe spinal cord injury. J Extracell Vesicles. 2021;10:e12137
288. Lu Y, Zhou Y, Zhang R, Wen L, Wu K, Li Y. et al. Bone Mesenchymal Stem Cell-Derived Extracellular Vesicles Promote Recovery Following Spinal Cord Injury via Improvement of the Integrity of the Blood-Spinal Cord Barrier. Front Neurosci. 2019;13:209
289. Mu J, Li L, Wu J, Huang T, Zhang Y, Cao J. et al. Hypoxia-stimulated mesenchymal stem cell-derived exosomes loaded by adhesive hydrogel for effective angiogenic treatment of spinal cord injury. Biomater Sci. 2022;10:1803-11
290. Huang JH, Xu Y, Yin XM, Lin FY. Exosomes Derived from miR-126-modified MSCs Promote Angiogenesis and Neurogenesis and Attenuate Apoptosis after Spinal Cord Injury in Rats. Neuroscience. 2020;424:133-45
291. Lee JR, Kyung JW, Kumar H, Kwon SP, Song SY, Han IB. et al. Targeted Delivery of Mesenchymal Stem Cell-Derived Nanovesicles for Spinal Cord Injury Treatment. Int J Mol Sci. 2020 21
292. Li C, Qin T, Jin Y, Hu J, Yuan F, Cao Y. et al. Cerebrospinal fluid-derived extracellular vesicles after spinal cord injury promote vascular regeneration via PI3K/AKT signaling pathway. J Orthop Translat. 2023;39:124-34
293. Guo S, Perets N, Betzer O, Ben-Shaul S, Sheinin A, Michaelevski I. et al. Intranasal Delivery of Mesenchymal Stem Cell Derived Exosomes Loaded with Phosphatase and Tensin Homolog siRNA Repairs Complete Spinal Cord Injury. ACS Nano. 2019;13:10015-28
294. Kim HY, Kumar H, Jo MJ, Kim J, Yoon JK, Lee JR. et al. Therapeutic Efficacy-Potentiated and Diseased Organ-Targeting Nanovesicles Derived from Mesenchymal Stem Cells for Spinal Cord Injury Treatment. Nano Lett. 2018;18:4965-75
295. Zhang M, Wang L, Huang S, He X. Exosomes with high level of miR-181c from bone marrow-derived mesenchymal stem cells inhibit inflammation and apoptosis to alleviate spinal cord injury. J Mol Histol. 2021;52:301-11
296. Li C, Jiao G, Wu W, Wang H, Ren S, Zhang L. et al. Exosomes from Bone Marrow Mesenchymal Stem Cells Inhibit Neuronal Apoptosis and Promote Motor Function Recovery via the Wnt/β-catenin Signaling Pathway. Cell Transplant. 2019;28:1373-83
297. Yerrapragada SM, Sawant H, Chen S, Bihl T, Wang J, Bihl JC. The protective effects of miR-210 modified endothelial progenitor cells released exosomes in hypoxia/reoxygenation injured neurons. Exp Neurol. 2022;358:114211
298. Li Y, Wang J, Chen S, Wu P, Xu S, Wang C. et al. miR-137 boosts the neuroprotective effect of endothelial progenitor cell-derived exosomes in oxyhemoglobin-treated SH-SY5Y cells partially via COX2/PGE2 pathway. Stem Cell Res Ther. 2020;11:330
299. Shao M, Jin M, Xu S, Zheng C, Zhu W, Ma X. et al. Exosomes from Long Noncoding RNA-Gm37494-ADSCs Repair Spinal Cord Injury via Shifting Microglial M1/M2 Polarization. Inflammation. 2020;43:1536-47
300. Li C, Li X, Zhao B, Wang C. Exosomes derived from miR-544-modified mesenchymal stem cells promote recovery after spinal cord injury. Arch Physiol Biochem. 2020;126:369-75
301. Liu W, Rong Y, Wang J, Zhou Z, Ge X, Ji C. et al. Exosome-shuttled miR-216a-5p from hypoxic preconditioned mesenchymal stem cells repair traumatic spinal cord injury by shifting microglial M1/M2 polarization. J Neuroinflammation. 2020;17:47
302. Kang J, Guo Y. Human Umbilical Cord Mesenchymal Stem Cells Derived Exosomes Promote Neurological Function Recovery in a Rat Spinal Cord Injury Model. Neurochem Res. 2022;47:1532-40
303. Liu C, Hu F, Jiao G, Guo Y, Zhou P, Zhang Y. et al. Dental pulp stem cell-derived exosomes suppress M1 macrophage polarization through the ROS-MAPK-NFκB P65 signaling pathway after spinal cord injury. J Nanobiotechnology. 2022;20:65
304. Seliktar D. Designing cell-compatible hydrogels for biomedical applications. Science. 2012;336:1124-8
305. Fan L, Liu C, Chen X, Zheng L, Zou Y, Wen H. et al. Exosomes-Loaded Electroconductive Hydrogel Synergistically Promotes Tissue Repair after Spinal Cord Injury via Immunoregulation and Enhancement of Myelinated Axon Growth. Adv Sci (Weinh). 2022;9:e2105586
306. Liu W, Liu Q, Li Z, Zhang C, Li Z, Ke H. et al. Multifunctional magneto-electric and exosome-loaded hydrogel enhances neuronal differentiation and immunoregulation through remote non-invasive electrical stimulation for neurological recovery after spinal cord injury. Bioact Mater. 2025;48:510-28
307. Kim DY, Liu Y, Kim G, An SB, Han I. Innovative Strategies in 3D Bioprinting for Spinal Cord Injury Repair. Int J Mol Sci. 2024 25
308. Wang Q, Liu K, Cao X, Rong W, Shi W, Yu Q. et al. Plant-derived exosomes extracted from Lycium barbarum L. loaded with isoliquiritigenin to promote spinal cord injury repair based on 3D printed bionic scaffold. Bioeng Transl Med. 2024;9:e10646
309. Li D, Xie X, Ou Y, Sun P, Lin J, Yu C. et al. Bone marrow mesenchymal stem cells-derived exosomal miR-24-3p alleviates spinal cord injury by targeting MAPK9 to inhibit the JNK/c-Jun/c-Fos pathway. Arch Biochem Biophys. 2025;769:110434
310. Lu X, Xu G, Lin Z, Zou F, Liu S, Zhang Y. et al. Engineered exosomes enriched in netrin-1 modRNA promote axonal growth in spinal cord injury by attenuating inflammation and pyroptosis. Biomater Res. 2023;27:3
311. Xiao Y, Hu X, Jiang P, Qi Z. Thermos-responsive hydrogel system encapsulated engineered exosomes attenuate inflammation and oxidative damage in acute spinal cord injury. Front Bioeng Biotechnol. 2023;11:1216878
312. Chen H, Sun H, Yang Y, Wang P, Chen X, Yin J. et al. Engineered melatonin-pretreated plasma exosomes repair traumatic spinal cord injury by regulating miR-138-5p/SOX4 axis mediated microglia polarization. J Orthop Translat. 2024;49:230-45
313. Fu GQ, Wang YY, Xu YM, Bian MM, Zhang L, Yan HZ. et al. Exosomes derived from vMIP-II-Lamp2b gene-modified M2 cells provide neuroprotection by targeting the injured spinal cord, inhibiting chemokine signals and modulating microglia/macrophage polarization in mice. Exp Neurol. 2024;377:114784
Author contact
![]() Corresponding authors: Xiaodong Wang (Email: ZSSL_70com), Huimin Hu (Email: dochuhuimincom), and Dingjun Hao (Email: haodingjunxjtu.edu.cn).
Corresponding authors: Xiaodong Wang (Email: ZSSL_70com), Huimin Hu (Email: dochuhuimincom), and Dingjun Hao (Email: haodingjunxjtu.edu.cn).

 Global reach, higher impact
Global reach, higher impact