10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2025; 21(9):4117-4128. doi:10.7150/ijbs.113161 This issue Cite
Research Paper
Apelin facilitates integrin αvβ3 production and enhances metastasis in prostate cancer by activating STAT3 and inhibiting miR-8070
1. School of Post-Baccalaureate Chinese Medicine, Tzu Chi University, Hualien, Taiwan.
2. Department of Urology, Buddhist Tzu Chi General Hospital Taichung Branch, Taichung, Taiwan.
3. Institute of Biomedical Sciences, Mackay Medical College, New Taipei City, Taiwan.
4. Department of Medical Laboratory Science and Biotechnology, Asia University, Taichung, Taiwan.
5. Department of Pharmacology, School of Medicine, China Medical University, Taichung, Taiwan.
6. Department of Sports Medicine, College of Health Care, China Medical University, Taichung, Taiwan.
7. Department of Orthopedic Surgery, China Medical University Hospital, Taichung, Taiwan.
8. Department of Orthopedic Surgery, China Medical University Beigang Hospital, Yunlin, Taiwan.
9. School of Medicine, China Medical University, Taichung, Taiwan.
10. Department of Urology, China Medical University Hospital, Taichung, Taiwan.
11. Department of Urology, China Medical University Beigang Hospital, Beigang, Yunlin, Taiwan.
12. School of Oral Hygiene, College of Oral Medicine, Taipei Medical University, Taipei, Taiwan.
13. Translational Medicine Center, Shin Kong Wu Ho-Su Memorial Hospital, Taipei, Taiwan.
14. Chinese Medicine Research Center, China Medical University, Taichung, Taiwan.
15. Department of Family Medicine, China Medical University Hospital, Taichung, Taiwan.
Received 2025-3-4; Accepted 2025-5-29; Published 2025-6-12
Abstract
Prostate cancer is the most common cancer in men and is often associated with distant metastasis in its later stages. Cell surface receptors called integrins function as adhesion molecules that mediate both cell adhesion and motility. The adipokine apelin has been implicated in cancer progression and metastasis. However, the mechanisms by which apelin regulates integrin production and metastasis in prostate cancer remain unclear. Here, we found that apelin and integrin αvβ3 expression levels were elevated in prostate cancer samples compared to those in normal individuals. Apelin stimulation enhances integrin αvβ3-dependent prostate cancer migration. The activation of STAT3 and inhibition of miR-8070 via the MAPK pathway mediate apelin-facilitated integrin synthesis and cell motility. Importantly, our in vivo study indicated that inhibiting apelin reduces integrin αvβ3 expression and prostate cancer metastasis. Our results suggest that the apelin/integrin axis is a novel therapeutic target for treating metastatic prostate cancer.
Keywords: prostate cancer, apelin, metastasis, integrin, miR-8070
Introduction
The most prevalent disease in males is prostate cancer, whose risk rises with age and fat [1, 2]. It has been predicted that by 2050, there would be more than 300,000 new cases detected annually in the United States of America alone. More than half of patients with prostate cancer eventually develop bone metastases, especially in advanced stages, although many people initially present with localized disease [2, 3]. Many patients with metastatic prostate cancer experience treatment resistance, even though the five-year relative survival rate for all diagnosed instances of the illness is 98%. These metastases are a major cause of death and can drastically lower a patient's quality of life [4]. A thorough understanding of the intricate processes behind the spread of prostate cancer and distant metastases is essential for future advancements in early prevention and intervention.
The process by which tumor cells leave their original site, move through the bloodstream, and develop into a new tumor in a different organ is known as metastasis. Cell surface receptors called integrins function as adhesion molecules that can mediate connections between the extracellular matrix and prostate epithelial cells [5]. Integrin heterodimers made up of α and β subunits connected by noncovalent bonds are seen in normal prostate basal cells [6]. There are a total of 24 distinct pairings formed by the 18 α and 8 β subunits that have been found thus far [7, 8]. Cell adhesion and motility are modulated by integrins in a range of biological processes [7]. The progression, migration, invasion, and metastasis of prostate cancer cells are linked to the integrins α2β1, αvβ3, α5β1, and α6β1 [9, 10].
The APLN gene encodes the peptide known as apelin [11], which is released by adipose tissue and contains a 77-amino acid preprotein precursor [12]. Numerous biological processes, such as angiogenesis [13], blood pressure regulation [14], and cellular metabolism [15], have been linked to the different molecular forms of apelin. Additionally, apelin has been linked to the pathological advancement of cancer, obesity, and diabetes [16]. By controlling variables related to tumor growth and motility, apelin influences tumor development and metastasis [17]. It has been demonstrated that apelin causes tumor growth, migration, and invasion in ovarian and gastric cancer, cholangiocarcinoma, lung cancer, and oral squamous cell carcinoma [18]. We previously demonstrated that apelin enhances prostate cancer motility [19]; however, the effects of apelin on integrin expression and distant metastasis of prostate cancer remain unclear. Here, we found that apelin and integrin αvβ3 are upregulated in prostate cancer patients compared to healthy individuals. Apelin enhances wound healing and cell migration in prostate cancer by promoting integrin αvβ3 production. Activation of STAT3 and inhibition of miR-8070 through the MAPK pathway are involved in apelin-induced prostate cancer migration. Inhibiting apelin reduces prostate metastasis in vivo, indicating that the apelin/integrin αvβ3 axis is a promising target for treating prostate cancer metastasis.
Materials and Methods
Materials
JNK (SC-474), p38 (SC-4972), ERK (SC-1647), STAT3 (SC-482), p-JNK (SC-6254), p-p38 (SC-166182), p-ERK (SC-7383) and integrin αvβ3 (SC-7312) antibodies as well as JNK (sc-29380), p38 (sc-29433), STAT3 (sc-29493), integrin αv (sc-29373), integrin β3 (sc-29375) and apelin (sc-44741) siRNA were purchased from Santa Cruz Biotechnology (CA, USA). Antibody against the phosphorylated form of STAT3 (AP0705) was obtained from Abclonal technology (Woburn, MA, USA). Apelin antibody was acquired from NOVUS biologicals (Centennial, CO, USA). Recombinant human apelin was acquired from PerpoTech (Rocky Hill, NJ, USA). All other chemicals utilized in this study were obtained from Sigma-Aldrich (St. Louis, MO, USA).
Cell culture
The American Type Culture Collection provided the human prostate cancer cell lines (PC3 and DU145). Both of PC3 and DU145 cells are Androgen-insensitive cell lines, while PC3 cells showed higher metastatic potential compared to DU145 cells. In RPMI-1640 media supplemented with 20 mM HEPES, 10% heat-inactivated fetal calf serum, 2 mM glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin, cells were kept at 37 °C with 5% CO2.
Bioinformatics analysis
Apelin levels were analyzed using the GSE6919 dataset obtained from the Gene Expression Omnibus (GEO) database [20]. This dataset provided expression levels of target genes in healthy prostate tissue and prostate tumor tissues. The GSE7930 dataset compared integrin αv and β3 levels between highly metastatic and poorly metastatic tumors from prostate cancer patients. The GSE7930 dataset was formatted and uploaded to QIAGEN Ingenuity Pathway Analysis (IPA) software (Hilden, Germany) for further exploration. The imported datasets were analyzed using the “Core Analysis” module and filtered for “gene expression.” Analysis of mRNA expression changes in IPA identified pathways with a strong positive correlation to prostate cancer. miRNA candidates were mined from the miRWalk database (Hilden, Germany). To identify miRNA regulators, the official gene symbols for integrin αv and β3 were used in the search process. To accurately identify miRNAs binding to integrin αv and β3, results were filtered using TargetScan and miRDB. After the data processing phase, subsequent experiments enabled us to pinpoint specific miRNAs regulated by apelin.
Real-time quantitative PCR analysis
The TRIzol reagent (MDBio; Taipei, Taiwan) was used to extract total RNA from prostate cancer cells. In summary, oligo-dT primers were used to reverse-transcribe 1 μg of RNA into cDNA in compliance with the manufacturer's instructions (Invitrogen; Carlsbad, CA, USA). The Mir-XTM miRNA First Strand Synthesis Kit (Takara Bio Inc.) was used to create cDNA from 100 ng of total RNA for the miRNA assay. The endogenous control gene was used to achieve relative quantification of gene expression. The fractional cycle number at which the fluorescence exceeded the predetermined threshold was known as the threshold cycle (CT). The comparative CT approach was used to calculate relative expression [21, 22]. The primer sequence was provided in Supplementary Table S1.
Western blot analysis
After electrophoretic separation on SDS-PAGE gels (7.5-12%), protein samples were transferred onto PVDF membranes (Merck; Darmstadt, Germany). Before the membranes were incubated with primary antibodies for an entire night at 4°C, they were blocked using 5% nonfat milk. After that, the membranes were treated for an hour at room temperature with certain secondary antibodies. An ECL kit (Millipore, USA) was used to detect the target protein's expression, and an ImageQuantTM LAS 4000 biomolecular imager was used to visualize it [23, 24].
Cell transfection
After seeding the cells (5 × 10⁵ cells/well) onto 6-well plates, the cells were transfected with different miRNA mimics or genetic siRNAs using Lipofectamine 2000 transfection reagent (Waltham, Massachusetts, USA) in accordance with the manufacturer's instructions.
Prostate cancer migration assay
Transwell inserts (Costar, NY; 8-mm pore size) were used in 24-well plates for the migration experiment. Cells were pretreated with varying inhibitors for 30 minutes before to the migration assay. The upper compartment contained about 1 × 104 cells in 200 μl of serum-free media, whereas the lower chamber had 300 μl of the same medium with varying apelin concentrations. The cells were fixed in 3.7% formaldehyde for five minutes, incubated for twenty-four hours at 37 °C in 5% CO2, and stained with 0.05% crystal violet in PBS. Cotton-tipped swabs were used to remove cells from the upper side of the filters, and PBS was used to wash the filters [25].
Wound-healing migration assay
Cells were seeded at a density of 1 × 105 cells/well in culture media onto 12-well plates for the wound-healing migration test. The monolayer cells were manually scratched with a pipette blue tip 24 hours after seeding in order to make long, distinct scratches in the middle of the dishes with a clear, brilliant field. The cells were washed once with PBS in order to eliminate the unattached cells. Each dish was supplemented with serum-free media, either with or without apelin. The photos were obtained for each point after a 24-hour migration, and the number of migratory cells was then counted and averaged for each experimental condition.
Immunohistochemistry (IHC)
Immunohistochemistry assays were conducted on tissue specimens obtained from prostate cancer patients and mouse tissues. The primary antibodies employed in the immunohistochemistry procedure were against apelin and integrin αvβ3. Apelin and integrin αvβ3 expression illustrated on the tumor of prostate cancer patients or mouse tissue were quantified by Fiji software. The darker 3, 3'-diaminobenzidine (DAB) expression gained the higher OD value during quantified procedure. Then, the sum of the OD values, which were quantified from the pathological section, divided by the area of the target distribution area to obtain the average optical density (AOD) value. The AOD value reflects the concentration per unit area of the target genes [26].
Statistics
All values are presented as mean ± standard deviation (SD). Statistical significance between experiment groups was assessed using the student's t-test. Comparisons involving more than two groups with a single variable were performed using one-way analysis of variance (ANOVA) followed by Bonferroni's post hoc test. Differences between groups were considered significant if the p-value was < 0.05.
Results
Apelin promotes prostate cancer migration through upregulating integrins αvβ3
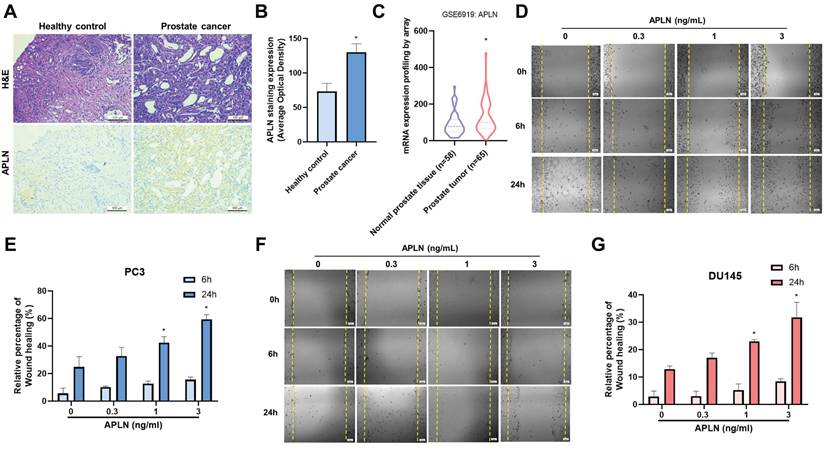
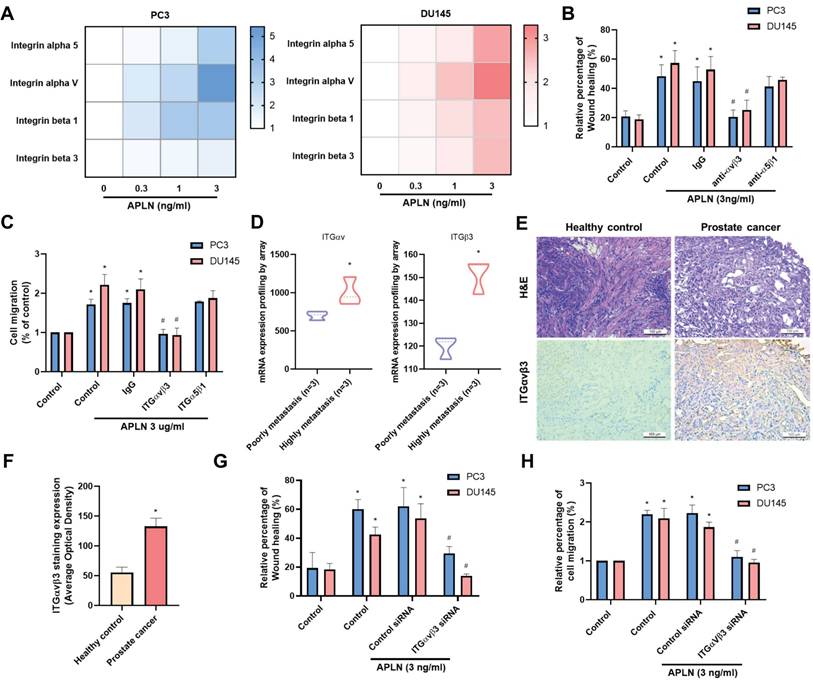
Apelin has been found to be important for the development of many types of cancer [27, 28], however it is yet unknown how apelin affects prostate cancer metastases. Human prostate cancer tissue samples have considerably higher apelin expression levels than normal tissue samples as determined by H&E and IHC staining (Fig. 1A&B). The data from GEO dataset also confirmed the similar results (Fig. 1C). Next, we used a wound healing migration assay to examine the migratory effects of apelin in prostate cancer cells. Apelin promotes wound healing migration in both PC3 and DU145 prostate cancer cells in a concentration-dependent manner (Fig. 1D-G). However, apelin treatment did not affect the cell viability in prostate cancer cell lines (Supplementary Fig. S1). Integrin level is linked to metastasis and correlates with various stages of human prostate cancer [5]. Stimulation of PC3 and DU145 cells enhances the expression of integrins α5, αv, β1, and β3 in a concentration-dependent manner (Fig. 2A). Integrins α5, αv, β1, and β3 form two unique complexes, namely αvβ3 and α5β1. Cells treated with antibodies that block integrin αvβ3, but not α5β1, exhibited inhibited apelin-induced promotion of wound healing and cell migration (Fig. 2B&C). Expression levels of integrins αv and β3 in highly metastatic prostate cancer patient samples from the GEO database were significantly higher than those in patients with poorly metastatic prostate cancer (Fig. 2D). In addition, H&C and IHC staining revealed that integrin αvβ3 levels are higher in human prostate cancer tissue samples than in normal tissue samples (Fig. 2E&F). Co-transfection of cells with integrin αv and β3 siRNAs diminished apelin-promoted cell motility (Fig. 2G&H), indicating that apelin enhances prostate cancer motility by promoting integrin αvβ3 production.
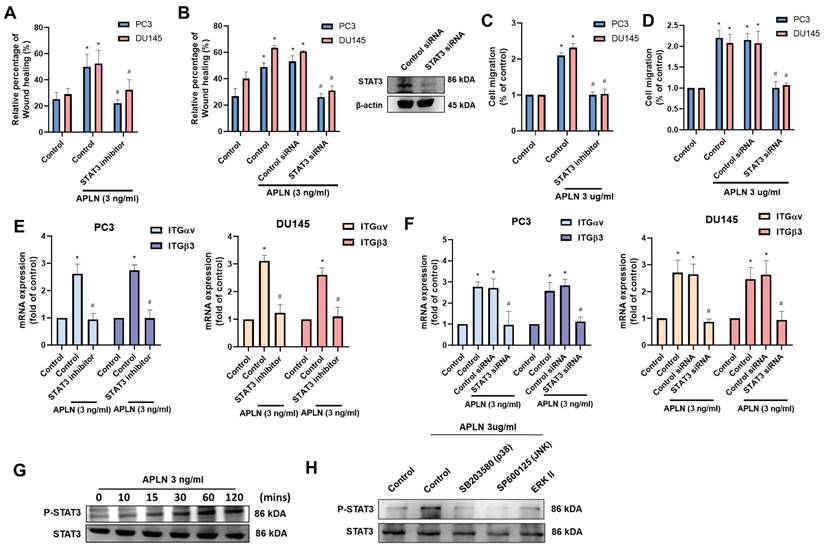
MAPK and STAT3 signaling pathways are mediated apelin-enhanced integrin expression and cell migration
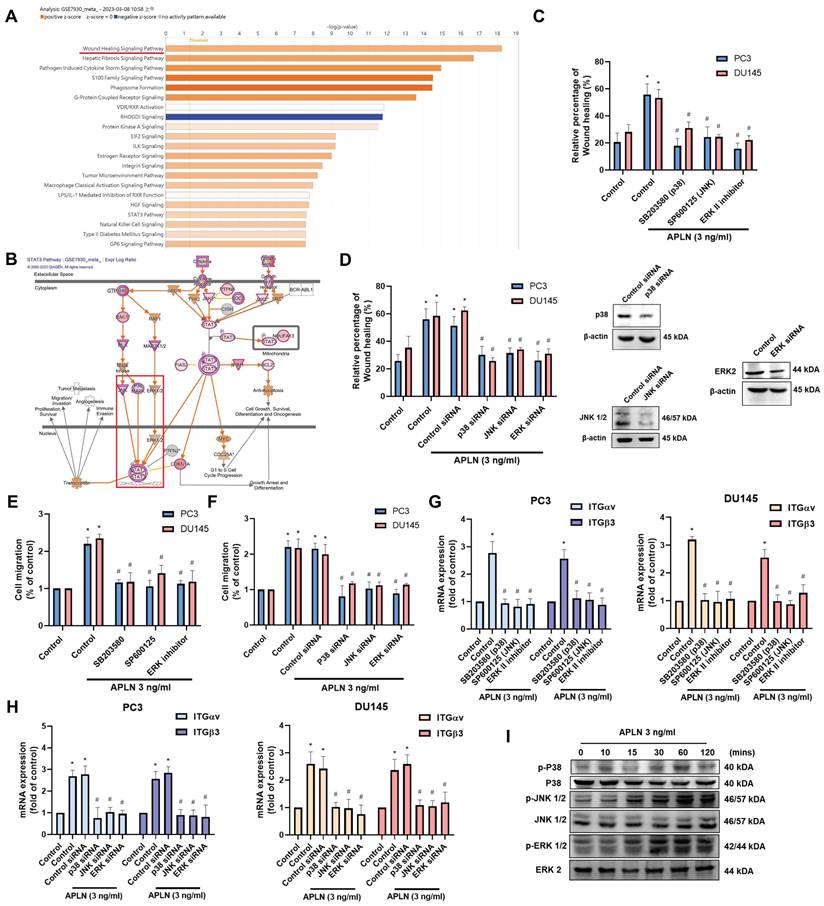
Next, we use IPA software to examine signaling pathways in the GSE7930 database in order to learn more about the mechanism behind cell motility. The findings showed that the major signaling pathways in the wound healing signaling system are MAPK (ERK, p38, JNK) and STAT3 (Fig. 3A&B). Stimulating cells with ERK (ERK II inhibitor), p38 (SB203580), and JNK (SP600125) inhibitors blocked apelin-induced wound healing and cell migration (Fig. 3C-F). Transfection with ERK, p38, and JNK siRNAs inhibited apelin-promoted cell motility (Fig. 3D&F). These inhibitors and siRNAs also diminished apelin-induced integrin expression (Fig. 3G&H). On the other hand, treatment of PC3 cells with apelin induced phosphorylation of ERK, p38, and JNK (Fig. 3I). Furthermore, both STAT3 inhibitor and STAT3 siRNA reduced apelin-facilitated cell motility and integrin generation (Fig. 4A-F). STAT3 phosphorylation was enhanced in a time-dependent manner after apelin stimulation (Fig. 4G). ERK, p38, and JNK inhibitors reduced apelin-enhanced STAT3 phosphorylation (Fig. 4H). Thus, the MAPK and STAT3 pathways are involved in apelin-induced integrin production and cell motility.
Apelin promotes wound healing migration in prostate cancer cells. (A&B) Representative images showing the results of IHC staining for apelin in tissue samples from healthy individuals (n=3) and prostate cancer patients (n=3). (C) Apelin gene levels in normal and prostate cancer patients retrieved from the GEO database. (D-G) Cells were treated with apelin, the wound healing migration was examined (n=3). * p < 0.05 compared with the control group.
Apelin enhances integrin αvβ3-dependent prostate cancer motility. (A) Cells were treated with apelin for 24 hours, the indicated integrin expression was examined by qPCR (n=3). (B&C) Cells were treated with integrin αvβ3 or α5β1 antibody then with apelin, the wound healing and cell migration was examined (n=3). (D) Integrin αv and β3 gene levels in normal and prostate cancer patients retrieved from the GEO database. (E&F) Representative images showing the results of IHC staining for integrin αvβ3 in tissue samples from healthy individuals (n=3) and prostate cancer patients (n=3). (G&H) Cells were co-transfected with integrin αv and β3 siRNA then with apelin, the wound healing and cell migration was examined (n=3). * p < 0.05 compared with the control group. # p < 0.05 compared with the apelin-treated group.
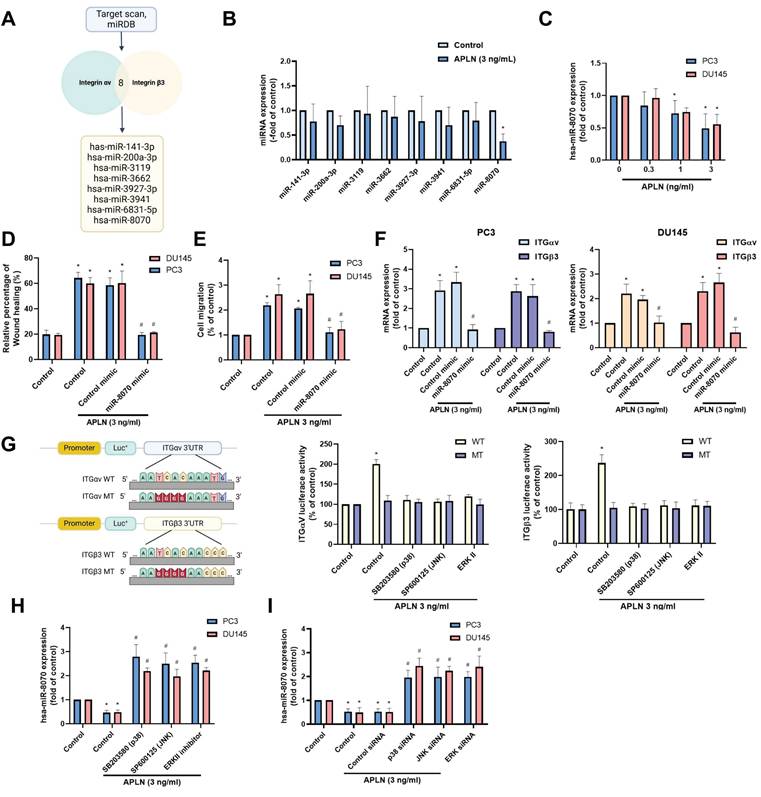
Apelin enhances integrin-dependent prostate cancer motility by suppressing miR-8070 generation
The metastasis of prostate cancer has been linked to dysregulated miRNAs [29]. Using two bioinformatics tools (TargetScan and miRDB), we identified eight miRNAs that directly bind to the 3' UTRs of both integrin αv and β3 (Fig. 5A). Among them, apelin treatment predominantly downregulated miR-8070 expression (Fig. 5B). Additionally, incubating PC3 or DU145 cells with apelin led to a concentration-dependent reduction in miR-8070 expression (Fig. 5C). To determine whether apelin enhances integrin expression through the regulation of miR-8070, further experiments were conducted involving the transfection of prostate cancer cells with a miR-8070 mimic, which reversed the effects of apelin on wound healing, cell migration, and integrin synthesis (Fig. 5D-F). Luciferase reporter plasmids containing the wild-type (WT) integrin αv and β3 3'-UTRs, as well as regions with mismatches (MT) predicted for miR-8070, were used to evaluate the influence of miR-8070 on integrin αv and β3 gene transcription. Apelin induced luciferase activity in WT-integrin αv and β3 3'-UTR plasmids but not in MT-integrin αv and β3 3'-UTR plasmids (Fig. 5G). Moreover, incubation with ERK, p38, and JNK inhibitors counteracted the apelin-induced effects on miR-8070 synthesis but not on WT luciferase activity (Fig. 5G-I), suggesting that apelin promotes integrin-dependent prostate cancer motility by suppressing miR-8070 via the MAPK pathway.
MAPK pathway is regulated apelin-induced integrin expression and prostate cancer migration. (A&B) IPA pathway enrichment figure showing pathways that were changed in the GSE7930 dataset (Orange color indicated upregulated gene profile; blue color indicated downregulated gene profile). (C-H) Cells were treated with ERK (ERK II inhibitor; 10 μM), p38 (SB203580; 10 μM) and JNK (SP600125; 10 μM) inhibitors or transfected with ERK, p38 and JNK siRNA for 24 hours then with apelin, the wound healing, cell migration and integrin mRNA expression was examined (n=3). (I) PC3 cells were stimulated with apelin and ERK, p38 and JNK phosphorylation was examined by Western blotting (n=3). * p < 0.05 compared with the control group. # p < 0.05 compared with the apelin-treated group.
Apelin promotes integrin production and cell motility via the STAT3 pathway. (A-F) Cells were treated with STAT3 inhibitor (10 μM) or transfected with STAT3 siRNA for 24 hours then with apelin, the wound healing, cell migration and integrin mRNA expression was examined (n=3). (G&H) PC3 cells were treated with apelin or pretreated with ERK, p38 and JNK inhibitor then with apelin, the STAT3 phosphorylation was examined by Western blotting (n=3). * p < 0.05 compared with the control group. # p < 0.05 compared with the apelin-treated group.
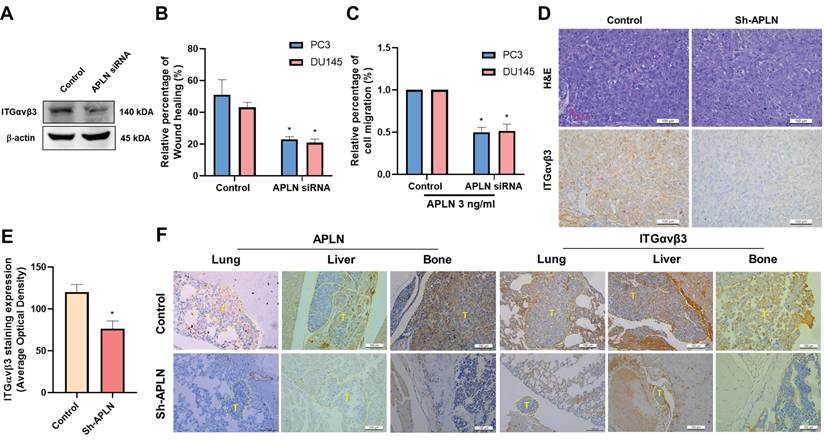
Blocking apelin inhibits metastasis of prostate cancer in vivo
To confirm the effects of apelin on integrin-dependent cell motility, PC3 cells were transfected with apelin siRNA. This transfection reduced integrin production, wound healing, and cell migration (Fig. 6A-C). We previously reported that apelin blockade reduces prostate cancer metastasis in tumor xenograft mouse models [19], the PC-3 cells were implanted into the anterior prostates of nude mice, and after 7 weeks, apelin blockade suppressed tumor growth by approximately 80% and 34%, as measured by IVIS imaging and manual tumor weight measurements, respectively. Ex vivo IVIS analysis revealed that apelin blockade inhibited prostate cancer metastasis to the liver and bone by 50% and 75%, respectively. Additionally, apelin blockade reduced metastatic tumor size in the lung, liver, and bone by 78.9%, 95.6%, and 96.1%, respectively, as determined by H&E staining, suggesting that inhibiting apelin suppresses prostate cancer metastasis. Here, we analyzed samples to examine integrin αvβ3 expression. IHC staining revealed that apelin blockade significantly reduced integrin αvβ3 expression levels in the prostate (Fig. 6D&E). Moreover, apelin blockade inhibited the occurrence of distant metastases and reduced the expression of apelin and integrin αvβ3 in the bone, liver, and lung (Fig. 6F), indicating that the apelin/integrin axis plays a crucial role in prostate cancer metastasis.
Discussion
Prostate cancer development is significantly influenced by adipose tissue [30]. The development, invasion, and metastasis of tumor cells have been thought to be influenced by direct or indirect interactions with adipocytes. In prostate cancer, bone tissue is the most common location of metastasis [31], and several investigations have suggested that bone marrow adipocytes have a role in the development and exacerbation of bone metastases [31]. Adipocytes produce the polypeptide apelin, which has a variety of biological functions and aids in tumor spreading by encouraging cell proliferation, motility, and survival [32]. It has been demonstrated that ovarian cancer tumor development and metastasis are facilitated by elevated apelin expression in ovarian tumor cells [33]. In this study, elevated apelin and integrin αvβ3 expression levels were detected in prostate cancer samples compared to those in normal individuals. Cell culture experiments demonstrated that apelin promotes integrin αvβ3-dependent prostate cancer migration by activating STAT3 and inhibiting miR-8070 via the MAPK pathway. Importantly, the in vivo study confirmed that inhibiting apelin reduces integrin expression and prostate cancer metastasis. We propose that the apelin/integrin axis represents a novel therapeutic target for treating metastatic prostate cancer. Our results are based on in vitro experiments and preclinical animal models; however, a limitation of this study is that the findings cannot be directly applied to clinical patients. Further development of therapies targeting the apelin/integrin axis will benefit prostate cancer patients undergoing treatment. Apelin is also a critical factor in obesity- and aging-related disorders [32]. Whether targeting apelin can also mitigate obesity- and aging-related disorders requires further investigation.
Apelin enhances integrin expression and promotes cell migration by inhibiting miR-8070 expression. (A) The diagrams depict the selection of miRNA candidates targeting integrin αv and β3. (B) PC3 cells were treated with apelin, the miRNAs expression was examined by qPCR (n=3). (C) Cells were treated with apelin for 24 hours, the miR-8070 expression was examined by qPCR (n=3). (D-F) Cells were transfected with miR-8070 mimic for 24 hours then with apelin, the wound healing, cell migration and integrin mRNA expression was examined (n=3). (G-I) Cells were treated with ERK, p38 and JNK inhibitor or siRNA then with apelin, the 3'UTR activity and miRNA expression was examined by luciferase activity and qPCR (n=3). * p < 0.05 compared with the control group. # p < 0.05 compared with the apelin-treated group.
Apelin blockade inhibits prostate cancer metastasis in vivo. (A-C) Cells were transfected with apelin siRNA, the integrin expression, wound healing and cell migration was examined (n=3). (D&E) IHC analysis of prostate cancer tissue samples stained with integrin αvβ3 antibody (n=3). (F) IHC analysis of leg bone (n=3), liver (n=3), and lung (n=3) sections stained with apelin and integrin αvβ3 antibodies. * p < 0.05 compared with the control group.
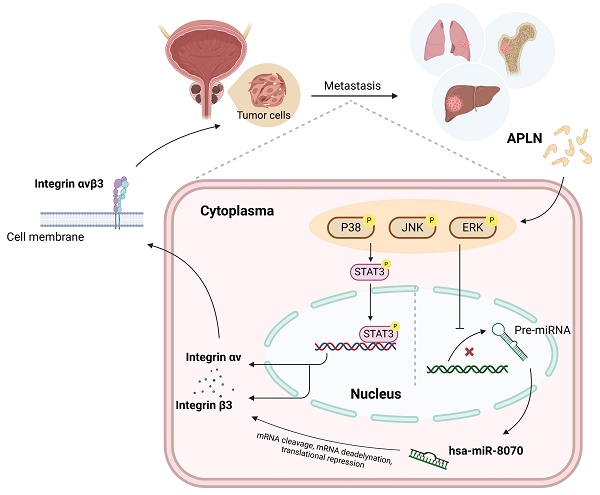
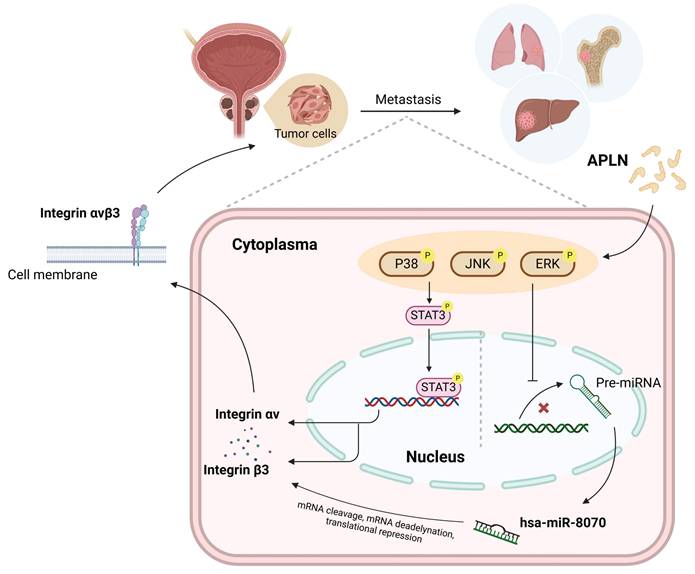
Schematic diagram illustrating the mechanism underlying the effects of apelin in prostate cancer metastasis. Apelin stimulation enhances integrin αvβ3-dependent prostate cancer migration and metastasis. The activation of STAT3 and inhibition of miR-8070 via the ERK, p38 and JNK pathways mediate apelin-facilitated integrin synthesis and cell motility.
As tumors grow and spread, changes in integrin expression are controlled [10]. Prostate cancer cell motility and proliferation in bone are facilitated by increases in integrin expression, indicating that integrin plays a critical role in prostate cancer metastasis to bone [34]. Furthermore, integrin makes prostate cancer malignancies more aggressive, and other studies have shown that prostate cancer has high levels of integrin expression, which can activate and cause metastases [34, 35]. Therefore, integrin seems to be a suitable target for therapeutic inhibition of metastases of prostate cancer. Our results reveal that apelin increases the production of integrins α5, αv, β1, and β3. An antibody against αvβ3, but not α5β1, blocks apelin-induced cell motility. Genetic inhibition of integrin αvβ3 produces similar effects. Apelin blockade inhibits integrin αvβ3 expression, cell migration, and metastasis both in vitro and in vivo. Therefore, integrin αvβ3 is a critical mediator of apelin-regulated prostate cancer metastasis.
MAPK signaling pathways are essential for many biological functions, including proliferation, growth, differentiation, and apoptosis [36, 37]. According to our IPA analysis of the GSE7930 database, the major wound healing signaling system was linked to the MAPK and STAT3 signaling pathways. We documented that apelin administration enhances the phosphorylation of MAPK molecules, including ERK, p38, and JNK. Treatment with pharmacological inhibitors of ERK, p38, and JNK suppressed apelin-induced integrin expression and cell migration. Transfection with their respective siRNAs produced consistent results. STAT3 transactivation is a key process in regulating tumor metastasis [38, 39]. Similarly, both STAT3 inhibitors and siRNA reversed apelin-mediated effects. Apelin-induced STAT3 activation was blocked by treatment with ERK, p38, and JNK inhibitors, indicating that apelin enhances integrin-dependent prostate cancer migration via the MAPK and STAT3 pathways.
At the post-transcriptional level, small, non-coding miRNAs are essential for regulating gene expression l [40]. This regulation controls physiological and pathological processes, including cancer, by destroying or inhibiting the translation of target mRNAs [41-43]. A promising treatment strategy to stop tumor metastasis is to alter miRNA expression through pharmacological interventions, which may be utilized to stop cancer cells from migrating [44, 45]. This study's investigation of miRNA database software revealed that miR-8070 disrupts integrin αv and β3 transcription. Subsequent studies showed that apelin administration decreased miR-8070 expression and that the introduction of a miR-8070 mimic into prostate cancer cells reversed apelin-induced integrin αv and β3 production, thereby reducing cell motility. Additionally, apelin-induced inhibition of miR-8070 synthesis was reversed by blocking ERK, p38, and JNK expression, indicating that apelin regulates integrin production and cell motility in prostate cancer by reducing miR-8070 levels through the MAPK pathway. We demonstrated that the activation of STAT3 and inhibition of miR-8070 via the ERK, p38, and JNK pathways mediate apelin-facilitated integrin synthesis and cell motility. The miRWalk database revealed that STAT3 does not contain an miR-8070 binding site, suggesting that miR-8070 cannot directly regulate STAT3 expression. Whether miR-8070 and STAT3 interact through other mechanisms requires further investigation.
In conclusion, we reveal that apelin increases integrin αvβ3 synthesis and promotes prostate cancer metastasis by activating STAT3 and inhibiting miR-8070 via the MAPK pathway (Fig. 7). Targeting the apelin/integrin αvβ3 axis may serve as a potential therapeutic strategy for treating metastatic prostate cancer.
Supplementary Material
Supplementary figure and table.
Acknowledgements
This work was supported by the Ministry of Science and Technology, Taiwan (MOST 111-2314-B-039-051), National Science and Technology Council (NSTC 112-2320-B-039-035-MY3; NSTC 113-2320-B-039-049-MY3 and NSTC 113-2314-B-715-009), and China Medical University (CMU111-ASIA-05; CMU112-ASIA-10; CMU113-ASIA-01; CMU113-ASIA-05; DMR-112-059; DMR-113-070; DMR-114-003; DMR-114-019).
Ethics approval and consent to participate
All animal-related procedures were approved by the Institutional Animal Care and Use Committee and conducted in strict adherence to the Guidelines of Animal Experimentation established by China Medical University (CMUIACUC-2020-353).
Patient consent for publication
Paraffin-embedded specimens containing 6 prostate cancer tissues and 3 normal tissues were collected, with patient consent, at the Department of Urology, China Medical University Hospital, Taiwan. The study was conducted under the Declaration of Helsinki and was approved by the Institutional Review Board of China Medical University Hospital (approval no. CMUH109-REC2-142). All specimens were collected from male patients diagnosed with prostate cancer. The patients' ages ranged from 57 to 82 years old, with a median of 73 and an average age of 72. All the patients were verbally explained and offered written informed permit before participation, with approval from the Institutional Review Board (IRB) of China Medical University Hospital (approval no. CMUH109-REC2-142). The authors inform that all processes followed relevant guidelines and regulations.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Force USPST, Grossman DC, Curry SJ, Owens DK, Bibbins-Domingo K, Caughey AB. et al. Screening for Prostate Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319:1901-13
2. Uehara H, Kobayashi T, Matsumoto M, Watanabe S, Yoneda A, Bando Y. Adipose tissue:Critical contributor to the development of prostate cancer. J Med Invest. 2018;65:9-17
3. Furesi G, Rauner M, Hofbauer LC. Emerging Players in Prostate Cancer-Bone Niche Communication. Trends Cancer. 2021;7:112-21
4. DiNatale A, Fatatis A. The Bone Microenvironment in Prostate Cancer Metastasis. Adv Exp Med Biol. 2019;1210:171-84
5. Hamidi H, Ivaska J. Every step of the way: integrins in cancer progression and metastasis. Nature reviews Cancer. 2018;18:533-48
6. Haywood-Reid PL, Zipf DR, Springer WR. Quantification of integrin subunits on human prostatic cell lines-comparison of nontumorigenic and tumorigenic lines. The Prostate. 1997;31:1-8
7. Li M, Wang Y, Li M, Wu X, Setrerrahmane S, Xu H. Integrins as attractive targets for cancer therapeutics. Acta pharmaceutica Sinica B. 2021;11:2726-37
8. Chen JC, Fong YC, Tang CH. Novel strategies for the treatment of chondrosarcomas: targeting integrins. Biomed Res Int. 2013;2013:396839
9. Drivalos A, Emmanouil G, Gavriatopoulou M, Terpos E, Sergentanis TN, Psaltopoulou T. Integrin expression in correlation to clinicopathological features and prognosis of prostate cancer: A systematic review and meta-analysis. Urologic oncology. 2021;39:221-32
10. Juan-Rivera MC, Martinez-Ferrer M. Integrin Inhibitors in Prostate Cancer. Cancers. 2018;10:44
11. Lee DK, Cheng R, Nguyen T, Fan T, Kariyawasam AP, Liu Y. et al. Characterization of apelin, the ligand for the APJ receptor. Journal of neurochemistry. 2000;74:34-41
12. Wysocka MB, Pietraszek-Gremplewicz K, Nowak D. The Role of Apelin in Cardiovascular Diseases, Obesity and Cancer. Frontiers in physiology. 2018;9:557
13. Zhang L, Takara K, Yamakawa D, Kidoya H, Takakura N. Apelin as a marker for monitoring the tumor vessel normalization window during antiangiogenic therapy. Cancer science. 2016;107:36-44
14. Tatemoto K, Takayama K, Zou MX, Kumaki I, Zhang W, Kumano K. et al. The novel peptide apelin lowers blood pressure via a nitric oxide-dependent mechanism. Regulatory peptides. 2001;99:87-92
15. Bertrand C, Valet P, Castan-Laurell I. Apelin and energy metabolism. Frontiers in physiology. 2015;6:115
16. Yang Y, Lv SY, Ye W, Zhang L. Apelin/APJ system and cancer. Clin Chim Acta. 2016;457:112-6
17. Masoumi J, Jafarzadeh A, Khorramdelazad H, Abbasloui M, Abdolalizadeh J, Jamali N. Role of Apelin/APJ axis in cancer development and progression. Adv Med Sci. 2020;65:202-13
18. Hall C, Ehrlich L, Venter J, O'Brien A, White T, Zhou T. et al. Inhibition of the apelin/apelin receptor axis decreases cholangiocarcinoma growth. Cancer letters. 2017;386:179-88
19. Lin TH, Chang SL, Khanh PM, Trang NTN, Liu SC, Tsai HC. et al. Apelin Promotes Prostate Cancer Metastasis by Downregulating TIMP2 via Increases in miR-106a-5p Expression. Cells. 2022;11:3285
20. Lin LW, Lin TH, Swain S, Fang JK, Guo JH, Yang SF. et al. Melatonin Inhibits ET-1 Production to Break Crosstalk Between Prostate Cancer and Bone Cells: Implication for Osteoblastic Bone Metastasis Treatment. J Pineal Res. 2024;76:e70000
21. Lee HP, Chen PC, Wang SW, Fong YC, Tsai CH, Tsai FJ. et al. Plumbagin suppresses endothelial progenitor cell-related angiogenesis in vitro and in vivo. Journal of Functional Foods. 2019;52:537-44
22. Lee HP, Wang SW, Wu YC, Lin LW, Tsai FJ, Yang JS. et al. Soya-cerebroside inhibits VEGF-facilitated angiogenesis in endothelial progenitor cells. Food Agr Immunol. 2020;31:193-204
23. Lee KT, Su CH, Liu SC, Chen BC, Chang JW, Tsai CH. et al. Cordycerebroside A inhibits ICAM-1-dependent M1 monocyte adhesion to osteoarthritis synovial fibroblasts. Journal of food biochemistry. 2022;46:e14108
24. Liu SC, Tsai CH, Wu TY, Tsai CH, Tsai FJ, Chung JG. et al. Soya-cerebroside reduces IL-1β-induced MMP-1 production in chondrocytes and inhibits cartilage degradation: implications for the treatment of osteoarthritis. Food Agr Immunol. 2019;30:620-32
25. Chang AC, Chen PC, Lin YF, Su CM, Liu JF, Lin TH. et al. Osteoblast-secreted WISP-1 promotes adherence of prostate cancer cells to bone via the VCAM-1/integrin alpha4beta1 system. Cancer Lett. 2018;426:47-56
26. Su C-H, Lin C-Y, Tsai C-H, Lee H-P, Lo L-C, Huang W-C. et al. Betulin suppresses TNF-α and IL-1β production in osteoarthritis synovial fibroblasts by inhibiting the MEK/ERK/NF-κB pathway. Journal of Functional Foods. 2021;86:104729
27. Zhao H, Tian X, He L, Li Y, Pu W, Liu Q. et al. Apj(+) Vessels Drive Tumor Growth and Represent a Tractable Therapeutic Target. Cell Rep. 2018;25:1241-54.e5
28. Sorli SC, Le Gonidec S, Knibiehler B, Audigier Y. Apelin is a potent activator of tumour neoangiogenesis. Oncogene. 2007;26:7692-9
29. Tang Y, Pan J, Huang S, Peng X, Zou X, Luo Y. et al. Downregulation of miR-133a-3p promotes prostate cancer bone metastasis via activating PI3K/AKT signaling. J Exp Clin Cancer Res. 2018;37:160
30. Ku HC, Cheng CF. Role of adipocyte browning in prostate and breast tumor microenvironment. Tzu Chi Med J. 2022;34:359-66
31. Gandaglia G, Abdollah F, Schiffmann J, Trudeau V, Shariat SF, Kim SP. et al. Distribution of metastatic sites in patients with prostate cancer: A population-based analysis. Prostate. 2014;74:210-6
32. Booth A, Magnuson A, Fouts J, Foster M. Adipose tissue, obesity and adipokines: role in cancer promotion. Hormone molecular biology and clinical investigation. 2015;21:57-74
33. Neelakantan D, Dogra S, Devapatla B, Jaiprasart P, Mukashyaka MC, Janknecht R. et al. Multifunctional APJ Pathway Promotes Ovarian Cancer Progression and Metastasis. Molecular cancer research: MCR. 2019;17:1378-90
34. Hall CL, Dubyk CW, Riesenberger TA, Shein D, Keller ET, van Golen KL. Type I collagen receptor (alpha2beta1) signaling promotes prostate cancer invasion through RhoC GTPase. Neoplasia. 2008;10:797-803
35. Ryu S, Park KM, Lee SH. Gleditsia sinensis Thorn Attenuates the Collagen-Based Migration of PC3 Prostate Cancer Cells through the Suppression of alpha2beta1 Integrin Expression. International journal of molecular sciences. 2016;17:328
36. Li J, Zhao Z, Liu J, Huang N, Long D, Wang J. et al. MEK/ERK and p38 MAPK regulate chondrogenesis of rat bone marrow mesenchymal stem cells through delicate interaction with TGF-beta1/Smads pathway. Cell Prolif. 2010;43:333-43
37. Zhang Y, Pizzute T, Pei M. A review of crosstalk between MAPK and Wnt signals and its impact on cartilage regeneration. Cell Tissue Res. 2014;358:633-49
38. Jiang YJ, Chao CC, Chang AC, Chen PC, Cheng FJ, Liu JF. et al. Cigarette smoke-promoted increases in osteopontin expression attract mesenchymal stem cell recruitment and facilitate lung cancer metastasis. J Adv Res. 2022;41:77-87
39. Hu SL, Huang CC, Tseng TT, Liu SC, Tsai CH, Fong YC. et al. S1P facilitates IL-1beta production in osteoblasts via the JAK and STAT3 signaling pathways. Environ Toxicol. 2020;35:991-7
40. Tehrani SS, Zaboli E, Sadeghi F, Khafri S, Karimian A, Rafie M. et al. MicroRNA-26a-5p as a potential predictive factor for determining the effectiveness of trastuzumab therapy in HER-2 positive breast cancer patients. BioMedicine. 2021;11:30-9
41. Kanwal R, Plaga AR, Liu X, Shukla GC, Gupta S. MicroRNAs in prostate cancer: Functional role as biomarkers. Cancer letters. 2017;407:9-20
42. Kong YW, Ferland-McCollough D, Jackson TJ, Bushell M. microRNAs in cancer management. The Lancet Oncology. 2012;13:e249-58
43. He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nature reviews Genetics. 2004;5:522-31
44. Tsai HC, Lai YY, Hsu HC, Fong YC, Lien MY, Tang CH. CCL4 Stimulates Cell Migration in Human Osteosarcoma via the mir-3927-3p/Integrin alphavbeta3 Axis. International journal of molecular sciences. 2021;22:12737
45. Tzeng HE, Lin SL, Thadevoos LA, Lien MY, Yang WH, Ko CY. et al. Nerve growth factor promotes lysyl oxidase-dependent chondrosarcoma cell metastasis by suppressing miR-149-5p synthesis. Cell death & disease. 2021;12:1101
Author contact
![]() Corresponding authors: Chih-Hsin Tang, PhD; Email: chtangcmu.edu.tw. Chih-Hsueh Lin, MD, PhD; Email: 005496caaumed.org.tw.
Corresponding authors: Chih-Hsin Tang, PhD; Email: chtangcmu.edu.tw. Chih-Hsueh Lin, MD, PhD; Email: 005496caaumed.org.tw.

 Global reach, higher impact
Global reach, higher impact