10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2025; 21(11):5135-5163. doi:10.7150/ijbs.115518 This issue Cite
Review
Roles of SIRT3 in aging and aging-related diseases
1. School of Clinical Medicine, Tsinghua University, Beijing 100084, China.
2. Department of General Practice, Beijing Tsinghua Changgung Hospital, School of Clinical Medicine, Tsinghua University, Beijing 100084, China.
Received 2025-4-10; Accepted 2025-7-10; Published 2025-7-28
Abstract
Aging is an inexorable pathophysiological progression characterized by the overwhelming deterioration of tissue integrity and cellular function coupled with increased risks of various aging-related diseases. Demographic shifts toward extended longevity have precipitated a paradigm shift in disease epidemiology, in which neurodegenerative conditions and cardiovascular pathologies now constitute predominant determinants of morbidity and mortality in geriatric populations. These conditions severely erode functional autonomy in aging populations and strain healthcare infrastructures globally.
As a principal nicotine adenine dinucleotide-dependent deacetylase within mitochondria, sirtuin 3 (SIRT3) exerts multimodal regulatory effects spanning mitochondrial bioenergetics, oxidative stress, and epigenetic modifications associated with aging. This review summarizes recent discoveries regarding the involvement of SIRT3 in physiological aging and its pathophysiological intersections with major aging-related disorders, providing new insights and ample inspiration for future research aimed at slowing the aging process and improving outcomes in aging-related diseases.
Keywords: SITR3, Mitochondria, Aging, Aging-related Diseases, Neurodegenerative Diseases
Introduction
Sirtuins (SIRTs) comprise an evolutionarily conserved family of nicotine adenine dinucleotide (NAD+)-dependent deacylases that beneficially modulate lifespan and healthspan1. These enzymes orchestrate a wide range of physiological mechanisms that contribute to cellular and systemic homeostasis, encompassing DNA damage repair2, mitochondrial biogenesis3, genomic stability4, inflammatory response5, and metabolic homeostasis6. By integrating various stress response pathways, SIRTs serve as central regulators of cellular integrity and overall organismal well-being7. Consequently, these proteins have been identified as key determinants in the aging process and in the prevention of aging-related diseases.
Mammalian systems express seven phylogenetically conserved sirtuin variants (SIRT1-7), each occupying distinct subcellular compartments8, which underpins their diverse functional roles in cellular processes. Although these isoforms maintain a structurally conserved catalytic domain essential for enzymatic activity9, their divergent N- and C-terminal segments confer differential localizations and substrate specificities, enabling SIRTs to regulate a broad spectrum of cellular functions and respond to various physiological and environmental cues10. Originally characterized as class III histone deacetylases (HDACs), SIRTs are currently recognized as catalysts for multifaceted post-translational modifications, encompassing ADP-ribosylation, desuccinylation11, demalonylation12, depropionylation13, and debutyrylation14. These modifications allow SIRTs to exert profound epigenetic control over gene expression and regulate critical biological processes essential for cellular health15, such as maintaining genomic integrity16, controlling apoptosis17, metabolic adaptation18, managing inflammatory responses19, and combating oxidative stress20. Collectively, these activities have significant implications for the aging process and aging-related pathologies21.
Among SIRTs, SIRT3 is the only isoform directly linked to human longevity22. Its predominant localization in the mitochondria and its central role in energy metabolism make it a critical regulator of mitochondrial function and metabolic adaptation23. Human SIRT3 is encoded by a 399-residue polypeptide organized into two evolutionarily conserved functional domains, including an N-terminal Rossmann fold that binds NAD+ cofactors and a C-terminal zinc finger module essential for structural stabilization24. This unique structural configuration enables SIRT3 to catalyze several enzymatic reactions25, including deacetylation, ADP-ribosylasation, demalonylation, and desuccinylation26,27. Mitochondrial proteomic analyses have identified multiple substrates of SIRT3, confirming its pivotal role in regulating metabolic pathways, particularly under stress conditions such as fasting and exercise28.
Although the mitochondrial function of SIRT3 is well established, emerging evidence also indicates that SIRT3 translocates to the nucleus under certain conditions and helps maintain the heterochromatin structure and prevent cellular senescence, particularly in human mesenchymal stem cells (hMSCs)29. This dual subcellular localization positions SIRT3 as a sentinel regulator of mitochondrial-nuclear crosstalk, highlighting its versatility in maintaining cellular homeostasis across different compartments. SIRT3 also contributes to genomic stability and cellular longevity by facilitating reactive oxygen species (ROS) detoxification by activating superoxide dismutase 2 (SOD2)30 and promoting mitophagy31. These mechanisms substantiate SIRT3's pleiotropic functions in maintaining genomic stability and cellular longevity, reinforcing its therapeutic relevance in combating oxidative damage and mitigating the effects of aging.
This review aims to illuminate the structural and functional roles of SIRT3 in the aging process, particularly its involvement in major aging-related diseases. Furthermore, we explore the therapeutic potential of SIRT3, positioning it as a promising target for mitigating aging-related pathologies and highlighting its growing importance in the field of geroscience.
Method
To ensure a comprehensive and systematic review, we conducted a literature search using databases such as Web of Science, Scopus, and Google Scholar for studies published up to June 2025. Keywords including “SIRT3,” “aging,” and “aging-related diseases” were used in various combinations. We primarily focused on peer-reviewed journal articles published in English. The inclusion criteria were relevance to the core themes of this review, publication within the last 10 years, and the inclusion of original research or substantial theoretical discussion. All cited references are provided with complete bibliographic information, including authors' names, article titles, journal names, year of publication, and DOIs, to ensure transparency and traceability. Furthermore, we applied PRISMA and AMSTAR standards to assess the methodological quality of the included studies, enabling the selection of high-quality research that offers both theoretical insights and methodological guidance for this review.
1. SIRT3 and aging
Aging constitutes an evolutionarily conserved biological trajectory associated with progressive attrition of systemic functionality32. Cellular senescence, a hallmark of aging, refers to a permanent cessation of cell division induced by various stressors. This state is defined by the senescence-associated secretory phenotype (SASP), accumulation of macromolecular damage, and metabolic dysregulation33.
Accumulating evidence indicates that the molecular mechanisms underlying closely align with those governing organismal aging34, including chronic inflammation35, epigenetic alterations36, autophagy malfunction37, oxidative stress38, metabolic dysfunction39, and mitochondrial instability40. Molecular investigations have identified SIRT3 as a central coordinator that orchestrates multiple anti-senescence programs across these processes41. Through deacetylation of mitochondrial targets, SIRT3 enhances the cellular antioxidant capacity42, promotes metabolic flexibility43, and reduces ROS accumulation, all of which are critical for minimizing DNA damage and inhibiting the onset of senescence44.
Importantly, SIRT3 also regulates canonical markers, such as SA-β-gal and inhibits the p53/p21 axis45, thereby delaying the senescence initiation. Moreover, SIRT3 functions as a negative regulator of SASP via its control over mitochondrial function46. Experimental models have shown that the loss of SIRT3 results in increased expression of SASP components, including TNF-α, IL-1β, and matrix metalloproteinase-9 (MMP9)47. Conversely, activation of SIRT3 ameliorates inflammatory tissue damage. Notably, oxidative stress-induced upregulation of microRNA-494-3p (miR-494-3p) leads to decreased SIRT3 levels, establishing a feedback loop that further exacerbates senescence48,49.
Although cellular senescence and organismal aging are mechanistically intertwined, they present as distinct phenotypic entities50. From a translational standpoint, the pleiotropic roles of SIRT3 in fibrosis and apoptosis underscore its potential as a therapeutic target for aging-related pathologies, extending beyond the context of cellular senescence alone. To further explore the molecular crosstalk between SIRT3 and cellular senescence, we focus on the pathways through which SIRT3 exerts its anti-senescence effects. These include the regulation of inflammation, epigenetic modifications, metabolic homeostasis, oxidative stress, autophagy, fibrosis and apoptosis. Together, these interconnected pathways form the molecular foundation for SIRT3's ability to delay aging and alleviate aging-related diseases.
1.1 Metabolic regulation
Emerging evidence indicates that SIRT3 acts as a key metabolic regulatory that senses intracellular acetyl-coenzyme A (CoA) and NAD+ levels by transducing these cues through the deacetylation of mitochondrial proteins51. The downstream targets of SIRT3 span critical mitochondrial pathways, including the tricarboxylic acid (TCA) cycle52, amino acid catabolism53, lipid β-oxidation54, and electron transport chain (ETC)/oxidative phosphorylation (OXPHOS) complexes55,56.
Mice with SIRT3 knockout exhibited compromised triglyceride catabolism, diminished β-oxidation efficiency, and reduced generation of essential metabolic intermediates such as acetyl-CoA57,58. Under caloric restriction (CR) or fasting, elevated NAD+ levels promote SIRT3 deacetylase activity, optimizing mitochondrial enzymatic efficiency through post-translational modifications59. Diminished hepatic SIRT3 expression induces hepatic steatosis by facilitating intracellular triglyceride deposition in response to elevated fatty acid concentrations60.
In the context of high-fat diet consumption, preclinical models with pancreatic β-cell-selective SIRT3 knockout exhibited compromised glucose homeostasis, decreased glucose-stimulated insulin secretion, and reduced pancreatic β-cell function61. Transcriptomic profiling further revealed that β-cell-selective SIRT3 knockout mediates insulin secretion and liver lipid homeostasis through a serotonin-dependent mechanism62.
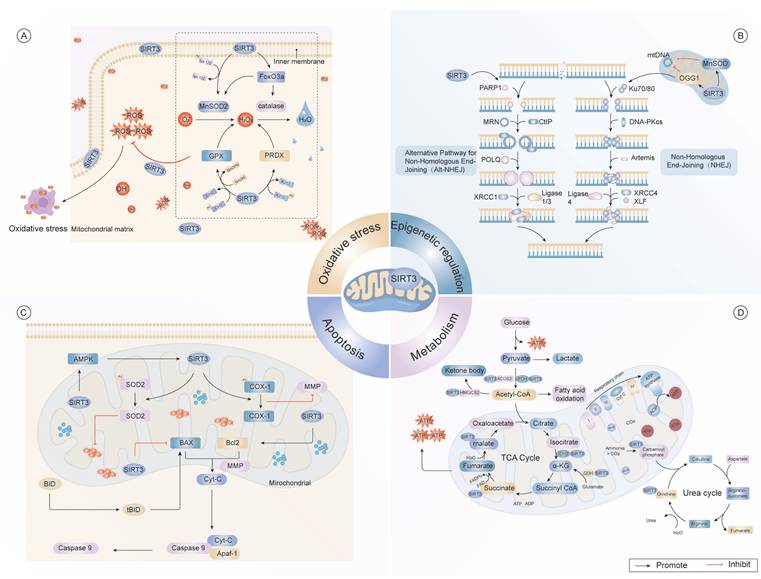
SIRT3 also regulates succinate dehydrogenase (SDH/complex II), a critical acetylation node within the mitochondrial matrix, which mediates crosstalk between metabolic flux control and mitochondrial energy transduction systems63. Through the modulation of SDH enzymatic activity, SIRT3 fine-tunes substrate utilization efficiency while maintaining electron transfer chain synchronization, thus preserving metabolic homeostasis64. Furthermore, experimental data have demonstrated that shRNA-induced SIRT3 knockdown significantly attenuates the cytoprotective effects of honokiol (HKL) on mitochondrial biogenesis, OXPHOS capacity, and bioenergetic output, underscoring its pivotal role in maintaining mitochondrial biogenesis and bioenergetics65 (Fig. 1).
These findings established SIRT3 as a crucial modulator of metabolic homeostasis through its multifaceted catalytic functions. Future research should focus on delineating the disease-specific mechanisms of SIRT3 and evaluating its therapeutic utility in metabolic and age-related diseases.
1.2 Oxidative stress
Oxidative stress represents a pathological condition defined by a systemic imbalance in which overproduction of reactive oxygen species (ROS) that overwhelms the detoxification potential of endogenous cytoprotective machinery66. Such perturbations initiate structural alterations in biomacromolecules, thereby disrupting cellular function and promoting aging-related pathologies67.
Previous studies have established that SIRT3 exerts potent deacetylase activity on ETC subunits and enzymes involved in oxidative stress response activity, serving as a critical factor in various underlying regulatory mechanisms68. Furthermore, SIRT3 augments ROS scavenging while maintaining organelle homeostasis, conferring cytoprotective effects against oxidative stress69. Experimental evidence has demonstrated that mitochondrial antioxidant enzymes display altered lysine acetylation modification in SIRT3-deficient conditions, exhibiting pronounced acetylation leading to enzymatic dysfunction, which exacerbates ROS generation and aggravates oxidative stress-induced damage70.
Emerging research has underscored the vital role of SIRT3 in mitigating oxidative stress through precise regulation of core antioxidant enzymes, including manganese superoxide dismutase 2 (MnSOD2), glutathione peroxidase, and isocitrate dehydrogenase 2 (IDH2)71. Under caloric restriction, SIRT3-mediated deacetylation dynamically regulates mitochondrial IDH2 activation, culminating in elevated NADPH biosynthesis72, prevents ETC overload and subsequent excessive ROS generation and potentiates mitochondrial antioxidant capacity, ultimately attenuating oxidative stress73,74. Notably, SIRT3 optimizes mitochondrial ROS clearance by deacetylating SOD75,76. Furthermore, SIRT3 enhances MnSOD activity through deacetylation, amplifying endogenous antioxidant responses77. Additionally, the SIRT3/forkhead box O3a (FOXO3a) signaling axis triggers mitochondrial DNA (mtDNA) expression, thereby alleviating oxidative damage78 (Fig. 1).
Hence, SIRT3 maintains mitochondrial redox homeostasis and attenuates oxidative stress-associated cellular damage through epigenetic regulation of antioxidant defense systems. The therapeutic potential of targeting SIRT3 in aging-related metabolic diseases and neurodegenerative disorders presents a promising avenue for future research.
1.3 Epigenetic regulation
Epigenetic regulation refers to a set of dynamic and reversible modifications spanning genomic DNA and nucleoprotein assemblies, orchestrated through intricate interactions between sequence-specific transcription factors and various epigenetic modifier complexes79. Accumulating evidence positions SIRT3 as a novel chromatin-modifying enzyme, exerting tripartite regulatory control over DNA methylation patterns, covalent histone modifications, and nucleosome remodeling80.
Mechanistically, SIRT3 modulates the enzymatic catalytic function of 8-oxo guanine DNA glycosylase-1 (OGG1) through lysine deacetylation, potentiating mitochondrial DNA damage remediation81. It also promotes the transcriptional activity of FOXO3a through deacetylation, thereby increasing its DNA-binding affinity and enhancing the expression of genes involved in cellular stress-responsive82. Notably, SIRT3 plays a critical geroprotectvive factor that maintains heterochromatin integrity in hMSCs, thereby postponing replicative senescence progression83. Furthermore, SIRT3 forms macromolecular assemblies with nuclear lamina constituents and heterochromatin-associated proteins, supporting chromatin compaction and genomic stability maintenance84.
Multidimensional regulatory network of SIRT3 in cellular homeostasis. (A) As a central redox regulator, SIRT3 orchestrates mitochondrial antioxidant defense through dual mechanisms. It regulates key antioxidant enzymes, including MnSOD2 and CAT, to counteract oxidative damage induced by ROS. Furthermore, SIRT3 modulates the activities of GPX and PRDX, thereby maintaining cellular redox homeostasis. (B) SIRT3 contributes to the regulation of epigenetic processes, particularly in DNA repair mechanisms via the NHEJ pathway. It interacts with critical DNA repair proteins, such as PARP1, Ku70/80, XRCC1, and DNA ligase 4, and facilitates the repair of mtDNA damage. (C) SIRT3 modulates apoptotic commitment through dynamic acetylation networks. SIRT3 regulates mitochondrial dynamics by deacetylating Bcl-2, thus preventing mitochondrial outer membrane permeabilization. Additionally, SIRT3 delays Cyt-C release kinetics the subsequent activation of caspases, thereby modulating apoptotic signaling pathways. (D) SIRT3 integrates metabolic flux through substrate channeling, exerting significant effects on the TCA cycle and promoting fatty acid oxidation. It regulates critical enzymes, such as AMPK, SOD2, and acetyl-CoA, and also modulates glucose metabolism by controlling the conversion of pyruvate to lactate. Moreover, SIRT3 supports the production of ketone bodies, which are essential for maintaining energy balance under conditions of metabolic stress.
The absence of SIRT3 triggers structural abnormalities in the nuclear envelope, disarray of heterochromatin organization, and reactivation of previously silenced repetitive sequences, collectively accelerating premature cellular aging85,86. Restoration of SIRT3 expression in deficient cells has been shown to re-establish chromatin organization and mitigate senescence-associated phenotypes87.
Advances in high-resolution chromatin profiling have illuminated previously uncharacterized mechanisms by which SIRT3 maintains genomic integrity88. Beyond its canonical deacetylase activity, SIRT3 possesses substantial lysine decrotonylase capacity89, selectively removing crotonyl groups from specific histone substrates90. This dynamic regulation of histone crotonylation adds a new dimension to SIRT3's epigenetic repertoire, particularly in modulating gene expression plasticity under both homeostatic and stress conditions91.
Moreover, SIRT3 undergoes stress-responsive chromatin redistribution92, influencing higher-order chromatin topology and initiating protective transcriptional programs in response to genotoxic stimuli93. Disruption of SIRT3 impairs the chromatin-silencing machinery, leading to altered transcriptional landscapes and compromised chromatin homeostasis94. Importantly, SIRT3 also facilitates DNA double-strand break repair by enhancing the recruitment of 53BP1, thus promoting the efficiency of the non-homologous end joining (NHEJ)95.
In the context of viral infection, SIRT3 dynamically modulates the transcriptional output and replicative capacity of viral genomes by guiding histone methyltransferase complexes to viral episomes96. Exogenous SIRT3 overexpression significantly attenuates virus gene expression, whereas deletion potentiates viral replication efficiency97. Additionally, SIRT3 decreases H3K27 crotonylation at metastasis-associated loci, such as ETS1, effectively constraining neoplastic dissemination98,99 (Fig. 1).
Taken together, these findings portray SIRT3 as a critical integrator of chromatin signaling pathways, positioned at the interface of epigenetic regulation and genome surveillance. Future investigations should aim to delineate the disease-specific chromatin landscapes modulated by SIRT3, with a particular focus on its roles in tumor biology, neurodegenerative disorders, and viral pathogenesis.
1.4 Apoptosis
Apoptosis exhibits paradoxical roles in maintaining organismal homeostasis100. As a bifunctional regulator of cellular dynamics, apoptosis maintains tissue homeostasis through precisely orchestrated mitochondrial101 and endoplasmic reticulum stress responses pathways102.
These processes yield divergent outcomes depending on whether the cellular environment is physiological or pathophysiological103.
In the context of senescent cells, sustained low-grade stress induces resistance to apoptosis signals, fostering a pro-inflammatory and senescence-associated phenotype, which accelerates systemic aging104,105.
Emerging evidence highlights the context-dependent manner of SIRT3 in modulating apoptotic responses106. Under stress conditions, SIRT3 predominantly exerts anti-apoptotic effects by orchestrating apoptotic regulatory hubs, such as the GSK-3β/Bax, Bax/Bcl-2, and caspase-9 pathways107,108. Through these interactions, SIRT3 safeguards mitochondrial integrity and promoting cell viability109. Notably, the AMPK/SENP1/SIRT3 axis governs mitochondrial apoptosis via SOD2 deacetylation, thereby mitigating oxidative stress-induced apoptosis110.
Conversely, under certain pathological conditions such as malignancy, SIRT3 exerts pro-apoptotic effects by facilitating mitochondrial apoptotic signaling, thereby impeding tumor proliferation and viability111. In sepsis models, genetic ablation of SIRT3 has been associated with intensified apoptotic responses, as evidenced by increased Bax and caspase-3 levels alongside reduced Bcl-2 expression112,113. This pro-apoptotic function is further potentiated by FOXO1 deacetylation, which upregulates transcription of apoptosis-promoting genes114,115. Moreover, non-coding RNAs, such as miR-297116 and miR-421117, have emerged as upstream modulators of SIRT3, modulating its expression and consequently influencing apoptosis-related pathways (Fig. 1).
Collectively, these insights underscore the dualistic and context-dependent roles of SIRT3 in apoptosis regulation. Serving as a pivotal node in apoptotic signaling networks, SIRT3 orchestrates cellular fate decisions with implications across aging and disease states. Future investigations should aim to dissect the tissue-specific functions of SIRT3, explore its molecular crosstalk with other apoptotic regulators, and evaluate its potential as a therapeutic target in aging-related disorders such as cancer.
1.5 Fibrosis
Fibrosis represents a hallmark of aging that arises from a progressive decline of tissue restorative and regenerative competence118,119. In senescent organs, injury-induced signals increasingly favor pro-fibrotic cascades rather than activating regenerative programs, leading to excessive extracellular matrix (ECM) deposition and increased tissue rigidity120,121. This maladaptive remodeling disturbs normal tissue architecture and function, ultimately driving multi-organ dysfunction in older individuals122.
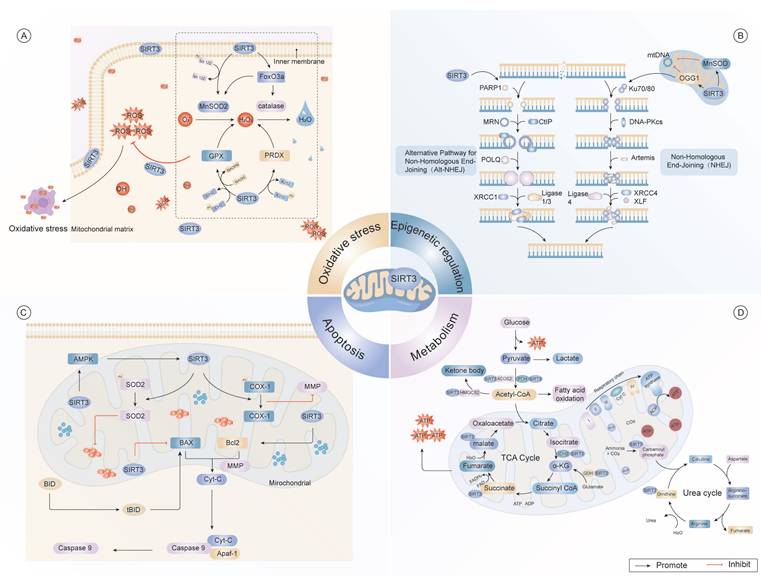
Recent investigations have identified SIRT3 as a critical suppressor of fibrogenesis, operating at the interface between mitochondrial homeostasis and nuclear transcriptional regulation123. Across a range of experimental fibrosis models, including peritoneal, cardiac, pulmonary, and hepatic fibrosis, SIRT3 consistently act as a central suppressor of fibrotic progression124. Mechanistically, SIRT3 mediates its anti-fibrotic effect through the targeted deacetylation of glycogen synthase kinase 3β (GSK-3β) at Lys15, effectively preventing Smad3 transcriptional complex formation and subsequent fibrogenic programming125. In cardiomyocytes, SIRT3 also attenuates fibrosis by inhibiting the FOS/AP-1126 and transcription 3 (STAT3)/NFATc2 signaling pathways125. Conversely, genetic deletion of SIRT3 exacerbates susceptibility to fibrosis127, particularly in the myocardium, where its absence leads to increased GSK-3β acetylation128 and hyperactivation of the TGF-β signaling axis129. These alterations culminate in enhanced transcription of ECM-related genes.
Notably, the pro-fibrotic consequences of SIRT3 deficiency are not confined to cardiac tissue. Similar signaling disruptions have observed in renal and pulmonary fibrosis models, where SIRT3 restoration effectively reverses fibrotic pathology by rebalancing mitochondrial redox states and repressing Smad3-mediated transcription activity130.
In pulmonary tissue, SIRT3 overexpression has been shown to mitigate bleomycin-induced fibrosis, primarily by preserving mtDNA integrity and suppressing TGF-β1-dependent signaling pathways131-133. In hepatic fibrosis models, SIRT3 has been implicated in mediating the anti-fibrotic effects of withaferin A134, an effect that is abolished in SIRT3-knockout mice. This finding reinforces the indispensable role of SIRT3 as a molecular effector of pharmacological anti-fibrotic agents126 (Fig. 2).
Collectively, these findings establish SIRT3 as a master regulatory nexus in the pathogenesis of fibrosis across multiple organ systems. By integrating mitochondrial integrity, redox homeostasis, and nuclear transcriptional control, SIRT3 orchestrates protective responses against fibrotic remodeling. Moving forward, research should focus on elucidating the organ-specific and context-dependent mechanisms by which SIRT3 modulates fibrosis. Particular attention should be directed toward the interplay between SIRT3's mitochondrial and nuclear activities during the transition from tissue injury to fibrotic remodeling, as well as its potential crosstalk with ECM-sensing mechanisms. These insights may pave the way for developing targeted therapeutic interventions aimed at modulating SIRT3 activity in fibrotic disease.
1.6 Inflammation
Low-grade inflammatory states have emerged as pathognomonic features of organismal senescence and primary drivers in geriatric pathobiology135. SIRT3 serves as a critical molecular determinant by modulating aging-related inflammation through its regulation of mitochondrial homeostasis and inflammatory signaling pathways136.
Studies revealed greater ROS accumulation, amplified NLRP3 inflammasome oligomerization, and exacerbated mitochondrial structural abnormalities in SIRT-knockout murine models compared with the findings in their wild-type counterparts124,137. Conversely, SIRT3 overexpression attenuates inflammation by inhibiting IκBα phosphorylation138 and modulating the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB)/TGF-β1/Smad axis139, thereby suppressing NLRP3 inflammasome formation and subsequent inflammatory responses116,140.
Notably, SIRT3 activation consistently correlates with reduced expression of pro-inflammatory cytokines, such as TNF-α, IL-6, and MIP-2, as well as diminished polymorphonuclear leukocyte infiltration in various preclinical models141,142. In murine models with chronic sodium overload, SIRT3 overexpression induced persistent disruption of chronic sodium overload, SIRT3 overexpression disrupted immune cell migration by modulating key metabolic and immune signaling pathways143. These anti-inflammatory effects were sustained even in the absence of continued dietary interventions, indicating that SIRT3 mediates durable transcriptional reprogramming via NF-κB and STAT3 suppression144,145 (Fig. 2).
Taken together, these findings position SIRT3 as a mitochondrial checkpoint in the regulation of inflammation during aging. Future investigations should aim to elucidate the mechanisms by which SIRT3 interfaces with tissue-specific immune circuits and determine whether pharmacologic activation of SIRT3 can confer resilience against aging-related inflammatory disorders such as atherosclerosis, neuroinflammation, and sarcopenia. Additionally, further research is warranted to uncover the upstream regulatory networks that govern SIRT3 activity under conditions of inflammatory stress.
Multifaceted regulatory mechanisms of SIRT3 in cellular homeostasis. (A) SIRT3 regulates autophagy through the deacetylation of FOXO1 and FOXO3a, which interact with E3 ligase and PARKIN to promote mitochondrial fission. Additionally, SIRT3 activates the LKB1/AMPK axis, thereby modulating autophagy via mTOR inhibition and Raptor activation, maintaining mitochondrial homeostasis and cellular integrity. Furthermore, SIRT3 influences mitochondrial dynamics by promoting the expression of MnSOD, thus alleviating oxidative damage caused by ROS. (B) SIRT3 suppresses pro-inflammatory signaling by deacetylating transcription factors NF-κB and AP-1, thereby attenuating NLRP3 inflammasome assembly and subsequent interleukin release. SIRT3 modulates the chemotactic proteins levels such as TGF-β1 in response to stress signals such as Ang-II. (C) In fibrosis, SIRT3 regulates the expression of fibrotic markers by deacetylating histone H3, thereby influencing gene transcription. It affects pathways related to extracellular matrix remodeling, including directly modulating TGF-β1 expression, a key driver of fibrosis. Metabolically, SIRT3 coordinates glycolysis, TCA cycle flux, and oxidative phosphorylation. by deacetylating key enzymes such as MnSOD, ensuring efficient energy production. By dynamically regulating mitochondrial protein acetylation, SIRT3 enables adaptive metabolic reprogramming during cellular stress.
1.7 Autophagy
Autophagy, a phylogenetically conserved lysosomal clearance mechanism, sustains proteostasis via the selective elimination of dysfunctional organelles and misfolded protein aggregates146. SIRT3 has emerged as a pivotal regulator of autophagic flux dynamics, modulating evolutionarily conserved signaling pathways, including the AMPK/mTOR and FOXO3a pathways147. Genetic ablation of SIRT3 impairs autophagic signaling, notably disrupting AMPK/mTOR-mediated transduction concomitant with the paradoxical activation of glutathione peroxidase 4 activity, which suppresses autophagy initiation148.
Mechanistically, SIRT3 facilitates mitochondrial quality control via AMPK/ULK1 phosphorylation while antagonizing mTOR-mediated autophagic repression, thereby establishing dual checkpoints for organelle surveillance149. Moreover, a positive feedback loop exists in which autophagy activation induces SIRT3 expression via FOXO3a-mediated transcriptional programming, thereby promoting PTEN-induced kinase (PINK1)/Parkin-mediated mitochondrial quality control to reduce mtROS levels and restore hematopoietic stem cell repopulation efficiency through the enhanced lysosomal clearance of damaged mitochondria150-152(Fig. 2).
Taken together, these findings underscore SIRT3 as a central integrator of the autophagy-lysosome network, orchestrating mitochondrial fidelity, proteostasis, and stem cell maintenance during aging. Future investigations should focus on elucidating the tissue-specific roles of SIRT3 in autophagy regulation and exploring whether pharmacological modulation of SIRT3 can be leveraged to enhance autophagic function in aging-related degenerative conditions.
2. The role of SIRT3 in aging-related diseases
Aging-related disorders arise within tissue microenvironments due to chronic inflammation, stem cell exhaustion, and structural remodeling associated with aging153. As an integrative coordinator of senescence mechanisms, SIRT3 has emerged as a pleiotropic safeguard against aging-related pathological progression through multimodal cytoprotective mechanisms55.
2.1 SIRT3 and neurodegenerative diseases
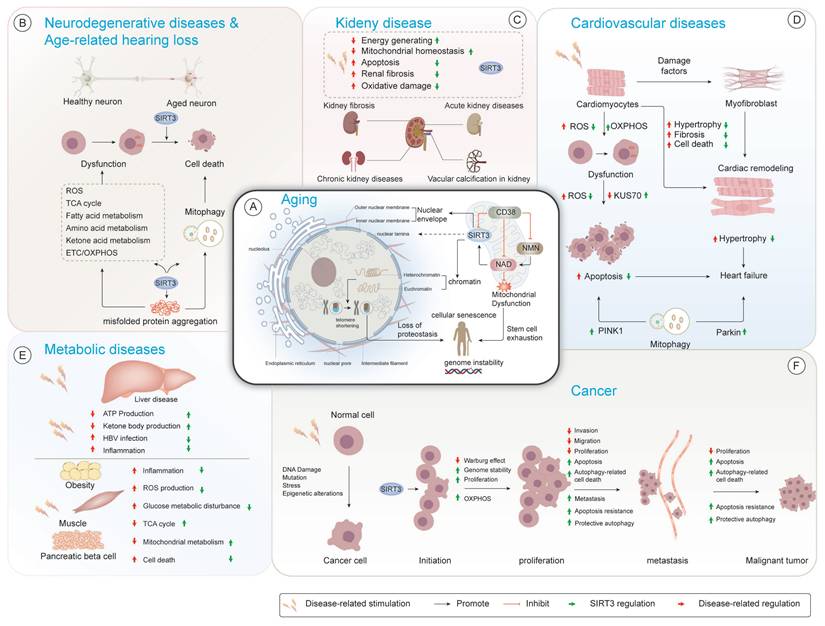
Neurodegenerative disorders represent a diverse array of pathophysiological entities defined by the time-dependent degeneration of specific neuronal populations within functionally interconnected neural networks154. This disease category is clinically exemplified by four principal clinical-pathological entities: Alzheimer's disease (AD), Parkinson's disease (PD), amyotrophic lateral sclerosis (ALS), and Huntington's disease (HD)155. Quantitative proteomic analyses demonstrated evolutionarily conserved maintenance of cerebral SIRT3 expression trajectories throughout ontogenic development and senescence43,156, thereby underscoring its regulatory nexus within neurodegenerative pathophysiology157 (Fig.3).
Regulatory mechanisms of SIRT3 in aging-related pathologies. (A) Cellular senescence mechanisms. Aging is associated with mitochondrial dysfunction and nuclear instability. SIRT3 regulates essential cellular processes involved in the maintenance of nuclear architecture, chromatin remodeling, and proteostasis, thereby influencing cellular senescence, stem cell exhaustion, and genome instability. (B) Neurodegenerative diseases and age-related hearing loss. SIRT3 exerts regulatory control over mitochondrial dynamics through the precise modulation of TCA cycle intermediates, lipid catabolism, and amino acid metabolic pathways, effectively counteracting ROS-induced oxidative stress. Additionally, SIRT3 mitigates the aggregation of misfolded proteins, which is essential for preserving neuronal function during aging. (C) Renal pathophysiology. Under chronic renal pathological conditions, SIRT3 demonstrates nephroprotective effects by enhancing mitochondrial bioenergetics, attenuating oxidative damage, and improving cellular energy transduction mechanisms. Its role in controlling renal fibrosis and apoptosis is crucial for preventing acute kidney injury and mitigating kidney dysfunction. (D) Cardiovascular pathology. SIRT3 protects cardiomyocytes by modulating ROS levels, oxidative phosphorylation, and KUS70 expression. SIRT3's cardioprotective actions encompass suppression of pathological hypertrophy and apoptotic signaling via selective mitochondrial quality control mechanisms, particularly through PINK1-mediated autophagic pathways. (E) Metabolic dysregulation. SIRT3 serves as a master regulator of bioenergetic processes, including ATP synthesis, gluconeogenic pathways, and ketone body production, particularly in metabolic disorders including obesity and non-alcoholic fatty liver disease. Its ability to mitigate ROS production and restore mitochondrial function in muscle and pancreatic β-cells underscores its importance in metabolic homeostasis. (F) Oncogenic processes. SIRT3 is integral to cancer progression, as it modulates cancer-specific metabolic adaptations including aerobic glycolysis (Warburg effect) and glutaminolysis and increases oxidative phosphorylation efficiency through complex I/III activity modulation. SIRT3 orchestrates key cell fate decisions via cell proliferation, apoptosis, and autophagy, thereby modulating cancer cell survival and metastasis.
2.1.1 AD
AD, the predominant aging-related neurodegenerative disorder, is pathologically marked by the extracellular deposition of amyloid-beta (Aβ) plaques and intraneuronal neurofibrillary tangles composed of hyperphosphorylated tau proteins158,159, which synergistically drive irreversible mnemonic deterioration and cognitive decline160. Among the sirtuin family of proteins, SIRT3 is the most abundantly expressed in the central nervous system161, but its mRNA expression exhibits a progressive decline spanning from clinical specimens to experimental rodents162-164. Recent findings revealed that SIRT3 plays a multifaceted and context-dependent neuroprotective role in the context of AD. SIRT3 regulates critical processes such as neurogenesis, neuroinflammation, and mitochondrial homeostasis165-167, thus contributing to its neuroprotective effects.
Notably, translational research has demonstrated an inverse association between SIRT3 levels and tau protein deposition168, indicating a potential modulatory role of SIRT3 in tauopathies. Mechanistically, SIRT3 has been shown to enhance mitochondrial bioenergetics, which are disrupted by Aβ toxicity169, partially through the deacetylation of mitochondrial p53 at Lys320170. This modification is crucial for maintaining energy homeostasis and neuronal survival. Meanwhile, SIRT3 knockout exacerbates synaptic degeneration by disrupting mitochondrial energy regulation171.
In addition to its role in metabolic processes, SIRT3 also plays a critical role in regulating neuronal oxidative stress by activating MnSOD and stabilizing mitochondrial dynamics through key post-translational modifications163. Furthermore, SIRT3 contribute to mitochondrial quality control through the PINK1/Parkin pathway, facilitating the mitophagic clearance of damaged mitochondria and mitigating both amyloidogenesis and tau pathology172. Interestingly, SIRT3 also modulates neuropeptide signaling pathways. Its interaction with pituitary adenylate cyclase-activating polypeptide enhances neuroprotective effects by alleviating Aβ toxicity173, indicating that SIRT3 can bridge neurotrophic and metabolic signaling in the aging brain.
Although the involvement of SIRT3 in the regulation of AD pathology regulation is becoming increasingly evident, the precise upstream cues that drive its downregulation in AD remain poorly understood. Future studies should investigate whether early exposure to Aβ directly impairs SIRT3 transcription or post-translational stability and whether glial SIRT3 is involved in modulating microglial or astrocytic responses during neuroinflammation. Additionally, the therapeutic potential of SIRT3 activation warrants further investigation. Lastly, the possibility of SIRT3 serving as a biomarker for early mitochondrial dysfunction in the progression of AD is an exciting avenue for future research.
2.1.2 PD
PD represents a multifactorial neurodegenerative condition with multifactorial etiology, pathologically defined by selective degeneration of dopaminergic neurons in the nigrostriatal pathway174, leading to the cardinal motor symptom triad of bradykinesia, resting tremor, and rigidity175. Accumulating evidence indicates that mitochondrial dysfunction, oxidative stress, and chronic neuroinflammation are central contributors to PD pathogenesis. Among the key regulators of these processes, SIRT3 has emerged as a critical upstream regulator25,177.
Preclinical investigations have shown that elevated SIRT3 expression confers substantial neuroprotection to dopaminergic (DA) neurons through multiple converging mechanisms178. One critical pathway involves the deacetylation of dynamin-related protein 1 (DRP1), a mitochondrial fission protein implicated in the pathological fragmentation of mitochondria in PD176. SIRT3-mediated modulation of DRP1 activity restores mitochondrial dynamics, protects against DA neuronal loss, and ameliorates motor deficits in murine models179. Concurrently, SIRT3 enhances mitochondrial resilience by scavenging ROS, preserving ETC integrity, and promoting mitochondrial autophagy, thereby attenuating neurodegeneration in midbrain dopaminergic populations180. Mechanistic investigations further elucidated that SIRT3 governs mitochondrial biogenesis by activating the peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) transcriptional activation, which in turn maintains mitochondrial DNA integrity in murine models181.
Beyond its roles in mitochondrial homeostasis, SIRT3 also contributes to the clearance of misfolded α-synuclein aggregates, a pathological hallmark of PD182. Furthermore, SIRT3 mitigates neuroinflammation by suppressing NLRP3 inflammasome activation183. Together, these diverse actions highlight the translational potential of targeting SIRT3 in PD therapy.
Despite compelling evidence from preclinical models supporting SIRT3's neuroprotective effects, its precise role in the initiation and early progression of PD remains unclear. Future research is required to determine whether alterations in SIRT3 expression precede dopaminergic neurodegeneration and to delineate its specific functions across neuronal and glial cell populations. Moreover, the development of selective, brain-permeable SIRT3 activators could open promising avenues for translational intervention in PD.
2.1.3 ALS
ALS represents a fatal neurological deterioration featuring the concurrent involvement of both upper motor neurons and lower motor neurons, progressing to neuromuscular junction disintegration, progressive myofiber degeneration, and premature fatality184.
Mechanistic studies revealed that dysregulation of SIRT3 plays a significant role in ALS pathogenesis. Specifically, loss of SIRT3 activity promotes aberrant mitochondrial protein hyperacetylation, thereby compromising OXPHOS complex stoichiometry and ETC capacity185. Pharmacological potentiation of SIRT3 by nicotinamide (NAM)186 has been shown to preserve neuronal ultrastructural integrity and viability in ALS models187. Notably, overexpression of SIRT3 in mutant SOD1 (G93A)-induced ALS models, ameliorates disease phenotypes188, suggesting a potential causal relationship between SIRT3 function and ALS progression.
Beyond its neuronal effects, SIRT3 dysregulation disrupts the fidelity of NAD+ salvage pathways within striated muscle microenvironments, perpetuating metabolic disturbances that exacerbate neuromuscular degeneration in ALS progression25. Collectively, these findings underscore the pivotal role of SIRT3 in modulating mitochondrial metabolism and preserving neuromuscular function in the context of ALS.
Despite robust evidence from preclinical models supporting the neuroprotective role of SIRT3, its temporal dynamics and cell-type specific actions in ALS remain insufficiently defined. Future research should clarify whether SIRT3 exerts distinct effects in motor neurons versus skeletal muscle, and determine whether therapeutic activation of SIRT3 yields greater benefit in early versus late disease stages of the disease. Furthermore, validation in patient-derived tissues will be critical to assess the clinical relevance of SIRT3 as a potential biomarker or therapeutic target in ALS.
2.1.4 HD
HD is an autosomal dominant trinucleotide repeat disorder with the neuropathological hallmark of a distinct symptom triad featuring hyperkinetic movement disorders including choreodystonic movements and gait ataxia, progressive cognitive impairment, and neuropsychiatric manifestations189,190. Post-mortem analyses of human brain tissue and investigations using transgenic animal models have identified aberrant SIRT3 expression as a consistent molecular signature associated with HD pathology191.
Emerging evidence suggests that SIRT3 plays a protective role in mitigating the pathogenic effects of mutant huntingtin protein. Overexpression of SIRT3 has been shown to ameliorate clinical symptoms and support the survival of striatal neurons192, whereas SIRT3 deficiency aggravates mitochondrial impairment and heightens neuronal susceptibility to excitotoxic damage193. Mechanistically, SIRT3 enhances mitochondrial bioenergetics, reduces oxidative stress, and prevents glutamate-induced apoptosis. These protective effects are mediated, at least in part, by regulation of NAD⁺ metabolism125,194 and activation of AMPK phosphorylation, which promotes PGC-1α-dependent transcriptional programs to sustain redox homeostasis and neuronal energy metabolism195. Furthermore, genetic ablation of SIRT3 exacerbates oxidative damage markers, whereas pharmacological redox modulators restore physiological SIRT3 levels via nuclear factor erythroid 2-related factor 2 (Nrf2)-mediated transcriptional feedback loops68.
In addition to metabolic regulation, SIRT3 also maintains mitochondrial dynamics by suppressing fission regulators, such as Fis1 and Drp1, thereby preserving mitochondrial morphology and promoting efficient axonal transport196,197. In Drosophila melanogaster HD models, transgenic expression of the SIRT3 ortholog dSirt2 significantly attenuated neuropil degeneration and prolonged organismal longevity193, reinforcing its conserved neuroprotective functions.
Despite compelling preclinical findings, the spatiotemporal dynamics and cell-type specificity of SIRT3 activity in HD remain poorly understood. Future research should investigate whether modulation of SIRT3 can delay disease onset or influence progression in vivo, and evaluate its potential synergistic effects with existing mutant huntingtin-lowering therapeutic strategies. The development of selective SIRT3 agonists with optimized blood-brain barrier permeability holds promise as a novel approach for HD management.
2.2 SIRT3 and cardiovascular disease (CVD)
CVD is the principal contributor to global mortality, imposing a substantial socioeconomic burden with elevated healthcare expenditures and workforce attrition in aging demographics198. Nevertheless, the geriatric-specific pathophysiology mechanisms underlying CVD progression persists as a poorly characterized domain.
Growing evidence implicates that SIRT3 as a key contributor to aging-related CVD, whereas SIRT3 upregulation by exogenous factors ameliorates disease progression in preclinical models199. Mechanistically, SIRT3 exerts pleiotropic cardioprotective effects, including the regulation of mitochondrial protein deacetylation, attenuation of oxidative stress, and modulation of ECM remodeling200. These functions are essential for preserving cardiovascular homeostasis and tissue integrity during aging. Taken together, these findings establish the therapeutic promise of SIRT3 as a potential molecular target for aging-related CVD pathologies (Fig.3).
2.2.1 SIRT3 and atherosclerosis
Atherosclerosis is a multifactorial vascular disease arises from dynamic interactions among chronic vascular inflammation, endothelial cell (EC) dysfunction, and dysregulated lipid homeostasis201. As a crucial mitochondrial deacetylase, SIRT3 deficiency increases oxidative stress and promotes plaque formation, thereby accelerating atherosclerosis pathological process202.
Mechanistically, SIRT3 regulates lipid metabolism through multiple interrelated signaling pathways203. Specifically, SIRT3 promotes AMPK activation and modulates the activity of uncoupling protein-1 (UCP1), thereby suppressing ox-LDL-induced foam cell formation204-207. Moreover, gut microbiota-derived metabolites can influence the SIRT3/SOD2/FOXO3A axis, which antagonizes lipogenic programming governed by the SREBP1c/FAS/DGAT2 cascade208. This indicates a functional link between SIRT3 activity and host-microbiome interactions in atherosclerosis. However, paradoxical findings from LDL receptor-knockout murine models featured no significant alteration in atherosclerotic lesion development upon SIRT3 deletion209, suggesting that the role of SIRT3 in atherosclerosis may be a context-dependent manner. Beyond lipid regulation, SIRT3 plays a pivotal role in maintaining EC function by preserving mitochondrial integrity via the SIRT3/ATG5 axis, thereby reducing ROS levels and sustaining nitric oxide bioavailability210. The absence of SIRT3 in EC-specific knockout models leads to exaggerated NLRP3 inflammasome activation and endothelial dysfunction211, thereby fostering a pro-inflammatory and pro-atherogenic phenotype.
Dietary intervention studies employing hyperlipidemic regimens in murine models demonstrated that SIRT3 deficiency further amplifies monocyte infiltration and cytokine production, primarily via dysregulation of NF-κB signaling208,212. At the molecular level, SIRT3-induced FOXO3a deacetylation and subsequent target catalase (CAT) activation help counteract oxidative stress213, reinforcing the anti-inflammatory function of SIRT3. Concurrently, the regulatory effects of SIRT3 on autophagic progress influence both foam cell formation and inflammation responses, key features of atherosclerotic disease progression214.
Collectively, these findings validate SIRT3 as a therapeutic target for vascular remodeling, thereby positioning pharmacological modulation of this deacetylase as a promising therapeutic strategy targeting vascular pathologies.
2.2.2 SIRT3 and heart failure (HF)
Chronic cardiac ischemia induced by diverse pathological conditions, such as hypertrophic cardiomyopathy, ischemic myocardial injury, and atherosclerotic coronary obstruction, progressively evolves into HF, a terminal phase of cardiovascular disorders responsible for substantial global morbidity and mortality215,216. Accumulating evidence indicates that cardiac SIRT3 expression is consistently decreased during HF pathogenesis, paralleling hyperacetylation-induced mitochondrial proteome dysfunction, impaired oxidative metabolism, and elevated ROS production217,218. Mechanistically, SIRT3 strengthens mitochondrial function by deacetylating key metabolic enzymes such as SOD2 and IDH2, thus optimizing TCA cycle efficiency and preserving cardiomyocyte viability under stress conditions, particularly in hypertensive HF models219. Uniquely among class III HDACs, SIRT3 attenuates pathological cardiac remodeling through FOXO3A activation, contrasting with the functional diversity exhibited by other HDAC subtypes in myocardial regulation220.
Pharmacological potentiation of SIRT3 via NAD+-dependent pathways and gut-derived metabolites such as indole-3-propionic acid, has been shown to enhance myocardial bioenergetics and improve diastolic function, especially in HF with preserved ejection fraction221-223. In preclinical models, SIRT3 overexpression significantly mitigates cardiac hypertrophy and fibrosis, whereas SIRT3 deficiency promotes the development of hypertrophic cardiomyopathy and progressive ventricular dysfunction224,225.
At the molecular level, SIRT3 orchestrates multiple cardioprotective pathways, including the FOXO3a-dependent transcriptional activation of MnSOD and CAT to counteract ROS accumulation226, inhibition of cyclophilin D-dependent mitochondrial permeability transition pore opening to preserve mitochondrial integrity and prevent apoptosis227, and interaction with the long noncoding RNA DACH1 to regulate mitochondrial oxidative damage and cell death228.
Beyond its roles in mitochondrial function and apoptosis, SIRT3 also suppresses myocardial fibrosis via inhibition of p53 acetylation and ferroptosis, along with deacetylation of pro-fibrotic genes229-231, such as COL1A1 and TGF-β1, ultimately reducing ECM deposition in pressure-overloaded cardiomyocytes126. Additionally, SIRT3 contributes to maintaining mitochondrial morphology through optic atrophy 1 (OPA1) deacetylation, maintaining cristae structure and preventing inflammasome activation in cardiac fibroblasts232. Through modulation of the β-catenin/peroxisome proliferator-activated receptor (PPAR)-γ and TGFβ/Smad3 axis233, SIRT3 further suppresses fibrotic remodeling and supports cardiac function128,234.
Despite its well-established cardioprotective functions, the temporal dynamics and tissue-specific roles of SIRT3 in different HF phenotypes remain incompletely understood. Future research should investigate strategies for cell-specific delivery of SIRT3 activators to cardiomyocytes and fibroblasts, as well as assess the translational potential of microbiota-derived SIRT3 modulators in human HF. These efforts might offer innovative therapeutic strategies leveraging SIRT3 as a metabolic and epigenetic checkpoint in HF.
2.3 SIRT3 and diabetes mellitus
Aging and diabetes converge to induce comparable patterns of multiorgan dysfunction through overlapping molecular pathways235, with mitochondrial dysfunction emerging as a central pathogenic mechanism driving the onset and progression of diabetic complications236,237. As the predominant mitochondrial NAD+-dependent deacetylase, SIRT3 serves as a metabolic gatekeeper, and is consistently found to be downregulated in clinical diabetic tissues, where its deficiency correlates with insulin resistance and disturbances in systemic energy homeostasis238-240.
In skeletal muscle, SIRT3 deficiency impairs insulin-stimulated glucose translocation, aggravating peripheral insulin resistance and contributing to the pathophysiology of type 2 diabetes241. Conversely, restoring SIRT3 expression via lifestyle interventions or fibroblast growth factor-21 signaling improves mitochondrial integrity and myocardial function57, pointing to a promising avenue for metabolic reprogramming in diabetic patients.
Growing evidence supports a cardioprotective role of SIRT3 in diabetic cardiomyopathy (DCM)242,243, where its activation improves mitochondrial respiratory capacity and suppresses oxidative stress, primarily through modulation of the AGO2/cytochrome b (CYTB) signaling pathway237. This regulatory mechanism uncouples excessive glucose levels from ETC dysfunction237, whereas SIRT3 downregulation leads to a breakdown of this axis, resulting in impaired ETC activity and accelerated progression of DCM244,245.
Within the central nervous system, chronic hyperglycemia suppresses SIRT3 expression in the hippocampus, contributing to cognitive deficits through mitochondrial Ca2+ overload and neuronal apoptosis246. SIRT3 overexpression mitigates neuronal function by inhibiting the VDAC1/GRP75/IP3R complex, thereby reducing mitochondria-associated endoplasmic reticulum membrane formation and protecting neurons from metabolic stress-induced apoptosis247.
Under conditions of hyperlipidemia and inflammation, SIRT3 also plays a pivotal role in preserving pancreatic β-cell viability and promoting osteogenic differentiation, thereby exerting antioxidative effects essential for the redox-modulating effects of irisin in diabetic periodontitis models248. Lentivirus-mediated SIRT3 silencing abolishes irisin's osteoprotective efficacy against osteoclastic alveolar bone resorption and ROS overproduction, establishing SIRT3 as the obligatory signaling node for irisin-mediated redox homeostasis and bone preservation240.
These findings underscore SIRT3's fundamental role in maintaining redox homeostasis and structural integrity across metabolically active organs.
Despite these organ-specific findings, a comprehensive understanding of SIRT3's integrated role in systemic diabetic complications remains incomplete. Future studies should prioritize characterizing SIRT3's cell type-specific SIRT3 functions in diabetic heart, brain, pancreas, and bone tissue, as well as developing targeted therapeutics (e.g., activators, gene therapy) capable of tissue-selective SIRT3 delivery. Ultimately, SIRT3 may represent a convergent therapeutic target for mitigating the multiorgan sequelae of diabetes via its dual roles in mitochondrial regulation and oxidative stress control (Fig.3).
2.4 SIRT3 and cancer
Aging represents an irreversible carcinogenic risk factor, with epidemiological data revealing an exponential rise in cancer incidence with advancing age249.
As a mitochondrial deacetylase, SIRT3 serves as a molecular nexus intersection of cellular senescence and oncogenesis, exerting tumor-suppressive effects by preserving genomic integrity through multiple regulatory mechanisms250,251. During early oncogenic transformation, SIRT3 stabilizes chromosomal integrity via OGG1-mediated DNA repair potentiation through lysine deacetylation252,253 and orchestrates chromatin remodeling by facilitating H3K56 deacetylation to enhance NHEJ fidelity55. Collectively, these mechanisms highlight SIRT3's role as a genomic sentinel in aging tissues prone to malignant transformation.
Beyond its role in genome stability, SIRT3 exerts metabolic control in a context-dependent manner, predominantly acting as a tumor suppressor. For instance, SIRT3 destabilizes hypoxia-inducible factor 1α, thereby attenuating the Warburg effect, a characteristic metabolic adaptation of rapidly proliferating tumors254-256. Transcriptomic profiling in castration-resistant prostate cancer reveal that SIRT3 suppresses aconitase 2 activation, disrupting glutamine-driven lipogenesis257.
In parallel, SIRT3 enhances OXPHOS efficiency via ETC assembly optimization in neoplasms, unveiling potential therapeutic vulnerabilities in OXPHOS-dependent malignancies such as pancreatic adenocarcinoma and BRAF-mutated melanomas258-261. However, emerging evidence indicates that SIRT3 can also exert oncogenic effects in certain genetic or microenvironmental contexts262, often mediated by post-translational modifications of metabolic enzymes and stress-response proteins258,263,264. For instance, SIRT3 catalyzes Lys228 deacetylation on pyrroline-5-carboxylate reductase 1, facilitating proline biosynthesis, a critical process for tumor cell proliferation265. In colorectal malignancies, SIRT3 optimizes serine metabolism via serine hydroxymethyltransferase 2 deacetylation at Lys95, enhances tumor aggressiveness266,267. Similarly, in glioblastoma models, SIRT3 promotes nucleotide biosynthesis by deacetylating glycine decarboxylase at Lys514, 268,269. SIRT3 also plays a critical role in modulating ROS homeostasis, which serve as a double-edged sword in tumor progression270.By deacetylating IDH2 at K143, SIRT3 suppresses ROS-mediated mutagenesis and tumorigenesis271.
Conversely, glioma stem cells inactivate SIRT3 to bypass oxidative growth constraints269. In chronic lymphocytic leukemia, SIRT3 enables metabolic adaptation confers chemoresistance via ROS buffering272, whereas in mammary carcinoma, SIRT3 inhibits Src kinase oxidation to suppress metastatic dissemination125.Moreover, SIRT3 exerts pleiotropic control over tumor microenvironmental reprogramming and cell death pathways, including fine-tuning apoptotic execution, autophagic quality control, and ferroptotic vulnerability landscapes273-275. Genetic ablation models confirmed that SIRT3 preserves cellular stress defense via activating key unfolded protein response mediators such as FOXO3a and MnSOD, ultimately restraining invasive tumor phenotypes276-278.
Despite extensive characterization, the dualistic, context-dependent roles of SIRT3 in cancer biology remain incompletely resolved. It has hypothesized that these opposing functions reflect a dynamic interplay between SIRT3's modulation of metabolic plasticity and redox equilibrium, influenced by tumor type, developmental stage, and microenvironmental conditions. Future research should focus on dissecting the spatiotemporal regulation of SIRT3 activity, as well as identifying interacting molecular co-factors or modifications that determine its tumor-suppressive versus pro-tumorigenic roles. Importantly, the development of context-specific SIRT3 modulators may offer a promising strategy for personalized cancer therapies (Fig.3).
2.5 SIRT3 and kidney diseases
As metabolically active organs, the kidneys sustain extensive mitochondrial networks to fuel filtration and reabsorption via robust energy production279. SIRT3 has emerged as a pivotal coordinator of renal bioenergetics, as it maintains mitochondrial homeostasis, enhances antioxidant defenses, and modulates ECM dynamics to counteract oxidative stress and fibrotic remodeling280. Clinical and experimental evidence consistently demonstrates that SIRT3 expression is reduced across various nephropathies, and its expression is negatively correlated with histological injury severity281. Conversely, SIRT3 overexpression was found to alleviate both acute kidney injury (AKI) and chronic kidney disease (CKD) progression in multiple preclinical models282(Fig.3).
2.5.1 SIRT3 and AKI
AKI is clinically defined by the rapid-onset deterioration of glomerular filtration capacity consequent to irreversible cellular necrosis within nephron components, and serves as a pivotal factor in the transition toward CKD283,284. Among the mechanistic underpinnings of AKI, mitochondrial dysfunction has emerged as a central contributor to its pathogenesis285, SIRT3, a key mitochondrial deacetylase, plays a protective role by safeguarding mitochondrial integrity and bioenergetic function286.
In preclinical models of sepsis-induced AKI, SIRT3 knockout exacerbates mitochondrial dysfunction in proximal tubules287, accompanied by enhanced epithelial cell apoptosis mediated via BAX oligomerization, caspase-3 activation, and BCL-2 network disruption112,288. These observations establish SIRT3 as a critical checkpoint regulator of mitochondrial apoptosis and oxidative stress equilibrium.
Furthermore, SIRT3 deficiency impairs fatty acid β-oxidation, thereby exacerbating parenchymal cell apoptosis and accelerating renal functional deterioration289,290. Conversely, pharmacological activation of SIRT3 improves OXPHOS efficiency, promoting ATP synthesis while simultaneously limiting ROS accumulation and lipid peroxidation288. Transgenic overexpression of SIRT3 confers renoprotective effects via multiple molecular pathways, including upregulation of the Nrf2 antioxidant pathway291, inhibition of NF-κB signaling through IκBα stabilization292, and preservation of tubular epithelial viability293. These interventions maintain mitochondrial membrane potential stability while suppressing cytochrome c-mediated apoptotic cascades294, ultimately attenuating ischemia-reperfusion (I/R)-induced mitochondrial damage in renal epithelia295.
In unilateral ureteral obstruction models, SIRT3 deficiency leads to aberrant acetylation of mitochondrial proteins and increased interstitial collagen accumulation, highlighting its involvement in ECM remodeling296. Furthermore, SIRT3 knockout accelerates the transition from AKI to CKD via early activation of the TGF-β/Smad3 pathway297,298, further supporting its role in renal fibrosis initiation and progression. In I/R-induced AKI, targeted SIRT3 activation produces multifaceted nephroprotective effects, including deacetylation of mitochondrial SOD2, enhancing antioxidative capacity, and suppressing pathological superoxide accumulation299. Concomitantly, SIRT3 activation also restores mitochondrial ATP synthase complex integrity and cristae morphology, thereby mitigating tubular epithelial apoptosis300. Additionally, SIRT3 attenuates inflammatory responses by inhibiting NLRP3 inflammasome activation and downregulating TGF-β1-driven fibrogenesis293, further supporting its anti-inflammatory and anti-fibrotic roles.
Intriguingly, metabolomic profiling of SIRT3-deficient specimens from models of AKI reveal elevated levels of glutathione biosynthesis precursors, suggesting a compensatory adaptation to oxidative stress301. Moreover, pharmacological AMPK activation using metformin promotes SENP1-mediated deSUMOylation of SIRT3, enhancing its mitochondrial localization and functional activity, which in turn reduces tubular cell apoptosis under metabolic stress302.
Collectively, these findings underscore SIRT3 as a master regulator of renal resilience, operating at the intersection of metabolic reprogramming, redox regulation, and anti-fibrotic signaling pathways. Future investigations should aim to unravel the tissue-specific post-translational modifications that fine-tune SIRT3 activity, as well as the potential for SIRT3-targeting agents within the AKI-CKD continuum for precision nephrology.
2.5.2 SIRT3 and CKD
CKD is defined as a progressive renal condition marked by sustained impairment in kidney function for at least 3 months303. SIRT3 orchestrates mitochondrial network remodeling, biogenetic processes, and metabolic adaptation to preserve cellular homeostasis, thereby supporting renal repair mechanisms and attenuating fibrotic pathogenesis304-306. Within the tubular epithelium, SIRT3 suppresses epithelial-mesenchymal transformation (EMT) through the activation of FOXO3a, a transcription factor that directly induces mitochondrial SOD2 and peroxisomal catalase expression213. This coordinated antioxidant enzyme induction mitigates intracellular oxidative stress, ultimately preventing the deposition of ECM components associated with early fibrotic lesions225,307. Moreover, SIRT3 acts as a downstream of UCP1, thereby stabilizing proteins and reducing ROS generation, which further contributes to inhibiting EMT and ECM deposition213. The regulatory capacity of SIRT3 extends to fibrogenesis through its ability to disrupt the NF-κB/TGF-β1/Smad axis139. Notably, SIRT3 deacetylates β-catenin, a pivotal transcriptional coactivator in fibroblast activation, facilitating the expression of MMP-7308 and plasminogen activator inhibitor-1309, two mediators implicated in ECM remodeling via EMT induction296. In the context of angiotensin II-induced nephropathy, SIRT3 exhibits nephroprotective by counteracting iron overload and inhibiting NADPH oxidase-mediated ROS overproduction, ultimately attenuating renal fibrogenesis and delaying CKD progression310.
Vascular calcification (VC), a hallmark of advanced CKD311, is another pathological process in which SIRT3 exerts protective roles. SIRT3 counteracts VC via mitochondrial function preservation and oxidative stress mitigation312. Notably, SIRT3 deacetylates proteins within the PGC-1α/TFAM pathway, promoting mitochondrial biogenesis while attenuating vascular smooth muscle cell calcification313. Genetic ablation studies demonstrated that the abrogation of VC is completely abolished in SIRT3-deficient models, whereas AMPK/SIRT3 axis activation attenuated mineralization through mitochondrial functional restoration and oxidative stress mitigation314. Biochemical analyses delineated a regulatory axis in which soluble epoxide hydrolase (sEH) modulates SIRT3 turnover via proteolytic degradation, with sEH deficiency preserving SIRT3 bioavailability to fulfill bioenergetic demands and impede renal VC progression315.
Taken together, these findings highlight SIRT3 as a master regulator of renal and vascular resilience in CKD. Enhancing SIRT3 activity may provide a promising therapeutic strategy to counteract mitochondrial dysfunction, fibrosis, and VC in affected patients. Future research should focus on developing selective SIRT3 activators, exploring cell-specific functional roles, and deciphering how post-translational modifications affect SIRT3's stability, localization, and bioactivity.
2.6 SIRT3 and age-related hearing loss (AHL)
AHL, the most prevalent form of auditory impairment among older adults, is tightly correlated with cumulative oxidative stress349. CR, a well-established anti-aging intervention, has been shown to alleviate AHL by reducing oxidative damage in cochlear cells350. However, this protective effect is abolished in SIRT3-deficient mice351, highlighting the indispensable role of SIRT3 in mediating CR-induced auditory resilience. Mechanistically, SIRT3 enhances the activity of IDH2 under CR, thereby boosting mitochondrial NADPH production and facilitating efficient ROS clearance352. This mitochondrial antioxidant defense mechanism plays a crucial role in preserving cochlear hair cell integrity and delays the progression of hearing loss353.
Overall, these findings underscore SIRT3 as a critical mediator of mitochondrial redox homeostasis within the auditory system. In light of the absence of effective interventions for AHL, SIRT3 represents a promising therapeutic target for aging-related hearing disorders. Future research should explore whether pharmacological activation of SIRT3 can replicate the benefits of CR in the cochlea, as well as explore its potential as a biomarker to predict AHL susceptibility (Fig.3).
2.7 SIRT3 and chronic obstructive pulmonary disease (COPD)
COPD is a progressive and largely irreversible respiratory disorder, representing a leading global cause of morbidity and mortality316. Aging has been identified as a key contributor to the pathogenesis of COPD317,318, primarily through its effects on oxidative stress regulation319 and mitochondrial dysfunction320.
SIRT3 is critical in maintaining mitochondrial homeostasis and counteracting oxidative stress, both of which are critical in the onset and progression of COPD321. The PGC-1α/SIRT3 signaling pathway is especially pivotal for mitochondrial biogenesis and redox equilibrium322. In murine models, SIRT3 deficiency exacerbates alveolar destruction, increases airway inflammation, and accelerates the declines in pulmonary function48, underscoring its protective effects in respiratory health. Mechanistically, SIRT3 enhances MnSOD activity through lysine deacetylation, thereby attenuating oxidative damage within mitochondria of airway epithelial cells323.
In cigarette smoke (CS)-induced lung injury models, SIRT3 upregulation promotes the deacetylation of SOD2, which reduces oxidative stress and attenuates lung structural damage and functional deterioration324. Conversely, SIRT3 inhibition modulates the Nrf2 pathway, decreasing inducible nitric oxide synthase expression and ROS accumulation, while activation of SIRT3 provides protection against CS-induced ferroptosis in bronchial epithelial cells325.Moreover, the expression of SIRT3 is regulated by microRNA pathways, which are closely linked to cellular senescence. Under oxidative stress conditions, miR-494-3p directly targets SIRT3 in small airway epithelial cells, leading to downregulation of SIRT3 levels, increased expression of p27, and accelerated cellular senescence, all of which contribute to the pathophysiology of COPD 49.
In conclusion, these findings emphasize the multifaceted role of SIRT3 in COPD, primarily through its functions in preserving mitochondrial integrity, suppressing oxidative injury, and delaying senescence in airway epithelial cells. These mechanisms are particularly relevant in the context of aging lung and environmental insults like CS. Future research should focus on developing therapeutic approaches that target SIRT3 activation or its downstream signaling pathways and further explore its interactions with non-coding RNAs and ferroptosis-related processes, particularly within the context of aging and environmental exposures. Such investigations will improve the understanding of SIRT3's contribution to pulmonary resilience, and pave the way for precision therapies aimed at treating aging-related COPD(Fig.4).
2.8 SIRT3 and degenerative spine and joint diseases
The musculoskeletal system plays a critical role in providing structural integrity and enabling movement326. Aging-related disruptions in bone homeostasis are commonly associated with an increased risk of degenerative conditions, such as intervertebral disc degeneration (IDD) and osteoarthritis (OA)327. These disorders are primary contributors to chronic pain and disability in older populations, significantly diminishing quality of life and imposing considerable socioeconomic burdens328.
Consequently, identifying molecular regulators, such as SIRT3, which may serve as potential therapeutic targets, is of paramount importance (Fig.4).
2.8.1 SIRT3 and IDD
Cellular senescence, mitochondrial dysfunction, and oxidative stress are key mechanisms involved in the pathogenesis of IDD329. SIRT3 expression is significantly reduced in degenerated intervertebral discs, and its knockdown further exacerbates the deterioration of nucleus pulposus cells (NPCs) under oxidative stress conditions330.Emerging studies suggest that lactate can downregulate SIRT3, which, in turn, increases the acyl-CoA synthetase long chain family member 4 lactylation, thereby promoting ferroptosis and accelerating the decline of NPC functionality331. Moreover, SIRT3 deficiency amplifies ROS-induced damage332, whereas SIRT3 overexpression has been shown to alleviate IDD severity in vivo20,330,333, highlighting its protective role.
Therapeutic strategies, such as hydrogel microspheres designed to upregulate both SIRT3 and SOD2, have exhibited promise in maintaining NPC viability334. Additionally, SIRT3 safeguards against advanced glycation end product-induced apoptosis335 and supports mitochondrial homeostasis via the AMPK/PGC-1α pathway336.
Taken together, these findings indicate that SIRT3 serves as a crucial regulator of NPC survival, orchestrating mitochondrial quality control, redox homeostasis, and apoptotic pathways. Despite these advances, the potential of SIRT3 as a biomarker for monitoring IDD progression or as a direct therapeutic target remains uncertain. Future studies should explore its role in human clinical samples, examine tissue-specific effects, and investigate the efficacy of SIRT3-activating compounds in translational models.
2.8.2 SIRT3 and OA
Mitochondrial dysfunction is increasingly recognized as a defining characteristic of OA337. SIRT3 preserves mitochondrial integrity by deacetylating cytochrome c oxidase subunit 4 isoform 2338, with its deletion correlating with the exacerbation of OA in both animal and human models339,340.
Modulatory mechanisms of SIRT3 in aging-related pathologies. (A) Chronic Obstructive Pulmonary Disease (COPD). Mitochondrial dysfunction induces excessive ROS production and mtDNA leakage, which activates the cGAS-STING pathway and triggers inflammatory cytokine release (e.g., IL-6, TNF). This promotes cellular senescence and the senescence-associated secretory phenotype (SASP). Impaired SIRT3 activity further aggravates oxidative stress by reducing SOD2 deacetylation, weakening mitochondrial antioxidant defenses and amplifying inflammation. (B) Intervertebral Disc Degeneration (IDD). Metabolic stress and AGE accumulation disrupt mitochondrial biogenesis and respiration. The AMPK-SIRT3-PGC-1α axis is central to maintaining mitochondrial function and antioxidant capacity. Its dysregulation leads to reduced expression of enzymes like SOD2 and catalase, increased ROS levels, and nucleus pulposus cell senescence, contributing to disc degeneration. (C) Osteoarthritis (OA). Oxidative stress impairs mitochondrial dynamics and activates the PINK1/Parkin-dependent mitophagy pathway. Inadequate mitophagy results in accumulation of dysfunctional mitochondria, elevated ROS, and chondrocyte apoptosis. Disrupted mitochondrial quality control exacerbates inflammation and cartilage matrix degradation, accelerating joint degeneration.
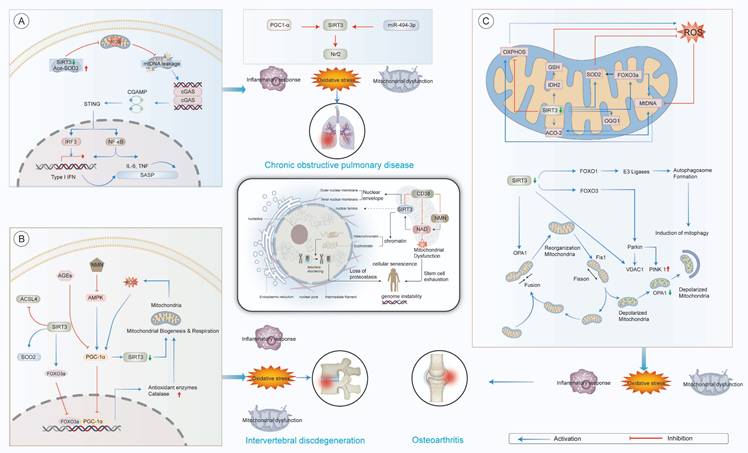
As aging progresses, autophagic activity declines, contributing to chondrocyte loss341. SIRT3 promotes mitophagy via the PINK/Parkin axis and maintains mitochondrial health by regulating FOXO3a hyperacetylation342. Furthermore, SIRT3 inhibits chondrocyte apoptosis through the PI3K/Akt/mTOR signaling pathway147.
Oxidative stress is another central pathological factor in OA343. SIRT3 facilitates rapid ROS clearance through antioxidant protein deacetylation and long-term redox homeostasis via FOXO3a activation344. Notably, reduced SIRT3 activity leads to SOD2 hyperacetylation, which results in increased ROS accumulation345.
Recent studies indicate that epigenetic modifications impair SIRT3 function346, such as through SUMOylation-mediated inhibition, thereby exacerbating mitochondrial dysfunction and cartilage degeneration347. SIRT3 also promotes mitochondrial fusion, facilitating mtDNA complementation and enhancing cartilage resilience348.
In summary, SIRT3 plays a multifaceted role in OA by regulating mitochondrial biogenesis, redox signaling, and chondrocyte viability. Nevertheless, the potential of SIRT3 activation as a disease-modifying strategy remains unclear. Future research should focus on elucidating the upstream regulatory mechanisms of SIRT3 in aging cartilage and evaluating the therapeutic potential of SIRT3-targeting interventions in both preclinical and clinical trials.
3. SIRT3 and therapeutic targets
The aforementioned evidence delineated the multifaceted regulatory roles of SIRT3 in biological aging processes and associated geriatric comorbidities. Its therapeutic potential is particularly evident in the context of neurodegenerative diseases, CVD, diabetes mellitus, and cancer, as well as kidney diseases. Accumulating research has focused on the pharmacological targeting of SIRT3, with the development of activators aimed at addressing these pathophysiological conditions that impose significant clinical burdens (Table 1).
3.1 Natural modulators
3.1.1 HKL
HKL, a naturally occurring bisphenolic lignan isolated from Magnolia officinalis, is a potent SIRT3 activator354. Previous studies revealed that HKL upregulates SIRT3 while augmenting its enzymatic activity355. Preclinical studies demonstrated that HKL can ameliorate pre-existing cardiac hypertrophy and suppresses SIRT3-dependent cardiac fibroblast proliferation218, suggesting that the beneficial effects of HKL are SIRT3-dependent.
Mechanistically, HKL-induced activation of SIRT3 mitigates silica-induced fibrosis and mtDNA damage via the cGAS/STING pathway356, providing further evidence for its therapeutic potential in conditions involving cellular damage and inflammation. Additionally, HKL possesses neuroprotective properties, likely attributable to its ability to cross the blood-brain barrier as a small molecule357,358. A prior study indicated that HKL promotes mitochondrial fusion and supports neural survival via the SIRT3/AMPK pathway in models of subarachnoid hemorrhage359. Moreover, HKL protects the brain from I/R injury in mice by reducing ROS production and enhancing mitochondrial function360. Furthermore, HKL ameliorates intracerebral hemorrhage-induced apoptosis and mitochondrial fission via SIRT3 activation354.
In murine models, HKL-activated SIRT3 upregulates mitochondrial GPX4 and decreases its acetylation, thereby inhibiting neuronal ferroptosis and mitigating perioperative neurocognitive disorders following anesthesia and surgery361. These findings further support the critical role of SIRT3 in regulating pyruvate dehydrogenase E1α deacetylation, bridging glycolysis and the TCA cycle in tubular epithelial cells during the progression of renal fibrosis362.
In conclusion, these findings highlight the promising therapeutic potential of HKL as a novel SIRT3-targeted agent for the prevention or even reversal of cardiac, pulmonary, and neurodegenerative diseases. However, further clinical investigations are required to evaluate its safety, efficacy, and broader applications in human diseases.
3.1.2 Resveratrol (RSV)
RSV, a phytoalexin extracted from Veratrum grandiflorum, has been reported to broadly activate SIRT proteins363. RSV significantly diminishes mtROS generation by enhancing the accumulation of SIRT3 within mitochondria, which subsequently upregulates of FOXO3A-dependent transcription of mitochondrial genes, including ATP6, CO1, Cytb, ND2, and ND5, which in turn improves complex I activity and ATP synthesis364.
In addition, RSV increases the expression of phosphorylated AMPK, PGC-1α, and SIRT3, as well as enhance SIRT3 transcription through the estrogen-related receptor-α (ERRα)-dependent transcription365. The beneficial of RSV on mitochondrial redox balance were abrogated when cells were treated with an AMPK inhibitor or transfected with siRNA targeting AMPK, PGC-1α, or SIRT3,suggesting that these protective mechanisms are mediated through the AMPK/PGC/1α/ERRα/SIRT3 signaling pathway366. This cascade ultimately helps attenuate oxidative injury in endothelial cells. In myocardial I/R injury models, RSV activates the SIRT3/FoxO pathway and downstream factors, such as Mfn2, Parkin, and PGC-1α, which together contribute to the restoration of mitochondrial integrity and normalization of autophagic flux367. Additionally, RSV alleviates cadmium-induced ultrastructural abnormalities and mitochondria dysfunction by upregulating SIRT3 expression, reversing the repression of PGC-1α, Nrf1, and TFAM, and PINK1/Parkin-mediated mitophagy initiation31. Experimental investigations have also elucidated the therapeutic efficacy of RSV in attenuating sepsis-induced AKI an a SIRT3/SOD2-dependent manner368, thereby maintaining mitochondrial homeostasis. Mechanistic studies further described the RSV-mediated modulation of SIRT3/FOXO3a signaling, leading to the transcriptional activation of PGC-1α and SOD2369,370.
Therapeutic agents targeting SIRT3 activation and their mechanisms of action in various diseases.
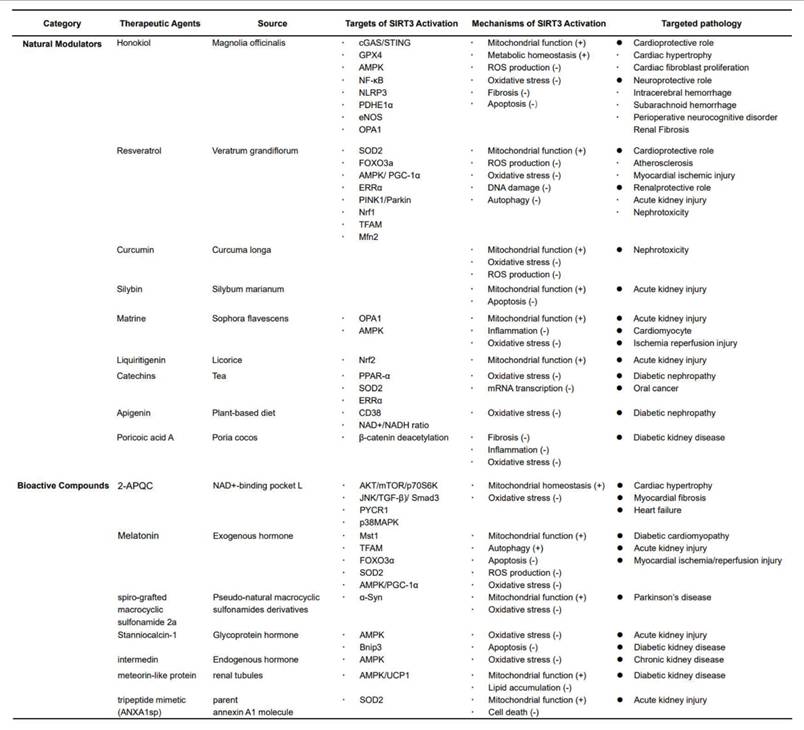
3.1.3 Other natural compounds
Curcumin modulates SIRT3 activity by coordinating mitochondrial quality control mechanisms, including bioenergetic regulation, optimization of mitochondrial dynamics, selective autophagy, and redox equilibrium371. These multifaceted actions highlight its therapeutic potential in preserving mitochondrial function and mitigating aging-related diseases. The Asteraceae-derived flavonoid silybin can attenuate cisplatin-induced nephrotoxicity by preserving renal tubular integrity and mitochondrial bioenergetics, likely via SIRT3-mediated mechanisms372, highlighting the potential therapeutic candidate of silybin in kidney-related pathologies. The Sophora flavescens-derived alkaloid matrine engages its protective effects against cisplatin-induced AKI via modulating mitochondrial membrane dynamics through the SIRT3/OPA1 pathway, combining antioxidant and anti-inflammatory mechanisms to preserve renal function373. Beyond nephroprotection, matrine also confers cardiovascular benefits via enhancing SIRT3/AMPK pathway, which preserving cardiomyocyte viability and limiting cell death in murine models374.
The licorice-derived compound liquiritigenin induces Nrf2 translocation, thereby potentiating SIRT3 activity and facilitating mitochondrial biogenesis while simultaneously suppressing apoptosis in AKI models375. This dual action highlights liquiritigenin's therapeutic value in mitigating oxidative stress injury and cell death. Catechins stimulate the SIRT3/SOD2 pathway through PPAR-α activation while stimulating ketogenesis-SIRT3 crosstalk concurrently to mitigate oxidative insults in diabetic nephropathy376. However, contrasting findings indicate that in cancer cells, catechins may repress SIRT3 expression at both mRNA and protein levels through ERRα modulation377, suggesting their biological effects are highly context-dependent. The dietary flavonoid apigenin, ubiquitous in plant-based diets, exerts nephroprotective effects by elevating the intracellular NAD+/NADH ratio and promoting SIRT3-mediated CD38 downregulation in diabetic kidney disease models378. These findings underscore apigenin's therapeutic potential as a metabolic modulator in nephropathy. Finally, Poria cocos-derived poricoic acid A upregulates SIRT3 and promotes β-catenin K49 deacetylation, effectively attenuating renal fibroblast activation and renal interstitial fibrosis in vivo and in vitro296, suggesting poricoic acid A may serve as a promising therapeutic candidate in fibrosis-related renal diseases.
3.2 Bioactive substances
3.2.1 2-Acetylphenolquinone congener (2-APQC)
Fu et al. identified 2-APQC as a novel pharmacological agonist of SIRT3. In a series of integrated preclinical models spanning cellular and organismal levels, 2-APQC significantly alleviated isoproterenol-induced pathological hypertrophy and fibrotic progression, whereas its efficacy was completely lost in SIRT3-knockout models, establishing causal dependence. Additionally, SIRT3 inhibition via NAM markedly attenuated the anti-hypertrophic effects of 2-APQC, thereby confirming SIRT3 as its primary molecular target. Mechanistically, further investigations delineated the multifaceted regulatory effects of SIRT3 activation, which worked in tandem with the suppression of key signaling pathways, including AKT/mTOR/p70S6K and JNK/TGF-β/Smad3, to attenuate pathological cardiac remodeling. This approach specifically targeted hypertrophic responses and maladaptive fibrotic deposition, suggesting a potential therapeutic avenue for targeted cardiac intervention379.
3.2.2 Melatonin
Accumulating evidence indicates that melatonin upregulates SIRT3, which in turn promotes mitophagy to mitigate oxidative stress-induced damage380. In preclinical models, exogenous melatonin supplementation preserved mitochondrial integrity and activate autophagy, thereby attenuating sepsis-induced multiorgan dysfunction through SIRT3 upregulation381. Notably, SIRT3 knockout models completely abolished the therapeutic benefits of melatonin against contrast-induced AKI, highlighting the essential role of SIRT3 in mediating melatonin's protective actions382.
Moreover, melatonin was found to effectively counteract adverse left ventricle remodeling and mitigate cardiac dysfunction in DCM. These benefits are attributed to the promotion of autophagy, reduction of apoptosis, and mitigation of mitochondrial dysfunction, which facilitated by the inhibition of Mst1 phosphorylation and upregulation of SIRT3 expression in DCM model383. Mechanistically, melatonin specifically activates the 28-kDa isoform of SIRT3, which enhances its enzymatic activity within mitochondria, thereby boosting its intramitochondrial catalytic efficiency384.
In depth analyses have outlined melatonin's orchestration of sequential mitophagy processes, with the SIRT3-mediated deacetylation of TFAM at Lys154 acting as the pivotal molecular switch to promote mitophagic flux382. Furthermore, endogenous melatonin demonstrates notable cytoprotective capacity through its involvement in the redox-sensitive SIRT3/FOXO3α/ROS regulatory triad, thereby effectively reducing macrophage cytotoxicity under metabolic-stress conditions385. Additionally, melatonin induced SOD2 deacetylation, leading to a reduction in oxidative stress, a process that was completely blocked in the absence of SIRT3381. The AMPK/PGC1α/SIRT3 axis has also been identified as a pivotal pathway in melatonin's protective effects against myocardial IR injury in rats with type 1 diabetes, primarily by preserving mitochondrial function386. Collectively, these findings establish melatonin's SIRT3-dependent therapeutic efficacy, underscoring its potential for clinical translation in treating diseases associated with mitochondrial dysfunction and oxidative stress.
3.2.3 Other bioactive compounds
Zhang et al. designed and synthesized an innovative class of pseudo-natural macrocyclic sulfonamides derivatives. In murine PD models, spiro-grafted macrocyclic sulfonamide 2a treatment significantly attenuated spontaneous locomotor deficits and motor coordination impairment compared to control mice387. These findings highlight the potential therapeutic role of SIRT3-dependent mechanisms, suggesting that SIRT3 activation could be pivotal in modulating PD pathologies.
Stanniocalcin-1, a glycoprotein hormone, has been demonstrated to orchestrate a hormetic AMPK/SIRT3 axis that mitigates oxidative stress and apoptosis388. This hormetic axis is crucial for maintaining cellular homeostasis and ameliorating renal injury in diabetic murine models by inhibiting the expression of Bnip3 through the AMPK/SIRT3 pathway389, further underscoring its therapeutic potential in kidney disease. Similarly, intermedin enhances mitochondrial bioenergetic capacity via AMPK/SIRT3-mediated cristae remodeling in VC models314, positioning it as a potent therapeutic strategy. Mechanistic investigations revealed that meteorin-like protein activates the PGC-1α/SIRT3 axis, which stimulates AMPK at Thr172 and increases UCP1-dependent proton leak, thereby preserving mitochondrial ultrastructural integrity and regulating energy metabolism, particularly in diabetic nephropathy207. Furthermore, the annexin A1-derived tripeptide Ac2-26 was reported to transcriptionally upregulate SIRT3, concurrently reducing oxidative damage while promoting mitochondrial biogenesis and mitophagy390.
These findings suggest that SIRT3 activation could be leveraged to enhance mitochondrial function, providing a promising avenue for therapeutic interventions aimed at combating oxidative stress and mitochondrial dysfunction.
Conclusion and perspective
The available research delineates SIRT3 as a pleiotropic regulatory hub within the cellular senescence and geropathological trajectories, establishing it as a critical determinant in a range of disorders spanning CVDs, neurodegenerative conditions, metabolic syndrome, cancer, kidney diseases, COPD, and degenerative spine and joint diseases. The multifaced regulation of metabolic adaptation, oxidative stress homeostasis, epigenetic integrity, and inflammatory responses by SIRT3 enables it to integrate systemic stress responses, thereby maintaining physiological resilience throughout the aging process.
Emerging evidence underscores SIRT3's pivotal role in preserving mitochondrial function by suppressing excessive ROS generation, maintaining ATP synthesis, and stabilizing mitochondrial dynamics. Additionally, SIRT3's regulation of the mitochondrial acetylome has a profound effect on chromatin remodeling and genome stability, establishing it as a crucial epigenetic modulator. Through these mechanisms, SIRT3 both protects cellular integrity and maintains organ function during aging, making it a promising target for therapeutic intervention.
Pharmacologic potentiation of SIRT3 deacetylase activity represents a burgeoning frontier in translational geroscience. Targeted activation strategies have increased therapeutic viability across multisystem aging-related morbidities. Beyond CR and pan-SIRT activators, innovative small molecules including 2-APQC, melatonin, and pseudo-natural macrocyclic sulfonamides displayed organ-specific protective effects in preclinical models. For instance, these compounds provide cardioprotection through the AMPK/PGC1α axis, neuroprotection via SOD2-mediated ROS suppression and renal protection by enhancing mitophagy. These findings both highlight the translational promise of SIRT3 agonism and emphasize its potential in combating mitochondrial dysfunction and oxidative damage, which are key drivers of aging and associated diseases.
Despite these encouraging advances, there remains a significant gap in translating these preclinical successes into clinical applications. The clinical development of SIRT3-targeted interventions remains in its nascent stage, being constrained by the paucity of robust, randomized human studies and the absence of aging-specific clinical endpoints. Priority research directions should adopt a three-pronged approach to bridge this gap, employing adaptive clinical trial designs that incorporate composite geriatric outcomes and functional endpoints relevant to older populations; implementing omics-based biomarker discovery platforms to stratify patients, monitor SIRT3 activity, and validate mechanisms of action; and establishing international longitudinal registries for long-term safety and efficacy surveillance. These methodologically rigorous initiatives will bridge critical knowledge gaps between preclinical findings to clinical implementation in aging populations.
Furthermore, the context-dependent nature of SIRT3 functions warrants further investigation. Although SIRT3 exerts cytoprotective effects in various cellular contexts, its oncogenic potential in specific tissue types and cellular environments must be explored in greater depth. Elucidating its interactions with other mitochondrial regulators and signaling pathways might uncover synergistic therapeutic opportunities. Moreover, the application of single-cell multiomics technologies offers an exciting avenue to dissect the cellular heterogeneity of SIRT3 activities across different tissue types and disease stages. This will provide deeper insights into its roles within both normal aging and pathological contexts, including cancer, neurodegeneration, and CVDs.
In conclusion, SIRT3 represents a compelling target for delaying or even reversing aging and aging-related diseases. By restoring mitochondrial function and mitigating oxidative damage, SIRT3 holds the potential to significantly extend healthspan. Ongoing preclinical and early-phase clinical studies will further support the selective activation of SIRT3 as a therapeutic strategy to improve health outcomes in aging populations. Ultimately, SIRT3 represents a focal point for future geroscience research, offering a promising pathway toward enhancing the quality of life in older adults and mitigating the burden of aging-related conditions.
Competing Interests
The authors have declared that no competing interest exists.
References
1. You Y, Liang W. SIRT1 and SIRT6: The role in aging-related diseases. Biochim Biophys Acta Mol Basis Dis. 2023;1869(7):166815 doi:10.1016/j.bbadis.2023.166815
2. Covarrubias AJ, Perrone R, Grozio A, Verdin E. NAD+ metabolism and its roles in cellular processes during ageing. Nat Rev Mol Cell Biol. 2021;22(2):119-141 doi:10.1038/s41580-020-00313-x
3. Imai S-i, Guarente L. NAD+ and sirtuins in aging and disease. Trends Cell Biol. 2014;24(8):464-471 doi:10.1016/j.tcb.2014.04.002
4. Korotkov A, Seluanov A, Gorbunova V. Sirtuin 6: linking longevity with genome and epigenome stability. Trends Cell Biol. 2021;31(12) doi:10.1016/j. tcb. 2021 06.009
5. Xi J, Chen Y, Jing J. et al. Sirtuin 3 suppresses the formation of renal calcium oxalate crystals through promoting M2 polarization of macrophages. J Cell Physiol. 2019;234(7):11463-11473 doi:10.1002/jcp.27803
6. Imai SA. et al. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403:795-800
7. Bosch-Presegué L, Vaquero A. Sirtuins in stress response: guardians of the genome. Oncogene. 2014;33(29):3764-3775 doi:10.1038/onc.2013.344
8. Grootaert MOJ, Bennett MR. Sirtuins in atherosclerosis: guardians of healthspan and therapeutic targets. Nat Rev Cardiol. 2022;19(10):668-683 doi:10.1038/s41569-022-00685-x
9. Fang Y, Tang S, Li X. Sirtuins in Metabolic and Epigenetic Regulation of Stem Cells. Trends Endocrinol Metab. 2019;30(3):177-188 doi:10.1016/j.tem.2018.12.002
10. Schwer B, Verdin E. Conserved metabolic regulatory functions of sirtuins. Cell Metab. 2008;7(2):104-112 doi:10.1016/j.cmet.2007.11.006
11. Park J, Chen Y, Tishkoff DX. et al. SIRT5-mediated lysine desuccinylation impacts diverse metabolic pathways. Mol Cell. 2013;50(6):919-930 doi:10.1016/j.molcel.2013.06.001
12. Słuczanowska-Głabowska S, Salmanowicz M, Staniszewska M, Pawlik A. The Role of Sirtuins in the Pathogenesis of Psoriasis. Int J Mol Sci. 2023 24(13) doi:10.3390/ijms241310782
13. Ding Q, Zhang Z, Li Y. et al. Propionate induces intestinal oxidative stress via Sod2 propionylation in zebrafish. iScience. 2021;24(6):102515 doi:10.1016/j.isci.2021.102515
14. Liu G, Chen H, Liu H, Zhang W, Zhou J. Emerging roles of SIRT6 in human diseases and its modulators. Med Res Rev. 2021;41(2):1089-1137 doi:10.1002/med.21753
15. Liao M, Sun X, Zheng W. et al. LINC00922 decoys SIRT3 to facilitate the metastasis of colorectal cancer through up-regulation the H3K27 crotonylation of ETS1 promoter. Mol Cancer. 2023;22(1):163 doi:10.1186/s12943-023-01859-y
16. Kim H-S, Patel K, Muldoon-Jacobs K. et al. SIRT3 is a mitochondria-localized tumor suppressor required for maintenance of mitochondrial integrity and metabolism during stress. Cancer Cell. 2010;17(1):41-52 doi:10.1016/j.ccr.2009.11.023
17. Zhao J, Wang G, Han K. et al. Mitochondrial PKM2 deacetylation by procyanidin B2-induced SIRT3 upregulation alleviates lung ischemia/reperfusion injury. Cell Death Dis. 2022;13(7):594 doi:10.1038/s41419-022-05051-w
18. Gong Y, Tang N, Liu P. et al. Newcastle disease virus degrades SIRT3 via PINK1-PRKN-dependent mitophagy to reprogram energy metabolism in infected cells. Autophagy. 2022;18(7):1503-1521 doi:10.1080/15548627.2021.1990515
19. Gao J, Huang C, Kong L. et al. SIRT3 Regulates Clearance of Apoptotic Cardiomyocytes by Deacetylating Frataxin. Circ Res. 2023;133(7):631-647 doi:10.1161/CIRCRESAHA.123.323160
20. Zhu J, Sun R, Sun K. et al. The deubiquitinase USP11 ameliorates intervertebral disc degeneration by regulating oxidative stress-induced ferroptosis via deubiquitinating and stabilizing Sirt3. Redox Biol. 2023;62:102707 doi:10.1016/j.redox.2023.102707
21. Wang Z, Zhang L, Liang Y. et al. Cyclic AMP Mimics the Anti-ageing Effects of Calorie Restriction by Up-Regulating Sirtuin. Sci Rep. 2015;5:12012 doi:10.1038/srep12012
22. Bellizzi D, Rose G, Cavalcante P. et al. A novel VNTR enhancer within the SIRT3 gene, a human homologue of SIR2, is associated with survival at oldest ages. Genomics. 2005;85(2):258-263
23. Zhou ZD, Tan EK. Oxidized nicotinamide adenine dinucleotide-dependent mitochondrial deacetylase sirtuin-3 as a potential therapeutic target of Parkinson's disease. Ageing Res Rev. 2020;62:101107 doi:10.1016/j.arr.2020.101107
24. Zhang J, Wang H, Slotabec L, Cheng F, Tan Y, Li J. Alterations of SIRT1/SIRT3 subcellular distribution in aging undermine cardiometabolic homeostasis during ischemia and reperfusion. Aging Cell. 2023;22(9):e13930 doi:10.1111/acel.13930
25. Anamika Khanna A, Acharjee P Acharjee A, Trigun SK. Mitochondrial SIRT3 and neurodegenerative brain disorders. J Chem Neuroanat. 2019;95:43-53 doi:10.1016/j.jchemneu.2017.11.009
26. Guarente L. Franklin H. Epstein Lecture: Sirtuins, aging, and medicine. N Engl J Med. 2011;364(23):2235-2244 doi:10.1056/NEJMra1100831
27. He M, Chiang H-H, Luo H. et al. An Acetylation Switch of the NLRP3 Inflammasome Regulates Aging-Associated Chronic Inflammation and Insulin Resistance. Cell Metab. 2020;31(3)doi:10.1016/j. cmet. 2020 01.009
28. Dai S, Wei J, Zhang H. et al. Intermittent fasting reduces neuroinflammation in intracerebral hemorrhage through the Sirt3/Nrf2/HO-1 pathway. J Neuroinflammation. 2022;19(1):122 doi:10.1186/s12974-022-02474-2
29. Hu J, Liu T, Fu F. et al. Omentin1 ameliorates myocardial ischemia-induced heart failure via SIRT3/FOXO3a-dependent mitochondrial dynamical homeostasis and mitophagy. J Transl Med. 2022;20(1):447 doi:10.1186/s12967-022-03642-x
30. Zhang J, Li W, Xue S. et al. Qishen granule attenuates doxorubicin-induced cardiotoxicity by protecting mitochondrial function and reducing oxidative stress through regulation of Sirtuin3. J Ethnopharmacol. 2024;319(Pt 1):117134 doi:10.1016/j.jep.2023.117134
31. Zhang Q, Zhang C, Ge J. et al. Ameliorative effects of resveratrol against cadmium-induced nephrotoxicity via modulating nuclear xenobiotic receptor response and PINK1/Parkin-mediated Mitophagy. Food Funct. 2020;11(2):1856-1868 doi:10.1039/c9fo02287b
32. Lehallier B, Gate D, Schaum N. et al. Undulating changes in human plasma proteome profiles across the lifespan. Nat Med. 2019;25(12):1843-1850 doi:10.1038/s41591-019-0673-2
33. Gorgoulis V, Adams PD, Alimonti A. et al. Cellular Senescence: Defining a Path Forward. Cell. 2019;179(4):813-827 doi:10.1016/j.cell.2019.10.005
34. Zumerle S, Sarill M, Saponaro M. et al. Targeting senescence induced by age or chemotherapy with a polyphenol-rich natural extract improves longevity and healthspan in mice. Nat Aging. 2024;4(9):1231-1248 doi:10.1038/s43587-024-00663-7
35. Yang N, Sen P. The senescent cell epigenome. Aging (Albany NY). 2018;10(11):3590-3609 doi:10.18632/aging.101617
36. Olivieri F, Prattichizzo F, Grillari J, Balistreri CR. Cellular Senescence and Inflammaging in Age-Related Diseases. Mediators Inflamm. 2018;2018:9076485 doi:10.1155/2018/9076485
37. Zou W, Lai M, Jiang Y. et al. Exosome Release Delays Senescence by Disposing of Obsolete Biomolecules. Adv Sci (Weinh). 2023;10(8):e2204826 doi:10.1002/advs.202204826
38. Zhu S, Jia L, Wang X. et al. Anti-aging formula protects skin from oxidative stress-induced senescence through the inhibition of CXCR2 expression. J Ethnopharmacol. 2024;318(Pt B):116996 doi:10.1016/j.jep.2023.116996
39. Fulop T, Larbi A, Dupuis G. et al. Immunosenescence and Inflamm-Aging As Two Sides of the Same Coin: Friends or Foes? Front Immunol. 2017;8:1960 doi:10.3389/fimmu.2017.01960
40. Zhang Y, Jiang Y, Yang X, Huang Y, Pan A, Liao Y. Adipose tissue senescence: Biological changes, hallmarks and therapeutic approaches. Mech Ageing Dev. 2024;222:111988 doi:10.1016/j.mad.2024.111988
41. Zhu J, Yang Q, Li H. et al. Sirt3 deficiency accelerates ovarian senescence without affecting spermatogenesis in aging mice. Free Radic Biol Med. 2022;193(Pt 2):511-525 doi:10.1016/j.freeradbiomed.2022.10.324
42. Tomczyk MM, Cheung KG, Xiang B. et al. Mitochondrial Sirtuin-3 (SIRT3) Prevents Doxorubicin-Induced Dilated Cardiomyopathy by Modulating Protein Acetylation and Oxidative Stress. Circ Heart Fail. 2022;15(5):e008547 doi:10.1161/CIRCHEARTFAILURE.121.008547
43. Wang C-L, Ohkubo R, Mu W-C. et al. The mitochondrial unfolded protein response regulates hippocampal neural stem cell aging. Cell Metab. 2023;35(6)doi:10.1016/j. cmet. 2023 04.012
44. Zhu J, Wu C, Yang L. Cellular senescence in Alzheimer's disease: from physiology to pathology. Transl Neurodegener. 2024;13(1):55 doi:10.1186/s40035-024-00447-4
45. Zhou Y, Li GY, Ren JP. et al. Protection of CD4+ T cells from hepatitis C virus infection-associated senescence via ΔNp63-miR-181a-Sirt1 pathway. J Leukoc Biol. 2016;100(5):1201-1211
46. Zhao L, Hu K, Liu W. et al. Anemonin ameliorates human diploid fibroblasts 2BS and IMR90 cell senescence by PARP1-NAD+-SIRT1 signaling pathway. Arch Gerontol Geriatr. 2024;117:105255 doi:10.1016/j.archger.2023.105255
47. Jiang L, Lu L, Xue C. et al. ACE2 deficiency inhibits thoracic aortic dissection by enhancing SIRT3 mediated inhibition of inflammation and VSCMs phenotypic switch. Mol Med. 2024;30(1):154 doi:10.1186/s10020-024-00926-4
48. Peng K, Yao Y-X, Lu X. et al. Mitochondrial dysfunction-associated alveolar epithelial senescence is involved in CdCl2-induced COPD-like lung injury. J Hazard Mater. 2024;476:135103 doi:10.1016/j.jhazmat.2024.135103
49. Zeng Q, Zeng J. Inhibition of miR-494-3p alleviates oxidative stress-induced cell senescence and inflammation in the primary epithelial cells of COPD patients. Int Immunopharmacol. 2021;92:107044 doi:10.1016/j.intimp.2020.107044
50. Zhao H, Liu Z, Chen H. et al. Identifying specific functional roles for senescence across cell types. Cell. 2024;187(25)doi:10.1016/j. cell. 2024 09.021
51. Wang T, Cao Y, Zheng Q. et al. SENP1-Sirt3 Signaling Controls Mitochondrial Protein Acetylation and Metabolism. Mol Cell. 2019;75(4) doi:10.1016/j. molcel. 2019 06.008
52. Shen K, Zhou H, Zuo Q. et al. GATD3A-deficiency-induced mitochondrial dysfunction facilitates senescence of fibroblast-like synoviocytes and osteoarthritis progression. Nat Commun. 2024;15(1):10923 doi:10.1038/s41467-024-55335-2
53. He X-D, Gong W, Zhang J-N. et al. Sensing and Transmitting Intracellular Amino Acid Signals through Reversible Lysine Aminoacylations. Cell Metab. 2018;27(1) doi:10.1016/j. cmet. 2017 10.015
54. Jia L, Peng J, Chen H. et al. TPTEP1 impedes the reprogramming of fatty acid metabolism in triple negative breast cancer via miR-1343-3p/SIRT3 axis. Int J Biol Macromol. 2024;280(Pt 2):135792 doi:10.1016/j.ijbiomac.2024.135792
55. Zhang J, Xiang H, Liu J, Chen Y, He R-R, Liu B. Mitochondrial Sirtuin 3: New emerging biological function and therapeutic target. Theranostics. 2020;10(18):8315-8342 doi:10.7150/thno.45922
56. Wu J, Zeng Z, Zhang W. et al. Emerging role of SIRT3 in mitochondrial dysfunction and cardiovascular diseases. Free Radic Res. 2019;53(2):139-149 doi:10.1080/10715762.2018.1549732
57. Jin L, Geng L, Ying L. et al. FGF21-Sirtuin 3 Axis Confers the Protective Effects of Exercise Against Diabetic Cardiomyopathy by Governing Mitochondrial Integrity. Circulation. 2022;146(20):1537-1557 doi:10.1161/CIRCULATIONAHA.122.059631
58. Gariani K, Menzies KJ, Ryu D. et al. Eliciting the mitochondrial unfolded protein response by nicotinamide adenine dinucleotide repletion reverses fatty liver disease in mice. Hepatology. 2016;63(4):1190-1204 doi:10.1002/hep.28245
59. Bonkowski MS, Sinclair DA. Slowing ageing by design: the rise of NAD+ and sirtuin-activating compounds. Nat Rev Mol Cell Biol. 2016;17(11):679-690 doi:10.1038/nrm.2016.93
60. Liu L, Xing D, Du X. et al. Sirtuin 3 improves fatty acid metabolism in response to high nonesterified fatty acids in calf hepatocytes by modulating gene expression. J Dairy Sci. 2020;103(7):6557-6568 doi:10.3168/jds.2019-17670
61. Cao H, Chung ACK, Ming X. et al. Autotaxin signaling facilitates β cell dedifferentiation and dysfunction induced by Sirtuin 3 deficiency. Mol Metab. 2022;60:101493 doi:10.1016/j.molmet.2022.101493
62. Ming X, Chung ACK, Mao D. et al. Pancreatic Sirtuin 3 Deficiency Promotes Hepatic Steatosis by Enhancing 5-Hydroxytryptamine Synthesis in Mice with Diet-Induced Obesity. Diabetes. 2021;70(1):119-131 doi:10.2337/db20-0339
63. Finley LWS, Haas W, Desquiret-Dumas V. et al. Succinate dehydrogenase is a direct target of sirtuin 3 deacetylase activity. PLoS One. 2011;6(8):e23295 doi:10.1371/journal.pone.0023295
64. Li Y, Kong E, Ding R. et al. Hyperglycemia-induced Sirt3 downregulation increases microglial aerobic glycolysis and inflammation in diabetic neuropathic pain pathogenesis. CNS Neurosci Ther. 2024;30(8):e14913 doi:10.1111/cns.14913
65. Wang D, Cao L, Zhou X. et al. Mitigation of honokiol on fluoride-induced mitochondrial oxidative stress, mitochondrial dysfunction, and cognitive deficits through activating AMPK/PGC-1α/Sirt3. J Hazard Mater. 2022;437:129381 doi:10.1016/j.jhazmat.2022.129381
66. Forman HJ, Zhang H. Targeting oxidative stress in disease: promise and limitations of antioxidant therapy. Nat Rev Drug Discov. 2021;20(9):689-709 doi:10.1038/s41573-021-00233-1
67. Black HS. A Synopsis of the Associations of Oxidative Stress, ROS, and Antioxidants with Diabetes Mellitus. Antioxidants (Basel). 2022 11(10)doi:10.3390/antiox11102003
68. Zhang S, Wu X, Wang J. et al. Adiponectin/AdiopR1 signaling prevents mitochondrial dysfunction and oxidative injury after traumatic brain injury in a SIRT3 dependent manner. Redox Biol. 2022;54:102390 doi:10.1016/j.redox.2022.102390
69. Yang W, Nagasawa K, Münch C. et al. Mitochondrial Sirtuin Network Reveals Dynamic SIRT3-Dependent Deacetylation in Response to Membrane Depolarization. Cell. 2016;167(4) doi:10.1016/j. cell. 2016 10.016
70. Zhang T, Liu J, Tong Q, Lin L. SIRT3 Acts as a Positive Autophagy Regulator to Promote Lipid Mobilization in Adipocytes via Activating AMPK. Int J Mol Sci. 2020 21(2) doi:10.3390/ijms21020372
71. Liu Y, Liu YL, Cheng W, Yin XM, Jiang B. The expression of SIRT3 in primary hepatocellular carcinoma and the mechanism of its tumor suppressing effects. Eur Rev Med Pharmacol Sci. 2017;21(5):978-998
72. Qian K, Tang J, Ling Y-J. et al. Exogenous NADPH exerts a positive inotropic effect and enhances energy metabolism via SIRT3 in pathological cardiac hypertrophy and heart failure. EBioMedicine. 2023;98:104863 doi:10.1016/j.ebiom.2023.104863
73. Zhang Q, Siyuan Z, Xing C, Ruxiu L. SIRT3 regulates mitochondrial function: A promising star target for cardiovascular disease therapy. Biomed Pharmacother. 2024;170:116004 doi:10.1016/j.biopha.2023.116004
74. Ning Y, Dou X, Wang Z. et al. SIRT3: A potential therapeutic target for liver fibrosis. Pharmacol Ther. 2024;257:108639 doi:10.1016/j.pharmthera.2024.108639
75. Toubai T, Tamaki H, Peltier DC. et al. Mitochondrial Deacetylase SIRT3 Plays an Important Role in Donor T Cell Responses after Experimental Allogeneic Hematopoietic Transplantation. J Immunol. 2018;201(11):3443-3455 doi:10.4049/jimmunol.1800148
76. Zheng Y, Shi B, Ma M, Wu X, Lin X. The novel relationship between Sirt3 and autophagy in myocardial ischemia-reperfusion. J Cell Physiol. 2019;234(5):5488-5495 doi:10.1002/jcp.27329
77. Pi H, Xu S, Reiter RJ. et al. SIRT3-SOD2-mROS-dependent autophagy in cadmium-induced hepatotoxicity and salvage by melatonin. Autophagy. 2015;11(7):1037-1051 doi:10.1080/15548627.2015.1052208
78. Liu GZ, Xu W, Zang YX. et al. Honokiol Inhibits Atrial Metabolic Remodeling in Atrial Fibrillation Through Sirt3 Pathway. Front Pharmacol. 2022;13:813272 doi:10.3389/fphar.2022.813272
79. Hogg SJ, Beavis PA, Dawson MA, Johnstone RW. Targeting the epigenetic regulation of antitumour immunity. Nat Rev Drug Discov. 2020;19(11):776-800 doi:10.1038/s41573-020-0077-5
80. Zhao Y, Lu Z, Zhang H. et al. Sodium-glucose exchanger 2 inhibitor canagliflozin promotes mitochondrial metabolism and alleviates salt-induced cardiac hypertrophy via preserving SIRT3 expression. J Adv Res. 2024 doi:10.1016/j.jare.2024.04.030
81. Bindu S, Pillai VB, Kanwal A. et al. SIRT3 blocks myofibroblast differentiation and pulmonary fibrosis by preventing mitochondrial DNA damage. Am J Physiol Lung Cell Mol Physiol. 2017;312(1):L68-L78 doi:10.1152/ajplung.00188.2016
82. Zhou Y, Zhao Q, Zhang Y. et al. A new andrographolide derivative ADA targeting SIRT3-FOXO3a signaling mitigates cognitive impairment by activating mitophagy and inhibiting neuroinflammation in Apoe4 mice. Phytomedicine. 2024;124:155298 doi:10.1016/j.phymed.2023.155298
83. Zhang D-Y, Zhang C-F, Fu B-C. et al. Sirtuin3 protects aged human mesenchymal stem cells against oxidative stress and enhances efficacy of cell therapy for ischaemic heart diseases. J Cell Mol Med. 2018;22(11):5504-5517 doi:10.1111/jcmm.13821
84. Diao Z, Ji Q, Wu Z. et al. Correction to 'SIRT3 consolidates heterochromatin and counteracts senescence'. Nucleic Acids Research. 2021;49(15):9004-9006 doi:10.1093/nar/gkab698
85. Pérez-Hernández M, van Opbergen CJM, Bagwan N. et al. Loss of Nuclear Envelope Integrity and Increased Oxidant Production Cause DNA Damage in Adult Hearts Deficient in PKP2: A Molecular Substrate of ARVC. Circulation. 2022;146(11):851-867 doi:10.1161/CIRCULATIONAHA.122.060454
86. Tatone C, Di Emidio G, Barbonetti A. et al. Sirtuins in gamete biology and reproductive physiology: emerging roles and therapeutic potential in female and male infertility. Hum Reprod Update. 2018;24(3):267-289 doi:10.1093/humupd/dmy003
87. Liu F, Yuan L, Li L. et al. S-sulfhydration of SIRT3 combats BMSC senescence and ameliorates osteoporosis via stabilizing heterochromatic and mitochondrial homeostasis. Pharmacol Res. 2023;192:106788 doi:10.1016/j.phrs.2023.106788
88. Wei T, Gao J, Huang C, Song B, Sun M, Shen W. SIRT3 (Sirtuin-3) Prevents Ang II (Angiotensin II)-Induced Macrophage Metabolic Switch Improving Perivascular Adipose Tissue Function. Arterioscler Thromb Vasc Biol. 2021;41(2):714-730 doi:10.1161/ATVBAHA.120.315337
89. Diao Z, Ji Q, Wu Z. et al. SIRT3 consolidates heterochromatin and counteracts senescence. Nucleic Acids Research. 2021;49(8):4203-4219 doi:10.1093/nar/gkab161
90. Liu Y, Liang J, Liu Z, Tian X, Sun C. Dihydrolipoyl dehydrogenase promotes white adipocytes browning by activating the RAS/ERK pathway and undergoing crotonylation modification. Int J Biol Macromol. 2024;265(Pt 1):130816 doi:10.1016/j.ijbiomac.2024.130816
91. Bao X, Wang Y, Li X. et al. Identification of 'erasers' for lysine crotonylated histone marks using a chemical proteomics approach. Elife. 2014 3doi:10.7554/eLife.02999
92. Yi X, Guo W, Shi Q. et al. SIRT3-Dependent Mitochondrial Dynamics Remodeling Contributes to Oxidative Stress-Induced Melanocyte Degeneration in Vitiligo. Theranostics. 2019;9(6):1614-1633 doi:10.7150/thno.30398
93. Yasuda T, Kagawa W, Ogi T. et al. Novel function of HATs and HDACs in homologous recombination through acetylation of human RAD52 at double-strand break sites. PLoS Genet. 2018;14(3):e1007277 doi:10.1371/journal.pgen.1007277
94. Iwahara T, Bonasio R, Narendra V, Reinberg D. SIRT3 functions in the nucleus in the control of stress-related gene expression. Mol Cell Biol. 2012;32(24):5022-5034 doi:10.1128/MCB.00822-12
95. Sengupta A, Haldar D. Human sirtuin 3 (SIRT3) deacetylates histone H3 lysine 56 to promote nonhomologous end joining repair. DNA Repair (Amst). 2018;61 doi:10.1016/j. dnarep. 2017 11.003
96. Bei J, Chen Y, Zhang Q. et al. HBV suppresses macrophage immune responses by impairing the TCA cycle through the induction of CS/PDHC hyperacetylation. Hepatol Commun. 2023 7(11) doi:10.1097/HC9.0000000000000294
97. Zhang A, Pan Y, Wang H. et al. Excessive processing and acetylation of OPA1 aggravate age-related hearing loss via the dysregulation of mitochondrial dynamics. Aging Cell. 2024;23(4):e14091 doi:10.1111/acel.14091
98. Toker L, Tran GT, Sundaresan J. et al. Genome-wide histone acetylation analysis reveals altered transcriptional regulation in the Parkinson's disease brain. Mol Neurodegener. 2021;16(1):31 doi:10.1186/s13024-021-00450-7
99. Ren J-H, Hu J-L, Cheng S-T. et al. SIRT3 restricts hepatitis B virus transcription and replication through epigenetic regulation of covalently closed circular DNA involving suppressor of variegation 3-9 homolog 1 and SET domain containing 1A histone methyltransferases. Hepatology. 2018;68(4):1260-1276 doi:10.1002/hep.29912
100. Liu K, Lan D, Li C. et al. A double-edged sword: role of apoptosis repressor with caspase recruitment domain (ARC) in tumorigenesis and ischaemia/reperfusion (I/R) injury. Apoptosis. 2023;28(3-4):313-325 doi:10.1007/s10495-022-01802-4
101. King LE, Hohorst L, García-Sáez AJ. Expanding roles of BCL-2 proteins in apoptosis execution and beyond. J Cell Sci. 2023 136(22) doi:10.1242/jcs.260790
102. Jiang Z, Wang H, Wang X. et al. TMED4 facilitates regulatory T cell suppressive function via ROS homeostasis in tumor and autoimmune mouse models. J Clin Invest. 2024 135(1) doi:10.1172/JCI179874
103. Bu X, Zhao W, Zou H, Li W, Li M, Wang G. Immune response and apoptosis of gibel carp (Carassius auratus gibelio) gills to Chilodonella hexasticha infection: Insights from histopathological and multi-omics analyses. Fish Shellfish Immunol. 2024;147:109429 doi:10.1016/j.fsi.2024.109429
104. He Y, Ye R, Peng Y. et al. Photobiomodulation ameliorates ovarian aging by alleviating oxidative stress and inflammation damage and improving mitochondrial function. J Photochem Photobiol B. 2024;260:113024 doi:10.1016/j.jphotobiol.2024.113024
105. Vo TTT, Huynh TD, Wang C-S. et al. The Potential Implications of Hydrogen Sulfide in Aging and Age-Related Diseases through the Lens of Mitohormesis. Antioxidants (Basel). 2022 11(8)doi:10.3390/antiox11081619
106. Alizadeh J, da Silva Rosa SC, Weng X. et al. Ceramides and ceramide synthases in cancer: Focus on apoptosis and autophagy. Eur J Cell Biol. 2023;102(3):151337 doi:10.1016/j.ejcb.2023.151337
107. Song CL, Tang H, Ran LK. et al. Sirtuin 3 inhibits hepatocellular carcinoma growth through the glycogen synthase kinase-3β/BCL2-associated X protein-dependent apoptotic pathway. Oncogene. 2016;35(5):631-641 doi:10.1038/onc.2015.121
108. Tao N-N, Zhou H-Z, Tang H. et al. Sirtuin 3 enhanced drug sensitivity of human hepatoma cells through glutathione S-transferase pi 1/JNK signaling pathway. Oncotarget. 2016;7(31):50117-50130 doi:10.18632/oncotarget.10319
109. Yang Z, Xie Y, Li M. et al. Ramelteon alleviates myocardial ischemia/reperfusion injury (MIRI) through Sirt3-dependent regulation of cardiomyocyte apoptosis. Biomed Pharmacother. 2024;172:116229 doi:10.1016/j.biopha.2024.116229
110. Zhu M, He J, Xu Y. et al. AMPK activation coupling SENP1-Sirt3 axis protects against acute kidney injury. Mol Ther. 2023;31(10):3052-3066 doi:10.1016/j.ymthe.2023.08.014
111. Mou Y, Chen Y, Fan Z. et al. Discovery of a novel small-molecule activator of SIRT3 that inhibits cell proliferation and migration by apoptosis and autophagy-dependent cell death pathways in colorectal cancer. Bioorg Chem. 2024;146:107327 doi:10.1016/j.bioorg.2024.107327
112. Fan H, Le J-W, Sun M, Zhu J-H. Sirtuin 3 deficiency promotes acute kidney injury induced by sepsis via mitochondrial dysfunction and apoptosis. Iran J Basic Med Sci. 2021;24(5):675-681 doi:10.22038/ijbms.2021.54905.12312
113. Cao Y, Li P, Wang H, Li L, Li Q. SIRT3 promotion reduces resistance to cisplatin in lung cancer by modulating the FOXO3/CDT1 axis. Cancer Med. 2021;10(4):1394-1404 doi:10.1002/cam4.3728
114. Reiter RJ, Sharma RN, Manucha W. et al. Dysfunctional mitochondria in age-related neurodegeneration: Utility of melatonin as an antioxidant treatment. Ageing Res Rev. 2024;101:102480 doi:10.1016/j.arr.2024.102480
115. Zhang R, Wen Y, Liu J. et al. The miR-15b-5p/miR-379-3p-FOXO axis regulates cell cycle and apoptosis in scleral remodeling during experimental myopia. J Transl Med. 2024;22(1):710 doi:10.1186/s12967-024-05523-x
116. Yue Y, Du Z, Tao J, Shi L. Inhibition of microRNA-297 alleviates THLE-2 cell injury induced by hypoxia/reoxygenation by inhibiting NLRP3 inflammasome activation via sirtuin 3. Can J Physiol Pharmacol. 2022;100(2):125-133 doi:10.1139/cjpp-2021-0287
117. Yang L, Liu S, He Y. et al. Exosomes regulate SIRT3-related autophagy by delivering miR-421 to regulate macrophage polarization and participate in OSA-related NAFLD. J Transl Med. 2024;22(1):475 doi:10.1186/s12967-024-05283-8
118. Yu L, Wan Q, Liu Q. et al. IgG is an aging factor that drives adipose tissue fibrosis and metabolic decline. Cell Metab. 2024;36(4)doi:10.1016/j. cmet. 2024 01.015
119. Chen Y, Pu Q, Ma Y. et al. Aging Reprograms the Hematopoietic-Vascular Niche to Impede Regeneration and Promote Fibrosis. Cell Metab. 2021;33(2)doi:10.1016/j. cmet. 2020 11.019
120. Vissers G, Giacomozzi M, Verdurmen W, Peek R, Nap A. The role of fibrosis in endometriosis: a systematic review. Hum Reprod Update. 2024;30(6):706-750 doi:10.1093/humupd/dmae023
121. Peng D, Fu M, Wang M, Wei Y, Wei X. Targeting TGF-β signal transduction for fibrosis and cancer therapy. Mol Cancer. 2022;21(1):104 doi:10.1186/s12943-022-01569-x
122. Yang Y, Lei W, Jiang S. et al. CircRNAs: Decrypting the novel targets of fibrosis and aging. Ageing Res Rev. 2021;70:101390 doi:10.1016/j.arr.2021.101390
123. Kundu A, Gali S, Sharma S. et al. Dendropanoxide Alleviates Thioacetamide-induced Hepatic Fibrosis via Inhibition of ROS Production and Inflammation in BALB/C Mice. Int J Biol Sci. 2023;19(9):2630-2647 doi:10.7150/ijbs.80743
124. Shen T, Wu Y, Wang X. et al. Activating SIRT3 in peritoneal mesothelial cells alleviates postsurgical peritoneal adhesion formation by decreasing oxidative stress and inhibiting the NLRP3 inflammasome. Exp Mol Med. 2022;54(9):1486-1501 doi:10.1038/s12276-022-00848-3
125. Qiao Y, Xu L, Tao X. et al. Protective effects of dioscin against fructose-induced renal damage via adjusting Sirt3-mediated oxidative stress, fibrosis, lipid metabolism and inflammation. Toxicol Lett. 2018;284:37-45 doi:10.1016/j.toxlet.2017.11.031
126. Palomer X, Román-Azcona MS, Pizarro-Delgado J. et al. SIRT3-mediated inhibition of FOS through histone H3 deacetylation prevents cardiac fibrosis and inflammation. Signal Transduct Target Ther. 2020;5(1):14 doi:10.1038/s41392-020-0114-1
127. Su H, Zeng H, Liu B, Chen J-X. Sirtuin 3 is essential for hypertension-induced cardiac fibrosis via mediating pericyte transition. J Cell Mol Med. 2020;24(14):8057-8068 doi:10.1111/jcmm.15437
128. Chen T, Li J, Liu J. et al. Activation of SIRT3 by resveratrol ameliorates cardiac fibrosis and improves cardiac function via the TGF-β/Smad3 pathway. Am J Physiol Heart Circ Physiol. 2015;308(5):H424-H434 doi:10.1152/ajpheart.00454.2014
129. Wang X, Wan W, Lu J. et al. Inhalable cryptotanshinone spray-dried swellable microparticles for pulmonary fibrosis therapy by regulating TGF-β1/Smad3, STAT3 and SIRT3 pathways. Eur J Pharm Biopharm. 2022;172:177-192 doi:10.1016/j.ejpb.2022.02.012
130. Dong L, Yu L, Zhong J. Histone lysine-specific demethylase 1 induced renal fibrosis via decreasing sirtuin 3 expression and activating TGF-β1/Smad3 pathway in diabetic nephropathy. Diabetol Metab Syndr. 2022;14(1):2 doi:10.1186/s13098-021-00771-z
131. Sosulski ML, Gongora R, Feghali-Bostwick C, Lasky JA, Sanchez CG. Sirtuin 3 Deregulation Promotes Pulmonary Fibrosis. J Gerontol A Biol Sci Med Sci. 2017;72(5):595-602 doi:10.1093/gerona/glw151
132. Jablonski RP, Kim S-J, Cheresh P. et al. SIRT3 deficiency promotes lung fibrosis by augmenting alveolar epithelial cell mitochondrial DNA damage and apoptosis. FASEB J. 2017;31(6):2520-2532 doi:10.1096/fj.201601077R
133. Rehan M, Kurundkar D, Kurundkar AR. et al. Restoration of SIRT3 gene expression by airway delivery resolves age-associated persistent lung fibrosis in mice. Nat Aging. 2021;1(2):205-217 doi:10.1038/s43587-021-00027-5
134. Xiao H, Xie Y, Xi K. et al. Targeting Mitochondrial Sirtuins in Age-Related Neurodegenerative Diseases and Fibrosis. Aging Dis. 2023;14(5):1583-1605 doi:10.14336/AD.2023.0203
135. Zhao Y, Simon M, Seluanov A, Gorbunova V. DNA damage and repair in age-related inflammation. Nat Rev Immunol. 2023;23(2):75-89 doi:10.1038/s41577-022-00751-y
136. Wei J, Xie J, He J. et al. Active fraction of Polyrhachis vicina (Roger) alleviated cerebral ischemia/reperfusion injury by targeting SIRT3-mediated mitophagy and angiogenesis. Phytomedicine. 2023;121:155104 doi:10.1016/j.phymed.2023.155104
137. Liu J, Li D, Zhang T, Tong Q, Ye RD, Lin L. SIRT3 protects hepatocytes from oxidative injury by enhancing ROS scavenging and mitochondrial integrity. Cell Death Dis. 2017;8(10):e3158 doi:10.1038/cddis.2017.564
138. Guan C, Huang X, Yue J. et al. SIRT3-mediated deacetylation of NLRC4 promotes inflammasome activation. Theranostics. 2021;11(8):3981-3995 doi:10.7150/thno.55573
139. Quan Y, Park W, Jin J, Kim W, Park SK, Kang KP. Sirtuin 3 Activation by Honokiol Decreases Unilateral Ureteral Obstruction-Induced Renal Inflammation and Fibrosis via Regulation of Mitochondrial Dynamics and the Renal NF-κBTGF-β1/Smad Signaling Pathway. Int J Mol Sci. 2020 21(2)doi:10.3390/ijms21020402
140. Liu Y, Qian X-M, He Q-C, Weng J-K. MiR-421 inhibition protects H9c2 cells against hypoxia/reoxygenation-induced oxidative stress and apoptosis by targeting Sirt3. Perfusion. 2020;35(3):255-262 doi:10.1177/0267659119870725
141. Kurundkar D, Kurundkar AR, Bone NB. et al. SIRT3 diminishes inflammation and mitigates endotoxin-induced acute lung injury. JCI Insight. 2019 4(1)doi:10.1172/jci.insight.120722
142. Zhai Q, Chen X, Fei D. et al. Nanorepairers Rescue Inflammation-Induced Mitochondrial Dysfunction in Mesenchymal Stem Cells. Adv Sci (Weinh). 2022;9(4):e2103839 doi:10.1002/advs.202103839
143. Wang C, Chen Q, Chen S. et al. Serine synthesis sustains macrophage IL-1β production via NAD+-dependent protein acetylation. Mol Cell. 2024;84(4)doi:10.1016/j. molcel. 2024 01.002
144. Abdel-Wahab BA, Zafaar D, Habeeb MS, El-Shoura EAM. Nicorandil mitigates arsenic trioxide-induced lung injury via modulating vital signalling pathways SIRT1/PGC-1α/TFAM, JAK1/STAT3, and miRNA-132 expression. Br J Pharmacol. 2024;181(17):3215-3231 doi:10.1111/bph.16414
145. Gao P, You M, Li L. et al. Salt-Induced Hepatic Inflammatory Memory Contributes to Cardiovascular Damage Through Epigenetic Modulation of SIRT3. Circulation. 2022;145(5):375-391 doi:10.1161/CIRCULATIONAHA.121.055600
146. Klionsky DJ, Petroni G, Amaravadi RK. et al. Autophagy in major human diseases. EMBO J. 2021;40(19):e108863 doi:10.15252/embj.2021108863
147. Xu K, He Y, Moqbel SAA, Zhou X, Wu L, Bao J. SIRT3 ameliorates osteoarthritis via regulating chondrocyte autophagy and apoptosis through the PI3K/Akt/mTOR pathway. Int J Biol Macromol. 2021;175:351-360 doi:10.1016/j.ijbiomac.2021.02.029
148. Han D, Jiang L, Gu X. et al. SIRT3 deficiency is resistant to autophagy-dependent ferroptosis by inhibiting the AMPK/mTOR pathway and promoting GPX4 levels. J Cell Physiol. 2020;235(11):8839-8851 doi:10.1002/jcp.29727
149. RETRACTION. Trehalose improves palmitic acid-induced apoptosis of osteoblasts by regulating SIRT3-medicated autophagy via the AMPK/mTOR/ULK1 pathway. FASEB J. 2024;38(7):e23516 doi:10.1096/fsb2.23516
150. Fang Y, An N, Zhu L. et al. Autophagy-Sirt3 axis decelerates hematopoietic aging. Aging Cell. 2020;19(10):e13232 doi:10.1111/acel.13232
151. Li Y, Sun T, Shen S, Wang L, Yan J. LncRNA DYNLRB2-2 inhibits THP-1 macrophage foam cell formation by enhancing autophagy. Biol Chem. 2019;400(8):1047-1057 doi:10.1515/hsz-2018-0461
152. Xia X, Zhou K, An L-Y. et al. Nicotinamide adenine dinucleotide rejuvenates septic bone marrow mesenchymal stem cells. World J Stem Cells. 2025;17(2):96893 doi:10.4252/wjsc.v17.i2.96893
153. Childs BG, Durik M, Baker DJ, van Deursen JM. Cellular senescence in aging and age-related disease: from mechanisms to therapy. Nat Med. 2015;21(12):1424-1435 doi:10.1038/nm.4000
154. Temple S. Advancing cell therapy for neurodegenerative diseases. Cell Stem Cell. 2023;30(5):512-529 doi:10.1016/j.stem.2023.03.017
155. Loh JS, Mak WQ, Tan LKS. et al. Microbiota-gut-brain axis and its therapeutic applications in neurodegenerative diseases. Signal Transduct Target Ther. 2024;9(1):37 doi:10.1038/s41392-024-01743-1
156. Ge Y, Wu X, Cai Y. et al. FNDC5 prevents oxidative stress and neuronal apoptosis after traumatic brain injury through SIRT3-dependent regulation of mitochondrial quality control. Cell Death Dis. 2024;15(5):364 doi:10.1038/s41419-024-06748-w
157. Falabella M, Vernon HJ, Hanna MG, Claypool SM, Pitceathly RDS. Cardiolipin, Mitochondria, and Neurological Disease. Trends Endocrinol Metab. 2021;32(4):224-237 doi:10.1016/j.tem.2021.01.006
158. Hao J, Guo Y, Guo K, Yang Q. Peripheral Inflammatory Biomarkers of Alzheimer's Disease. J Alzheimers Dis. 2022;88(2):389-398 doi:10.3233/JAD-215422
159. van der Kant R, Goldstein LSB, Ossenkoppele R. Amyloid-β-independent regulators of tau pathology in Alzheimer disease. Nat Rev Neurosci. 2020;21(1):21-35 doi:10.1038/s41583-019-0240-3
160. Younan D, Petkus AJ, Widaman KF. et al. Particulate matter and episodic memory decline mediated by early neuroanatomic biomarkers of Alzheimer's disease. Brain. 2020;143(1):289-302 doi:10.1093/brain/awz348
161. Xiaowei X, Qian X, Dingzhou Z. Sirtuin-3 activates the mitochondrial unfolded protein response and reduces cerebral ischemia/reperfusion injury. Int J Biol Sci. 2023;19(13):4327-4339 doi:10.7150/ijbs.86614
162. Tyagi A, Nguyen CU, Chong T. et al. SIRT3 deficiency-induced mitochondrial dysfunction and inflammasome formation in the brain. Sci Rep. 2018;8(1):17547 doi:10.1038/s41598-018-35890-7
163. Tyagi A, Pugazhenthi S. A Promising Strategy to Treat Neurodegenerative Diseases by SIRT3 Activation. Int J Mol Sci. 2023 24(2)doi:10.3390/ijms24021615
164. Duong A, Che Y, Ceylan D. et al. Regulators of mitochondrial complex I activity: A review of literature and evaluation in postmortem prefrontal cortex from patients with bipolar disorder. Psychiatry Res. 2016;236:148-157 doi:10.1016/j.psychres.2015.12.015
165. Pradeepkiran JA, Baig J, Seman A, Reddy PH. Mitochondria in Aging and Alzheimer's Disease: Focus on Mitophagy. Neuroscientist. 2024;30(4):440-457 doi:10.1177/10738584221139761
166. Scheltens P, De Strooper B, Kivipelto M. et al. Alzheimer's disease. Lancet. 2021;397(10284):1577-1590 doi:10.1016/S0140-6736(20)32205-4
167. Liu Q, Sun Y-M, Huang H. et al. Sirtuin 3 protects against anesthesia/surgery-induced cognitive decline in aged mice by suppressing hippocampal neuroinflammation. J Neuroinflammation. 2021;18(1):41 doi:10.1186/s12974-021-02089-z
168. Li S, Yin J, Nielsen M, Beach TG, Guo L, Shi J. Sirtuin 3 Mediates Tau Deacetylation. J Alzheimers Dis. 2019;69(2):355-362 doi:10.3233/JAD-190014
169. do Carmo JM, Omoto ACM, Dai X. et al. Sex differences in the impact of parental obesity on offspring cardiac SIRT3 expression, mitochondrial efficiency, and diastolic function early in life. Am J Physiol Heart Circ Physiol. 2021;321(3):H485-H495 doi:10.1152/ajpheart.00176.2021
170. Jin Y, Gu W, Chen W. Sirt3 is critical for p53-mediated ferroptosis upon ROS-induced stress. J Mol Cell Biol. 2021;13(2):151-154 doi:10.1093/jmcb/mjaa074
171. Lee J, Kim Y, Liu T. et al. SIRT3 deregulation is linked to mitochondrial dysfunction in Alzheimer's disease. Aging Cell. 2018 17(1)doi:10.1111/acel.12679
172. Sun K, Jing X, Guo J, Yao X, Guo F. Mitophagy in degenerative joint diseases. Autophagy. 2021;17(9):2082-2092 doi:10.1080/15548627.2020.1822097
173. Wang Q, Wang Y, Li S, Shi J. PACAP-Sirtuin3 alleviates cognitive impairment through autophagy in Alzheimer's disease. Alzheimers Res Ther. 2023;15(1):184 doi:10.1186/s13195-023-01334-2
174. Hayes MT. Parkinson's Disease and Parkinsonism. Am J Med. 2019;132(7):802-807 doi:10.1016/j.amjmed.2019.03.001
175. Tolosa E, Garrido A, Scholz SW, Poewe W. Challenges in the diagnosis of Parkinson's disease. Lancet Neurol. 2021;20(5):385-397 doi:10.1016/S1474-4422(21)00030-2
176. Zhu J-H, Ouyang S-X, Zhang G-Y. et al. GSDME promotes MASLD by regulating pyroptosis, Drp1 citrullination-dependent mitochondrial dynamic, and energy balance in intestine and liver. Cell Death Differ. 2024;31(11):1467-1486 doi:10.1038/s41418-024-01343-0
177. Zeng R, Wang X, Zhou Q. et al. Icariin protects rotenone-induced neurotoxicity through induction of SIRT3. Toxicol Appl Pharmacol. 2019;379:114639 doi:10.1016/j.taap.2019.114639
178. Duan W-J, Liang L, Pan M-H. et al. Theacrine, a purine alkaloid from kucha, protects against Parkinson's disease through SIRT3 activation. Phytomedicine. 2020;77:153281 doi:10.1016/j.phymed.2020.153281
179. Xi Y, Tao K, Wen X. et al. SIRT3-Mediated Deacetylation of DRP1K711 Prevents Mitochondrial Dysfunction in Parkinson's Disease. Adv Sci (Weinh). 2025;12(17):e2411235 doi:10.1002/advs.202411235
180. Gleave JA, Arathoon LR, Trinh D. et al. Sirtuin 3 rescues neurons through the stabilisation of mitochondrial biogenetics in the virally-expressing mutant α-synuclein rat model of parkinsonism. Neurobiol Dis. 2017;106:133-146 doi:10.1016/j.nbd.2017.06.009
181. Zhang S, Ma Y, Feng J. Neuroprotective mechanisms of ε-viniferin in a rotenone-induced cell model of Parkinson's disease: significance of SIRT3-mediated FOXO3 deacetylation. Neural Regen Res. 2020;15(11):2143-2153 doi:10.4103/1673-5374.282264
182. Park J-H, Burgess JD, Faroqi AH. et al. Alpha-synuclein-induced mitochondrial dysfunction is mediated via a sirtuin 3-dependent pathway. Mol Neurodegener. 2020;15(1):5 doi:10.1186/s13024-019-0349-x
183. Hou Y, Lautrup S, Cordonnier S. et al. NAD+ supplementation normalizes key Alzheimer's features and DNA damage responses in a new AD mouse model with introduced DNA repair deficiency. Proc Natl Acad Sci U S A. 2018;115(8):E1876-E1885 doi:10.1073/pnas.1718819115
184. Feldman EL, Goutman SA, Petri S. et al. Amyotrophic lateral sclerosis. Lancet. 2022;400(10360):1363-1380 doi:10.1016/S0140-6736(22)01272-7
185. Zhang H, Dai S, Yang Y. et al. Role of Sirtuin 3 in Degenerative Diseases of the Central Nervous System. Biomolecules. 2023 13(5)doi:10.3390/biom13050735
186. Lundt S, Ding S. Potential Therapeutic Interventions Targeting NAD+ Metabolism for ALS. Cells. 2024 13(17)doi:10.3390/cells13171509
187. Maniatis S, Äijö T, Vickovic S. et al. Spatiotemporal dynamics of molecular pathology in amyotrophic lateral sclerosis. Science. 2019;364(6435):89-93 doi:10.1126/science.aav9776
188. Harlan BA, Pehar M, Sharma DR, Beeson G, Beeson CC, Vargas MR. Enhancing NAD+ Salvage Pathway Reverts the Toxicity of Primary Astrocytes Expressing Amyotrophic Lateral Sclerosis-linked Mutant Superoxide Dismutase 1 (SOD1). J Biol Chem. 2016;291(20):10836-10846 doi:10.1074/jbc.M115.698779
189. Kim A, Lalonde K, Truesdell A. et al. New Avenues for the Treatment of Huntington's Disease. Int J Mol Sci. 2021 22(16)doi:10.3390/ijms22168363
190. Tabrizi SJ, Estevez-Fraga C, van Roon-Mom WMC. et al. Potential disease-modifying therapies for Huntington's disease: lessons learned and future opportunities. Lancet Neurol. 2022;21(7):645-658 doi:10.1016/S1474-4422(22)00121-1
191. Cheng Y, Zhao A, Li Y. et al. Roles of SIRT3 in cardiovascular and neurodegenerative diseases. Ageing Res Rev. 2025;104:102654 doi:10.1016/j.arr.2024.102654
192. Almalki WH, Alzahrani A, Mahmoud El-Daly ME-S, Fadel Ahmed A-SHF. The emerging potential of SIRT-3 in oxidative stress-inflammatory axis associated increased neuroinflammatory component for metabolically impaired neural cell. Chem Biol Interact. 2021;333:109328 doi:10.1016/j.cbi.2020.109328
193. Cheng A, Yang Y, Zhou Y. et al. Mitochondrial SIRT3 Mediates Adaptive Responses of Neurons to Exercise and Metabolic and Excitatory Challenges. Cell Metab. 2016;23(1):128-142 doi:10.1016/j.cmet.2015.10.013
194. Kumar V, Kundu S, Singh A, Singh S. Understanding the Role of Histone Deacetylase and their Inhibitors in Neurodegenerative Disorders: Current Targets and Future Perspective. Curr Neuropharmacol. 2022;20(1):158-178 doi:10.2174/1570159X19666210609160017
195. Lautrup S, Sinclair DA, Mattson MP, Fang EF. NAD+ in Brain Aging and Neurodegenerative Disorders. Cell Metab. 2019;30(4):630-655 doi:10.1016/j.cmet.2019.09.001
196. Oh J-M, Kim G, Jeong J, Chun S. Compound K promotes thermogenic signature and mitochondrial biogenesis via the UCP1-SIRT3-PGC1α signaling pathway. Biomed Pharmacother. 2025;183:117838 doi:10.1016/j.biopha.2025.117838
197. Naia L, Carmo C, Campesan S. et al. Mitochondrial SIRT3 confers neuroprotection in Huntington's disease by regulation of oxidative challenges and mitochondrial dynamics. Free Radic Biol Med. 2021;163:163-179 doi:10.1016/j.freeradbiomed.2020.11.031
198. Evans MA, Sano S, Walsh K. Cardiovascular Disease, Aging, and Clonal Hematopoiesis. Annu Rev Pathol. 2020;15:419-438 doi:10.1146/annurev-pathmechdis-012419-032544
199. Chen Z, Li Z, Xu R, Xie Y, Li D, Zhao Y. Design, Synthesis, and In vivo Evaluation of Isosteviol Derivatives as New SIRT3 Activators with Highly Potent Cardioprotective Effects. J Med Chem. 2024;67(8):6749-6768 doi:10.1021/acs.jmedchem.4c00345
200. Winnik S, Auwerx J, Sinclair DA, Matter CM. Protective effects of sirtuins in cardiovascular diseases: from bench to bedside. Eur Heart J. 2015;36(48):3404-3412 doi:10.1093/eurheartj/ehv290
201. Rizzacasa B, Amati F, Romeo F, Novelli G, Mehta JL. Epigenetic Modification in Coronary Atherosclerosis: JACC Review Topic of the Week. J Am Coll Cardiol. 2019;74(10):1352-1365 doi:10.1016/j.jacc.2019.07.043
202. Boniakowski AM, denDekker AD, Davis FM. et al. SIRT3 Regulates Macrophage-Mediated Inflammation in Diabetic Wound Repair. J Invest Dermatol. 2019;139(12)doi:10.1016/j. jid. 2019 05.017
203. Marfella R, Prattichizzo F, Sardu C. et al. Evidence of an anti-inflammatory effect of PCSK9 inhibitors within the human atherosclerotic plaque. Atherosclerosis. 2023;378:117180 doi:10.1016/j.atherosclerosis.2023.06.971
204. O'Brien C, Ling T, Berman JM. et al. Simultaneous inhibition of Sirtuin 3 and cholesterol homeostasis targets acute myeloid leukemia stem cells by perturbing fatty acid β-oxidation and inducing lipotoxicity. Haematologica. 2023;108(9):2343-2357 doi:10.3324/haematol.2022.281894
205. Ding Y, Gong W, Zhang S. et al. Protective role of sirtuin3 against oxidative stress and NLRP3 inflammasome in cholesterol accumulation and foam cell formation of macrophages with ox-LDL-stimulation. Biochem Pharmacol. 2021;192:114665 doi:10.1016/j.bcp.2021.114665
206. Wang X-H, Ning Z-H, Xie Z. et al. SIRT3/AMPK Signaling Pathway Regulates Lipid Metabolism and Improves Vulnerability to Atrial Fibrillation in Dahl Salt-Sensitive Rats. Am J Hypertens. 2024;37(11):901-908 doi:10.1093/ajh/hpae091
207. Zhou Y, Liu L, Jin B. et al. Metrnl Alleviates Lipid Accumulation by Modulating Mitochondrial Homeostasis in Diabetic Nephropathy. Diabetes. 2023;72(5):611-626 doi:10.2337/db22-0680
208. Ma L, Zhao Z, Zhao Y, Gao Y, Zhao L, Li S. Weizmannia coagulans JA845 improves atherosclerosis induced by vitamin D3 and high-fat diet in rats through modulating lipid metabolism, oxidative stress, and endothelial vascular injury. J Appl Microbiol. 2023 134(8)doi:10.1093/jambio/lxad165
209. Winnik S, Gaul DS, Preitner F. et al. Deletion of Sirt3 does not affect atherosclerosis but accelerates weight gain and impairs rapid metabolic adaptation in LDL receptor knockout mice: implications for cardiovascular risk factor development. Basic Res Cardiol. 2014;109(1):399 doi:10.1007/s00395-013-0399-0
210. Hu X, Li Y, Chen Q. et al. Sialic acids promote macrophage M1 polarization and atherosclerosis by upregulating ROS and autophagy blockage. Int Immunopharmacol. 2023;120:110410 doi:10.1016/j.intimp.2023.110410
211. Hao Y, Yang Z, Liu J. et al. Protective effects of 5-heptadecylresorcinol against adipocyte mitochondrial dysfunction through upregulation of Sirt3-mediated autophagy. J Nutr Biochem. 2022;103:108956 doi:10.1016/j.jnutbio.2022.108956
212. Cao X, Wu VWY, Han Y. et al. Role of Argininosuccinate Synthase 1 -Dependent L-Arginine Biosynthesis in the Protective Effect of Endothelial Sirtuin 3 Against Atherosclerosis. Adv Sci (Weinh). 2024;11(12):e2307256 doi:10.1002/advs.202307256
213. Xiong W, Xiong Z, Song A. et al. UCP1 alleviates renal interstitial fibrosis progression through oxidative stress pathway mediated by SIRT3 protein stability. J Transl Med. 2023;21(1):521 doi:10.1186/s12967-023-04376-0
214. Cong L, Gao Z, Zheng Y. et al. Electrical stimulation inhibits Val-boroPro-induced pyroptosis in THP-1 macrophages via sirtuin3 activation to promote autophagy and inhibit ROS generation. Aging (Albany NY). 2020;12(7):6415-6435 doi:10.18632/aging.103038
215. Tsutsui H. Recent advances in the pharmacological therapy of chronic heart failure: Evidence and guidelines. Pharmacol Ther. 2022;238:108185 doi:10.1016/j.pharmthera.2022.108185
216. Zhu L, Li C, Liu Q, Xu W, Zhou X. Molecular biomarkers in cardiac hypertrophy. J Cell Mol Med. 2019;23(3):1671-1677 doi:10.1111/jcmm.14129
217. Parodi-Rullán RM, Chapa-Dubocq XR, Javadov S. Acetylation of Mitochondrial Proteins in the Heart: The Role of SIRT3. Front Physiol. 2018;9:1094 doi:10.3389/fphys.2018.01094
218. Pillai VB, Samant S, Sundaresan NR. et al. Honokiol blocks and reverses cardiac hypertrophy in mice by activating mitochondrial Sirt3. Nat Commun. 2015;6:6656 doi:10.1038/ncomms7656
219. Dikalova AE, Pandey A, Xiao L. et al. Mitochondrial Deacetylase Sirt3 Reduces Vascular Dysfunction and Hypertension While Sirt3 Depletion in Essential Hypertension Is Linked to Vascular Inflammation and Oxidative Stress. Circ Res. 2020;126(4):439-452 doi:10.1161/CIRCRESAHA.119.315767
220. Han Y, Nie J, Wang DW, Ni L. Mechanism of histone deacetylases in cardiac hypertrophy and its therapeutic inhibitors. Front Cardiovasc Med. 2022;9:931475 doi:10.3389/fcvm.2022.931475
221. Ramachandra CJA, Cong S, Chan X, Yap EP, Yu F, Hausenloy DJ. Oxidative stress in cardiac hypertrophy: From molecular mechanisms to novel therapeutic targets. Free Radic Biol Med. 2021;166:297-312 doi:10.1016/j.freeradbiomed.2021.02.040
222. Dikalova AE, Itani HA, Nazarewicz RR. et al. Sirt3 Impairment and SOD2 Hyperacetylation in Vascular Oxidative Stress and Hypertension. Circ Res. 2017;121(5):564-574 doi:10.1161/CIRCRESAHA.117.310933
223. Wang Y-C, Koay YC, Pan C. et al. Indole-3-Propionic Acid Protects Against Heart Failure With Preserved Ejection Fraction. Circ Res. 2024;134(4):371-389 doi:10.1161/CIRCRESAHA.123.322381
224. Zhang B, Yang J, Li X. et al. Tetrahydrocurcumin ameliorates postinfarction cardiac dysfunction and remodeling by inhibiting oxidative stress and preserving mitochondrial function via SIRT3 signaling pathway. Phytomedicine. 2023;121:155127 doi:10.1016/j.phymed.2023.155127
225. Sundaresan NR, Gupta M, Kim G, Rajamohan SB, Isbatan A, Gupta MP. Sirt3 blocks the cardiac hypertrophic response by augmenting Foxo3a-dependent antioxidant defense mechanisms in mice. J Clin Invest. 2009;119(9):2758-2771 doi:10.1172/JCI39162
226. Gu C, Kong F, Zeng J, Geng X, Sun Y, Chen X. Remote ischemic preconditioning protects against spinal cord ischemia-reperfusion injury in mice by activating NMDAR/AMPK/PGC-1α/SIRT3 signaling. Cell Biosci. 2023;13(1):57 doi:10.1186/s13578-023-00999-4
227. Dikalova A, Fehrenbach D, Mayorov V. et al. Mitochondrial CypD Acetylation Promotes Endothelial Dysfunction and Hypertension. Circ Res. 2024;134(11):1451-1464 doi:10.1161/CIRCRESAHA.123.323596
228. Zhang Q, Li D, Dong X. et al. LncDACH1 promotes mitochondrial oxidative stress of cardiomyocytes by interacting with sirtuin3 and aggravates diabetic cardiomyopathy. Sci China Life Sci. 2022;65(6):1198-1212 doi:10.1007/s11427-021-1982-8
229. Zhang J, Lu Y, Yu P. et al. Therapeutic hypothermia alleviates myocardial ischaemia-reperfusion injury by inhibiting inflammation and fibrosis via the mediation of the SIRT3/NLRP3 signalling pathway. J Cell Mol Med. 2022;26(19):4995-5007 doi:10.1111/jcmm.17523
230. Zeng H, Chen J-X. Sirtuin 3, Endothelial Metabolic Reprogramming, and Heart Failure With Preserved Ejection Fraction. J Cardiovasc Pharmacol. 2019;74(4):315-323 doi:10.1097/FJC.0000000000000719
231. Su H, Cantrell AC, Chen J-X, Gu W, Zeng H. SIRT3 Deficiency Enhances Ferroptosis and Promotes Cardiac Fibrosis via p53 Acetylation. Cells. 2023 12(10)doi:10.3390/cells12101428
232. Suo M, Qi Y, Liu L. et al. SS31 Alleviates Pressure Overload-Induced Heart Failure Caused by Sirt3-Mediated Mitochondrial Fusion. Front Cardiovasc Med. 2022;9:858594 doi:10.3389/fcvm.2022.858594
233. Guo X, Yan F, Shan X. et al. SIRT3 inhibits Ang II-induced transdifferentiation of cardiac fibroblasts through β-catenin/PPAR-γ signaling. Life Sci. 2017;186:111-117 doi:10.1016/j.lfs.2017.07.030
234. Srivastava SP, Li J, Takagaki Y. et al. Endothelial SIRT3 regulates myofibroblast metabolic shifts in diabetic kidneys. iScience. 2021;24(5):102390 doi:10.1016/j.isci.2021.102390
235. Palmer AK, Gustafson B, Kirkland JL, Smith U. Cellular senescence: at the nexus between ageing and diabetes. Diabetologia. 2019;62(10):1835-1841 doi:10.1007/s00125-019-4934-x
236. Amorim JA, Coppotelli G, Rolo AP, Palmeira CM, Ross JM, Sinclair DA. Mitochondrial and metabolic dysfunction in ageing and age-related diseases. Nat Rev Endocrinol. 2022;18(4):243-258 doi:10.1038/s41574-021-00626-7
237. Zhan J, Jin K, Xie R. et al. AGO2 Protects Against Diabetic Cardiomyopathy by Activating Mitochondrial Gene Translation. Circulation. 2024;149(14):1102-1120 doi:10.1161/CIRCULATIONAHA.123.065546
238. Paulin R, Dromparis P, Sutendra G. et al. Sirtuin 3 deficiency is associated with inhibited mitochondrial function and pulmonary arterial hypertension in rodents and humans. Cell Metab. 2014;20(5):827-839 doi:10.1016/j.cmet.2014.08.011
239. Liu Y-T, Qiu H-L, Xia H-X. et al. Macrod1 suppresses diabetic cardiomyopathy via regulating PARP1-NAD+-SIRT3 pathway. Acta Pharmacol Sin. 2024;45(6):1175-1188 doi:10.1038/s41401-024-01247-2
240. Li G, Qin H, Zhou M. et al. Knockdown of SIRT3 perturbs protective effects of irisin against bone loss in diabetes and periodontitis. Free Radic Biol Med. 2023;200:11-25 doi:10.1016/j.freeradbiomed.2023.02.023
241. Lantier L, Williams AS, Williams IM. et al. SIRT3 Is Crucial for Maintaining Skeletal Muscle Insulin Action and Protects Against Severe Insulin Resistance in High-Fat-Fed Mice. Diabetes. 2015;64(9):3081-3092 doi:10.2337/db14-1810
242. Lu Q-B, Fu X, Liu Y. et al. Disrupted cardiac fibroblast BCAA catabolism contributes to diabetic cardiomyopathy via a periostin/NAP1L2/SIRT3 axis. Cell Mol Biol Lett. 2023;28(1):93 doi:10.1186/s11658-023-00510-4
243. Peng M-L, Fu Y, Wu C-W, Zhang Y, Ren H, Zhou S-S. Signaling Pathways Related to Oxidative Stress in Diabetic Cardiomyopathy. Front Endocrinol (Lausanne). 2022;13:907757 doi:10.3389/fendo.2022.907757
244. Klimova N, Long A, Kristian T. Nicotinamide mononucleotide alters mitochondrial dynamics by SIRT3-dependent mechanism in male mice. J Neurosci Res. 2019;97(8):975-990 doi:10.1002/jnr.24397
245. Wang X, Liu Z, Deng S. et al. SIRT3 alleviates high glucose-induced chondrocyte injury through the promotion of autophagy and suppression of apoptosis in osteoarthritis progression. Int Immunopharmacol. 2024;130:111755 doi:10.1016/j.intimp.2024.111755
246. Dai S-H, Chen T, Wang Y-H. et al. Sirt3 protects cortical neurons against oxidative stress via regulating mitochondrial Ca2+ and mitochondrial biogenesis. Int J Mol Sci. 2014;15(8):14591-14609 doi:10.3390/ijms150814591
247. Chang Y, Wang C, Zhu J. et al. SIRT3 ameliorates diabetes-associated cognitive dysfunction via regulating mitochondria-associated ER membranes. J Transl Med. 2023;21(1):494 doi:10.1186/s12967-023-04246-9
248. Zhou Y, Chung ACK, Fan R. et al. Sirt3 Deficiency Increased the Vulnerability of Pancreatic Beta Cells to Oxidative Stress-Induced Dysfunction. Antioxid Redox Signal. 2017;27(13):962-976 doi:10.1089/ars.2016.6859
249. Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74(1):12-49 doi:10.3322/caac.21820
250. Schumacker PT. A tumor suppressor SIRTainty. Cancer Cell. 2010;17(1):5-6 doi:10.1016/j.ccr.2009.12.032
251. Li S-T, Huang D, Shen S. et al. Myc-mediated SDHA acetylation triggers epigenetic regulation of gene expression and tumorigenesis. Nat Metab. 2020;2(3):256-269 doi:10.1038/s42255-020-0179-8
252. Mayo JC, Sainz RM, González Menéndez P, Cepas V, Tan D-X, Reiter RJ. Melatonin and sirtuins: A "not-so unexpected" relationship. J Pineal Res. 2017 62(2)doi:10.1111/jpi.12391
253. Dong Y-X, Li T-H, Wang S-S. et al. Bu zhong Yiqi Decoction ameliorates mild cognitive impairment by improving mitochondrial oxidative stress damage via the SIRT3/MnSOD/OGG1 pathway. J Ethnopharmacol. 2024;331:118237 doi:10.1016/j.jep.2024.118237
254. Wei X, Xu Y, Xu FF. et al. RelB Expression Determines the Differential Effects of Ascorbic Acid in Normal and Cancer Cells. Cancer Res. 2017;77(6):1345-1356 doi:10.1158/0008-5472.CAN-16-0785
255. De Rasmo D, Cormio A, Cormio G, Signorile A. Ovarian Cancer: A Landscape of Mitochondria with Emphasis on Mitochondrial Dynamics. Int J Mol Sci. 2023 24(2)doi:10.3390/ijms24021224
256. Xu L, Li Y, Zhou L. et al. SIRT3 elicited an anti-Warburg effect through HIF1α/PDK1/PDHA1 to inhibit cholangiocarcinoma tumorigenesis. Cancer Med. 2019;8(5):2380-2391 doi:10.1002/cam4.2089
257. Sawant Dessai A, Dominguez MP, Chen U-I. et al. Transcriptional Repression of SIRT3 Potentiates Mitochondrial Aconitase Activation to Drive Aggressive Prostate Cancer to the Bone. Cancer Res. 2021;81(1):50-63 doi:10.1158/0008-5472.CAN-20-1708
258. Torrens-Mas M, Hernández-López R, Pons D-G, Roca P, Oliver J, Sastre-Serra J. Sirtuin 3 silencing impairs mitochondrial biogenesis and metabolism in colon cancer cells. Am J Physiol Cell Physiol. 2019;317(2):C398-C404 doi:10.1152/ajpcell.00112.2019
259. Kunadis E, Piperi C. Exploring the Multi-Faceted Role of Sirtuins in Glioblastoma Pathogenesis and Targeting Options. Int J Mol Sci. 2022 23(21)doi:10.3390/ijms232112889
260. Greene J, Segaran A, Lord S. Targeting OXPHOS and the electron transport chain in cancer; Molecular and therapeutic implications. Semin Cancer Biol. 2022;86(Pt 2):851-859 doi:10.1016/j.semcancer.2022.02.002
261. Ashton TM, McKenna WG, Kunz-Schughart LA, Higgins GS. Oxidative Phosphorylation as an Emerging Target in Cancer Therapy. Clin Cancer Res. 2018;24(11):2482-2490 doi:10.1158/1078-0432.CCR-17-3070
262. Chae H-S, Hong S-T. Overview of Cancer Metabolism and Signaling Transduction. Int J Mol Sci. 2022 24(1)doi:10.3390/ijms24010012
263. Shi J, Xiong Z, Wang K. et al. HIF2α promotes tumour growth in clear cell renal cell carcinoma by increasing the expression of NUDT1 to reduce oxidative stress. Clin Transl Med. 2021;11(11):e592 doi:10.1002/ctm2.592
264. Tao R, Coleman MC, Pennington JD. et al. Sirt3-mediated deacetylation of evolutionarily conserved lysine 122 regulates MnSOD activity in response to stress. Mol Cell. 2010;40(6):893-904 doi:10.1016/j.molcel.2010.12.013
265. Ahmed MA, O'Callaghan C, Chang ED, Jiang H, Vassilopoulos A. Context-Dependent Roles for SIRT2 and SIRT3 in Tumor Development Upon Calorie Restriction or High Fat Diet. Front Oncol. 2019;9:1462 doi:10.3389/fonc.2019.01462
266. Chen S, Yang X, Yu M. et al. SIRT3 regulates cancer cell proliferation through deacetylation of PYCR1 in proline metabolism. Neoplasia. 2019;21(7):665-675 doi:10.1016/j.neo.2019.04.008
267. Wei Z, Song J, Wang G. et al. Deacetylation of serine hydroxymethyl-transferase 2 by SIRT3 promotes colorectal carcinogenesis. Nat Commun. 2018;9(1):4468 doi:10.1038/s41467-018-06812-y
268. Liu R, Zeng L-W, Gong R, Yuan F, Shu H-B, Li S. mTORC1 activity regulates post-translational modifications of glycine decarboxylase to modulate glycine metabolism and tumorigenesis. Nat Commun. 2021;12(1):4227 doi:10.1038/s41467-021-24321-3
269. Park H-K, Hong J-H, Oh YT. et al. Interplay between TRAP1 and Sirtuin-3 Modulates Mitochondrial Respiration and Oxidative Stress to Maintain Stemness of Glioma Stem Cells. Cancer Res. 2019;79(7):1369-1382 doi:10.1158/0008-5472.CAN-18-2558
270. Li X, Zhang W, Xing Z. et al. Targeting SIRT3 sensitizes glioblastoma to ferroptosis by promoting mitophagy and inhibiting SLC7A11. Cell Death Dis. 2024;15(2):168 doi:10.1038/s41419-024-06558-0
271. Onyiba CI, Scarlett CJ, Weidenhofer J. The Mechanistic Roles of Sirtuins in Breast and Prostate Cancer. Cancers (Basel). 2022 14(20)doi:10.3390/cancers14205118
272. Maiti GP, Sinha S, Mahmud H. et al. SIRT3 overexpression and epigenetic silencing of catalase regulate ROS accumulation in CLL cells activating AXL signaling axis. Blood Cancer J. 2021;11(5):93 doi:10.1038/s41408-021-00484-6
273. Liu L, Li Y, Cao D. et al. SIRT3 inhibits gallbladder cancer by induction of AKT-dependent ferroptosis and blockade of epithelial-mesenchymal transition. Cancer Lett. 2021;510doi:10.1016/j. canlet. 2021 04.007
274. Huang P, Zhao H, Pan X. et al. SIRT3-mediated autophagy contributes to ferroptosis-induced anticancer by inducing the formation of BECN1-SLC7A11 complex. Biochem Pharmacol. 2023;213:115592 doi:10.1016/j.bcp.2023.115592
275. Ma Z, Li Z, Wang S. et al. ZMAT1 acts as a tumor suppressor in pancreatic ductal adenocarcinoma by inducing SIRT3/p53 signaling pathway. J Exp Clin Cancer Res. 2022;41(1):130 doi:10.1186/s13046-022-02310-8
276. Kenny TC, Craig AJ, Villanueva A, Germain D. Mitohormesis Primes Tumor Invasion and Metastasis. Cell Rep. 2019;27(8)doi:10.1016/j. celrep. 2019 04.095
277. Mitchell S, Zhang P, Cannon M. et al. Anti-tumor NAMPT inhibitor, KPT-9274, mediates gender-dependent murine anemia and nephrotoxicity by regulating SIRT3-mediated SOD deacetylation. J Hematol Oncol. 2021;14(1):101 doi:10.1186/s13045-021-01107-0
278. Kenny TC, Hart P, Ragazzi M. et al. Selected mitochondrial DNA landscapes activate the SIRT3 axis of the UPRmt to promote metastasis. Oncogene. 2017;36(31):4393-4404 doi:10.1038/onc.2017.52
279. Saxena S, Dagar N, Shelke V, Lech M, Khare P, Gaikwad AB. Metabolic reprogramming: Unveiling the therapeutic potential of targeted therapies against kidney disease. Drug Discov Today. 2023;28(11):103765 doi:10.1016/j.drudis.2023.103765
280. Juszczak F, Arnould T, Declèves A-E. The Role of Mitochondrial Sirtuins (SIRT3, SIRT4 and SIRT5) in Renal Cell Metabolism: Implication for Kidney Diseases. Int J Mol Sci. 2024 25(13)doi:10.3390/ijms25136936
281. Kwon Y, Kim J, Lee C-Y, Kim H. Expression of SIRT1 and SIRT3 varies according to age in mice. Anat Cell Biol. 2015;48(1):54-61 doi:10.5115/acb.2015.48.1.54
282. Hobson S, Arefin S, Witasp A. et al. Accelerated Vascular Aging in Chronic Kidney Disease: The Potential for Novel Therapies. Circ Res. 2023;132(8):950-969 doi:10.1161/CIRCRESAHA.122.321751
283. Kellum JA, Prowle JR. Paradigms of acute kidney injury in the intensive care setting. Nat Rev Nephrol. 2018;14(4):217-230 doi:10.1038/nrneph.2017.184
284. Kellum JA, Romagnani P, Ashuntantang G, Ronco C, Zarbock A, Anders H-J. Acute kidney injury. Nat Rev Dis Primers. 2021;7(1):52 doi:10.1038/s41572-021-00284-z
285. Shen H, Holliday M, Sheikh-Hamad D. et al. Sirtuin-3 mediates sex differences in kidney ischemia-reperfusion injury. Transl Res. 2021;235:15-31 doi:10.1016/j.trsl.2021.03.015
286. Emma F, Montini G, Parikh SM, Salviati L. Mitochondrial dysfunction in inherited renal disease and acute kidney injury. Nat Rev Nephrol. 2016;12(5):267-280 doi:10.1038/nrneph.2015.214
287. Deng Z, He M, Hu H. et al. Melatonin attenuates sepsis-induced acute kidney injury by promoting mitophagy through SIRT3-mediated TFAM deacetylation. Autophagy. 2024;20(1):151-165 doi:10.1080/15548627.2023.2252265
288. Li M, Li C-M, Ye Z-C. et al. Sirt3 modulates fatty acid oxidation and attenuates cisplatin-induced AKI in mice. J Cell Mol Med. 2020;24(9):5109-5121 doi:10.1111/jcmm.15148
289. Perico L, Morigi M, Benigni A. Mitochondrial Sirtuin 3 and Renal Diseases. Nephron. 2016;134(1):14-19 doi:10.1159/000444370
290. Fan H, Le J-W, Sun M, Zhu J-H. N-acetylcysteine protects septic acute kidney injury by inhibiting SIRT3-mediated mitochondrial dysfunction and apoptosis. Iran J Basic Med Sci. 2024;27(7):850-856 doi:10.22038/IJBMS.2024.72882.15853
291. Huang C, Jiang S, Gao S. et al. Sirtuins: Research advances on the therapeutic role in acute kidney injury. Phytomedicine. 2022;101:154122 doi:10.1016/j.phymed.2022.154122
292. Gou XY, Li Y, Fan XP. The Role of Mdivi-1 in Reducing Mitochondrial Fission via the NF-kappaB/JNK/SIRT3 Signaling Pathway in Acute Kidney Injury. Physiol Res. 2025;74(1):79-92
293. Shen L, Zhang Q, Tu S, Qin W. SIRT3 mediates mitofusin 2 ubiquitination and degradation to suppress ischemia reperfusion-induced acute kidney injury. Exp Cell Res. 2021;408(2):112861 doi:10.1016/j.yexcr.2021.112861
294. Nežić L, Škrbić R, Amidžić L. et al. Protective Effects of Simvastatin on Endotoxin-Induced Acute Kidney Injury through Activation of Tubular Epithelial Cells' Survival and Hindering Cytochrome C-Mediated Apoptosis. Int J Mol Sci. 2020 21(19)doi:10.3390/ijms21197236
295. Wang Q, Xu J, Li X. et al. Sirt3 modulate renal ischemia-reperfusion injury through enhancing mitochondrial fusion and activating the ERK-OPA1 signaling pathway. J Cell Physiol. 2019;234(12):23495-23506 doi:10.1002/jcp.28918
296. Chen D-Q, Chen L, Guo Y. et al. Poricoic acid A suppresses renal fibroblast activation and interstitial fibrosis in UUO rats via upregulating Sirt3 and promoting β-catenin K49 deacetylation. Acta Pharmacol Sin. 2023;44(5):1038-1050 doi:10.1038/s41401-022-01026-x
297. Chen C, Gu J, Wang J. et al. Physcion 8-O-β-glucopyranoside ameliorates liver fibrosis through inflammation inhibition by regulating SIRT3-mediated NF-κB P65 nuclear expression. Int Immunopharmacol. 2021;90:107206 doi:10.1016/j.intimp.2020.107206
298. Mandala A, Chen WJ, Armstrong A. et al. PPARα agonist fenofibrate attenuates iron-induced liver injury in mice by modulating the Sirt3 and β-catenin signaling. Am J Physiol Gastrointest Liver Physiol. 2021;321(4):G262-G269 doi:10.1152/ajpgi.00129.2021
299. Xie S, Zou W, Liu S. et al. Site 1 protease aggravates acute kidney injury by promoting tubular epithelial cell ferroptosis through SIRT3-SOD2-mtROS signaling. FEBS J. 2024;291(7):1575-1592 doi:10.1111/febs.17057
300. Yuan Y, Zhu L, Li L. et al. S-Sulfhydration of SIRT3 by Hydrogen Sulfide Attenuates Mitochondrial Dysfunction in Cisplatin-Induced Acute Kidney Injury. Antioxid Redox Signal. 2019;31(17):1302-1319 doi:10.1089/ars.2019.7728
301. Guo Y-Y, Liang N-N, Zhang X-Y. et al. Mitochondrial GPX4 acetylation is involved in cadmium-induced renal cell ferroptosis. Redox Biol. 2024;73:103179 doi:10.1016/j.redox.2024.103179
302. He J, Shangguan X, Zhou W. et al. Glucose limitation activates AMPK coupled SENP1-Sirt3 signalling in mitochondria for T cell memory development. Nat Commun. 2021;12(1):4371 doi:10.1038/s41467-021-24619-2
303. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024;105(4S):S117-S314. doi:10.1016/j. kint. 2023 10.018
304. Li L, Fu H, Liu Y. The fibrogenic niche in kidney fibrosis: components and mechanisms. Nat Rev Nephrol. 2022;18(9):545-557 doi:10.1038/s41581-022-00590-z
305. Djudjaj S, Boor P. Cellular and molecular mechanisms of kidney fibrosis. Mol Aspects Med. 2019;65:16-36 doi:10.1016/j.mam.2018.06.002
306. Cheng L, Yang X, Jian Y. et al. SIRT3 deficiency exacerbates early-stage fibrosis after ischaemia-reperfusion-induced AKI. Cell Signal. 2022;93:110284 doi:10.1016/j.cellsig.2022.110284
307. Wu X, Liu M, Wei G. et al. Renal protection of rhein against 5/6 nephrectomied-induced chronic kidney disease: role of SIRT3-FOXO3α signalling pathway. J Pharm Pharmacol. 2020;72(5):699-708 doi:10.1111/jphp.13234
308. Zheng C-M, Lu K-C, Chen Y-J, Li C-Y, Lee Y-H, Chiu H-W. Matrix metalloproteinase-7 promotes chronic kidney disease progression via the induction of inflammasomes and the suppression of autophagy. Biomed Pharmacother. 2022;154:113565 doi:10.1016/j.biopha.2022.113565
309. Mizukami Y, Kawao N, Ohira T. et al. Effects of plasminogen activator inhibitor-1 deficiency on bone disorders and sarcopenia caused by adenine-induced renal dysfunction in mice. PLoS One. 2024;19(10):e0311902 doi:10.1371/journal.pone.0311902
310. Feng X, Su H, He X, Chen J-X, Zeng H. SIRT3 Deficiency Sensitizes Angiotensin-II-Induced Renal Fibrosis. Cells. 2020 9(11)doi:10.3390/cells9112510
311. Ye Y, Chen A, Li L. et al. Repression of the antiporter SLC7A11/glutathione/glutathione peroxidase 4 axis drives ferroptosis of vascular smooth muscle cells to facilitate vascular calcification. Kidney Int. 2022;102(6):1259-1275 doi:10.1016/j.kint.2022.07.034
312. Moellmann J, Krueger K, Wong DWL. et al. 2,8-Dihydroxyadenine-induced nephropathy causes hexosylceramide accumulation with increased mTOR signaling, reduced levels of protective SirT3 expression and impaired renal mitochondrial function. Biochim Biophys Acta Mol Basis Dis. 2024;1870(1):166825 doi:10.1016/j.bbadis.2023.166825
313. Guan Y-M, Diao Z-L, Huang H-D. et al. Bioactive peptide apelin rescues acute kidney injury by protecting the function of renal tubular mitochondria. Amino Acids. 2021;53(8):1229-1240 doi:10.1007/s00726-021-03028-1
314. Liu S-M, Zhang Y-R, Chen Y. et al. Intermedin Alleviates Vascular Calcification in CKD through Sirtuin 3-Mediated Inhibition of Mitochondrial Oxidative Stress. Pharmaceuticals (Basel). 2022 15(10)doi:10.3390/ph15101224
315. He W, Huang J, Liu Y. et al. Deletion of soluble epoxide hydrolase suppressed chronic kidney disease-related vascular calcification by restoring Sirtuin 3 expression. Cell Death Dis. 2021;12(11):992 doi:10.1038/s41419-021-04283-6
316. Zhou J-X, Peng Z-X, Zheng Z-Y, Ni H-G. Big picture thinking of global PM2.5-related COPD: Spatiotemporal trend, driving force, minimal burden and economic loss. J Hazard Mater. 2025;488:137321 doi:10.1016/j.jhazmat.2025.137321
317. Zhong S, Yang L, Liu N. et al. Identification and validation of aging-related genes in COPD based on bioinformatics analysis. Aging (Albany NY). 2022;14(10):4336-4356 doi:10.18632/aging.204064
318. Zhou J-S, Li Z-Y, Xu X-C. et al. Cigarette smoke-initiated autoimmunity facilitates sensitisation to elastin-induced COPD-like pathologies in mice. Eur Respir J. 2020 56(3)doi:10.1183/13993003.00404-2020
319. Ito S, Araya J, Kurita Y. et al. PARK2-mediated mitophagy is involved in regulation of HBEC senescence in COPD pathogenesis. Autophagy. 2015;11(3):547-559 doi:10.1080/15548627.2015.1017190
320. Meyer A, Zoll J, Charles AL. et al. Skeletal muscle mitochondrial dysfunction during chronic obstructive pulmonary disease: central actor and therapeutic target. Exp Physiol. 2013;98(6):1063-1078 doi:10.1113/expphysiol.2012.069468
321. Piao Y, Yun SY, Fu Z. et al. Recombinant Human HAPLN1 Mitigates Pulmonary Emphysema by Increasing TGF-β Receptor I and Sirtuins Levels in Human Alveolar Epithelial Cells. Mol Cells. 2023;46(9):558-572 doi:10.14348/molcells.2023.0097
322. Rato L, Duarte AI, Tomás GD. et al. Pre-diabetes alters testicular PGC1-α/SIRT3 axis modulating mitochondrial bioenergetics and oxidative stress. Biochim Biophys Acta. 2014;1837(3):335-344 doi:10.1016/j.bbabio.2013.12.008
323. Zhang M, Zhang Y, Roth M. et al. Sirtuin 3 Inhibits Airway Epithelial Mitochondrial Oxidative Stress in Cigarette Smoke-Induced COPD. Oxid Med Cell Longev. 2020;2020:7582980 doi:10.1155/2020/7582980
324. Liao S, Chen D, Long H. et al. Hydrogen sulfide attenuates oxidative stress-induced cellular senescence via the Sirt3/SOD2 signaling pathway in chronic obstructive pulmonary disease. Chin Med J (Engl). 2025 doi:10.1097/CM9.0000000000003452
325. Zi Y, Wang X, Zi Y. et al. Cigarette smoke induces the ROS accumulation and iNOS activation through deactivation of Nrf-2/SIRT3 axis to mediate the human bronchial epithelium ferroptosis. Free Radic Biol Med. 2023;200:73-86 doi:10.1016/j.freeradbiomed.2023.03.002
326. Zuo X, Zhao R, Wu M. et al. Multi-omic profiling of sarcopenia identifies disrupted branched-chain amino acid catabolism as a causal mechanism and therapeutic target. Nat Aging. 2025;5(3):419-436 doi:10.1038/s43587-024-00797-8
327. Wei X, Li H, Qiu J. et al. Tree shrew as a new animal model for musculoskeletal disorders and aging. Bone Res. 2025;13(1):5 doi:10.1038/s41413-024-00367-z
328. Xiang Q, Wu Z, Zhao Y. et al. Cellular and molecular mechanisms underlying obesity in degenerative spine and joint diseases. Bone Res. 2024;12(1):71 doi:10.1038/s41413-024-00388-8
329. Zhang G-Z, Deng Y-J, Xie Q-Q. et al. Sirtuins and intervertebral disc degeneration: Roles in inflammation, oxidative stress, and mitochondrial function. Clin Chim Acta. 2020;508:33-42 doi:10.1016/j.cca.2020.04.016
330. Zhou TY, Wu YG, Zhang YZ, Bao YW, Zhao Y. SIRT3 retards intervertebral disc degeneration by anti-oxidative stress by activating the SIRT3/FOXO3/SOD2 signaling pathway. Eur Rev Med Pharmacol Sci. 2019;23(21):9180-9188 doi:10.26355/eurrev_201911_19408
331. Sun K, Shi Y, Yan C. et al. Glycolysis-Derived Lactate Induces ACSL4 Expression and Lactylation to Activate Ferroptosis during Intervertebral Disc Degeneration. Adv Sci (Weinh). 2025;12(21):e2416149 doi:10.1002/advs.202416149
332. Lin J, Du J, Wu X. et al. SIRT3 mitigates intervertebral disc degeneration by delaying oxidative stress-induced senescence of nucleus pulposus cells. J Cell Physiol. 2021;236(9):6441-6456 doi:10.1002/jcp.30319
333. Silwal P, Nguyen-Thai AM, Mohammad HA. et al. Cellular Senescence in Intervertebral Disc Aging and Degeneration: Molecular Mechanisms and Potential Therapeutic Opportunities. Biomolecules. 2023 13(4)doi:10.3390/biom13040686
334. Yang Y, Guo J, Cao H. et al. Seeds-and-soil inspired hydrogel microspheres: A dual-action antioxidant and cellular therapy for reversing intervertebral disc degeneration. Biomaterials. 2025;321:123326 doi:10.1016/j.biomaterials.2025.123326
335. Song Y, Li S, Geng W. et al. Sirtuin 3-dependent mitochondrial redox homeostasis protects against AGEs-induced intervertebral disc degeneration. Redox Biol. 2018;19:339-353 doi:10.1016/j.redox.2018.09.006
336. Hu B, Wang P, Zhang S. et al. HSP70 attenuates compression-induced apoptosis of nucleus pulposus cells by suppressing mitochondrial fission via upregulating the expression of SIRT3. Exp Mol Med. 2022;54(3):309-323 doi:10.1038/s12276-022-00745-9
337. Gambarotto L, Metti S, Chrisam M. et al. Ambra1 deficiency impairs mitophagy in skeletal muscle. J Cachexia Sarcopenia Muscle. 2022;13(4):2211-2224 doi:10.1002/jcsm.13010
338. Zhang Y, Liu Y, Hou M. et al. Reprogramming of Mitochondrial Respiratory Chain Complex by Targeting SIRT3-COX4I2 Axis Attenuates Osteoarthritis Progression. Adv Sci (Weinh). 2023;10(10):e2206144 doi:10.1002/advs.202206144
339. Zhao Y, Lin D, Zhu X. et al. SDF-1 alleviates osteoarthritis by resolving mitochondrial dysfunction through the activation of the Sirt3/PGC-1α signalling pathway. Arthritis Res Ther. 2025;27(1):51 doi:10.1186/s13075-025-03509-8
340. Wang G-E, Liu X-T, Yang F. et al. Biochanin A ameliorated oleate-induced steatosis in HepG2 cells by activating the SIRT3/AMPK/ULK-1 signaling pathway. J Food Biochem. 2022;46(12):e14428 doi:10.1111/jfbc.14428
341. López de Figueroa P, Lotz MK, Blanco FJ, Caramés B. Autophagy activation and protection from mitochondrial dysfunction in human chondrocytes. Arthritis Rheumatol. 2015;67(4):966-976 doi:10.1002/art.39025
342. Yu W, Gao B, Li N. et al. Sirt3 deficiency exacerbates diabetic cardiac dysfunction: Role of Foxo3A-Parkin-mediated mitophagy. Biochim Biophys Acta Mol Basis Dis. 2017;1863(8):1973-1983 doi:10.1016/j.bbadis.2016.10.021
343. Lepetsos P, Papavassiliou AG. ROS/oxidative stress signaling in osteoarthritis. Biochim Biophys Acta. 2016;1862(4):576-591 doi:10.1016/j.bbadis.2016.01.003
344. He Y, Wu Z, Xu L. et al. The role of SIRT3-mediated mitochondrial homeostasis in osteoarthritis. Cell Mol Life Sci. 2020;77(19):3729-3743 doi:10.1007/s00018-020-03497-9
345. Fu Y, Kinter M, Hudson J. et al. Aging Promotes Sirtuin 3-Dependent Cartilage Superoxide Dismutase 2 Acetylation and Osteoarthritis. Arthritis Rheumatol. 2016;68(8):1887-1898 doi:10.1002/art.39618
346. Zhu X, Chen F, Lu K, Wei A, Jiang Q, Cao W. PPARγ preservation via promoter demethylation alleviates osteoarthritis in mice. Ann Rheum Dis. 2019;78(10):1420-1429 doi:10.1136/annrheumdis-2018-214940
347. Long D, Deng Z, Zhao X. et al. m7G-modified mt-tRF3b-LeuTAA regulates mitophagy and metabolic reprogramming via SUMOylation of SIRT3 in chondrocytes. Biomaterials. 2025;314:122903 doi:10.1016/j.biomaterials.2024.122903
348. Benigni A, Perico L, Macconi D. Mitochondrial Dynamics Is Linked to Longevity and Protects from End-Organ Injury: The Emerging Role of Sirtuin 3. Antioxid Redox Signal. 2016;25(4):185-199 doi:10.1089/ars.2016.6682
349. Lin FR. Age-Related Hearing Loss. N Engl J Med. 2024;390(16):1505-1512 doi:10.1056/NEJMcp2306778
350. Han C, Someya S. Maintaining good hearing: calorie restriction, Sirt3, and glutathione. Exp Gerontol. 2013;48(10):1091-1095 doi:10.1016/j.exger.2013.02.014
351. Fowler CG, Chiasson KB, Colman RJ, Kemnitz JW, Beasley TM, Weindruch RH. Hyperinsulinemia/diabetes, hearing, and aging in the University of Wisconsin calorie restriction monkeys. Hear Res. 2015;328:78-86 doi:10.1016/j.heares.2015.07.001
352. Yu W, Dittenhafer-Reed KE, Denu JM. SIRT3 protein deacetylates isocitrate dehydrogenase 2 (IDH2) and regulates mitochondrial redox status. J Biol Chem. 2012;287(17):14078-14086 doi:10.1074/jbc.M112.355206
353. Someya S, Yu W, Hallows WC. et al. Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell. 2010;143(5):802-812 doi:10.1016/j.cell.2010.10.002
354. Zheng X, Gao J, Zhao M. et al. Honokiol attenuates mitochondrial fission and cell apoptosis by activating Sirt3 in intracerebral hemorrhage. Chin Med J (Engl). 2023;136(6):719-731 doi:10.1097/CM9.0000000000002178
355. Huang X, Gou H, Xie J. et al. Sirt3 Rescues Porphyromonas gingivalis-Impaired Cementogenesis via SOD2 Deacetylation. Cell Prolif. 2025:e70022. doi:10.1111/cpr.70022.
356. Zhou Q, Yi G, Chang M. et al. Activation of Sirtuin3 by honokiol ameliorates alveolar epithelial cell senescence in experimental silicosis via the cGAS-STING pathway. Redox Biol. 2024;74:103224 doi:10.1016/j.redox.2024.103224
357. Chen C, Zhang Q-W, Ye Y, Lin L-G. Honokiol: A naturally occurring lignan with pleiotropic bioactivities. Chin J Nat Med. 2021;19(7):481-490 doi:10.1016/S1875-5364(21)60047-X
358. Lin J-W, Chen J-T, Hong C-Y. et al. Honokiol traverses the blood-brain barrier and induces apoptosis of neuroblastoma cells via an intrinsic bax-mitochondrion-cytochrome c-caspase protease pathway. Neuro Oncol. 2012;14(3):302-314 doi:10.1093/neuonc/nor217
359. Wu X, Luo J, Liu H, Cui W, Feng D, Qu Y. SIRT3 protects against early brain injury following subarachnoid hemorrhage via promoting mitochondrial fusion in an AMPK dependent manner. Chin Neurosurg J. 2020;6:1 doi:10.1186/s41016-019-0182-7
360. Chen C-M, Liu S-H, Lin-Shiau S-Y. Honokiol, a neuroprotectant against mouse cerebral ischaemia, mediated by preserving Na+, K+-ATPase activity and mitochondrial functions. Basic Clin Pharmacol Toxicol. 2007;101(2):108-116
361. Zeng L, Hu P, Wang X. et al. Sirtuin-3 activation by honokiol attenuated anesthesia/surgery-induced cognitive impairment and neuronal ferroptosis via inhibiting mitochondrial GPX4 acetylation. J Nanobiotechnology. 2025;23(1):414 doi:10.1186/s12951-025-03502-y
362. Zhang Y, Wen P, Luo J. et al. Sirtuin 3 regulates mitochondrial protein acetylation and metabolism in tubular epithelial cells during renal fibrosis. Cell Death Dis. 2021;12(9):847 doi:10.1038/s41419-021-04134-4
363. Wang X, Shen T, Lian J. et al. Resveratrol reduces ROS-induced ferroptosis by activating SIRT3 and compensating the GSH/GPX4 pathway. Mol Med. 2023;29(1):137 doi:10.1186/s10020-023-00730-6
364. Zhou X, Chen M, Zeng X. et al. Resveratrol regulates mitochondrial reactive oxygen species homeostasis through Sirt3 signaling pathway in human vascular endothelial cells. Cell Death Dis. 2014;5(12):e1576 doi:10.1038/cddis.2014.530
365. Zhong J, Fang G-Y, Wang Z-X, Chen P, Lu D-Y, Shi X-D. Yishen Huoxue decoction attenuates unilateral ureteric obstruction-induced renal fibrosis and hypoxia-induced reactive oxygen species generation via adenosine monophosphate-activated protein kinase / peroxisome proliferator-activated receptor coactivator-1α / silent mating-type information regulation 2 homolog 3 pathway. J Tradit Chin Med. 2021;41(6):875-882 doi:10.19852/j.cnki.jtcm.2021.06.006
366. Mathieu L, Lopes Costa A, Le Bachelier C. et al. Resveratrol attenuates oxidative stress in mitochondrial Complex I deficiency: Involvement of SIRT3. Free Radic Biol Med. 2016;96:190-198 doi:10.1016/j.freeradbiomed.2016.04.027
367. Zheng M, Bai Y, Sun X. et al. Resveratrol Reestablishes Mitochondrial Quality Control in Myocardial Ischemia/Reperfusion Injury through Sirt1/Sirt3-Mfn2-Parkin-PGC-1α Pathway. Molecules. 2022 27(17)doi:10.3390/molecules27175545
368. Zeng Z, Yang Y, Dai X. et al. Polydatin ameliorates injury to the small intestine induced by hemorrhagic shock via SIRT3 activation-mediated mitochondrial protection. Expert Opin Ther Targets. 2016;20(6):645-652 doi:10.1080/14728222.2016.1177023
369. Fu B, Zhao J, Peng W, Wu H, Zhang Y. Resveratrol rescues cadmium-induced mitochondrial injury by enhancing transcriptional regulation of PGC-1α and SOD2 via the Sirt3/FoxO3a pathway in TCMK-1 cells. Biochem Biophys Res Commun. 2017;486(1):198-204 doi:10.1016/j.bbrc.2017.03.027
370. Xu S, Gao Y, Zhang Q. et al. SIRT1/3 Activation by Resveratrol Attenuates Acute Kidney Injury in a Septic Rat Model. Oxid Med Cell Longev. 2016;2016:7296092 doi:10.1155/2016/7296092
371. Ortega-Domínguez B, Aparicio-Trejo OE, García-Arroyo FE. et al. Curcumin prevents cisplatin-induced renal alterations in mitochondrial bioenergetics and dynamic. Food Chem Toxicol. 2017;107(Pt A):373-385 doi:10.1016/j.fct.2017.07.018
372. Li Y, Ye Z, Lai W. et al. Activation of Sirtuin 3 by Silybin Attenuates Mitochondrial Dysfunction in Cisplatin-induced Acute Kidney Injury. Front Pharmacol. 2017;8:178 doi:10.3389/fphar.2017.00178
373. Yuan L, Yang J, Li Y. et al. Matrine alleviates cisplatin-induced acute kidney injury by inhibiting mitochondrial dysfunction and inflammation via SIRT3/OPA1 pathway. J Cell Mol Med. 2022;26(13):3702-3715 doi:10.1111/jcmm.17398
374. Lu Q, Lin X, Wu J, Wang B. Matrine attenuates cardiomyocyte ischemia-reperfusion injury through activating AMPK/Sirt3 signaling pathway. J Recept Signal Transduct Res. 2021;41(5):488-493 doi:10.1080/10799893.2020.1828914
375. Zhou M, Dai Y, Ma Y. et al. Protective Effects of Liquiritigenin against Cisplatin-Induced Nephrotoxicity via NRF2/SIRT3-Mediated Improvement of Mitochondrial Function. Molecules. 2022 27(12)doi:10.3390/molecules27123823
376. Xie J, Zhong F, Guo Z. et al. Hyperinsulinemia impairs the metabolic switch to ketone body utilization in proximal renal tubular epithelial cells under energy crisis via the inhibition of the SIRT3/SMCT1 pathway. Front Endocrinol (Lausanne). 2022;13:960835 doi:10.3389/fendo.2022.960835
377. Tao L, Park J-Y, Lambert JD. Differential prooxidative effects of the green tea polyphenol, (-)-epigallocatechin-3-gallate, in normal and oral cancer cells are related to differences in sirtuin 3 signaling. Mol Nutr Food Res. 2015;59(2):203-211 doi:10.1002/mnfr.201400485
378. Ogura Y, Kitada M, Xu J, Monno I, Koya D. CD38 inhibition by apigenin ameliorates mitochondrial oxidative stress through restoration of the intracellular NAD+/NADH ratio and Sirt3 activity in renal tubular cells in diabetic rats. Aging (Albany NY). 2020;12(12):11325-11336 doi:10.18632/aging.103410
379. Peng F, Liao M, Jin W. et al. 2-APQC, a small-molecule activator of Sirtuin-3 (SIRT3), alleviates myocardial hypertrophy and fibrosis by regulating mitochondrial homeostasis. Signal Transduct Target Ther. 2024;9(1):133 doi:10.1038/s41392-024-01816-1
380. Reiter RJ, Tan DX, Rosales-Corral S, Galano A, Jou M-J, Acuna-Castroviejo D. Melatonin Mitigates Mitochondrial Meltdown: Interactions with SIRT3. Int J Mol Sci. 2018 19(8)doi:10.3390/ijms19082439
381. Xu S, Li L, Wu J. et al. Melatonin Attenuates Sepsis-Induced Small-Intestine Injury by Upregulating SIRT3-Mediated Oxidative-Stress Inhibition, Mitochondrial Protection, and Autophagy Induction. Front Immunol. 2021;12:625627 doi:10.3389/fimmu.2021.625627
382. Zhang C, Suo M, Liu L. et al. Melatonin Alleviates Contrast-Induced Acute Kidney Injury by Activation of Sirt3. Oxid Med Cell Longev. 2021;2021:6668887 doi:10.1155/2021/6668887
383. Zhang M, Lin J, Wang S. et al. Melatonin protects against diabetic cardiomyopathy through Mst1/Sirt3 signaling. J Pineal Res. 2017 63(2)doi:10.1111/jpi.12418
384. Sundaresan NR, Samant SA, Pillai VB, Rajamohan SB, Gupta MP. SIRT3 is a stress-responsive deacetylase in cardiomyocytes that protects cells from stress-mediated cell death by deacetylation of Ku70. Mol Cell Biol. 2008;28(20):6384-6401 doi:10.1128/MCB.00426-08
385. Cong L, Liu X, Bai Y. et al. Melatonin alleviates pyroptosis by regulating the SIRT3/FOXO3α/ROS axis and interacting with apoptosis in Atherosclerosis progression. Biol Res. 2023;56(1):62 doi:10.1186/s40659-023-00479-6
386. Yu L, Gong B, Duan W. et al. Melatonin ameliorates myocardial ischemia/reperfusion injury in type 1 diabetic rats by preserving mitochondrial function: role of AMPK-PGC-1α-SIRT3 signaling. Sci Rep. 2017;7:41337 doi:10.1038/srep41337
387. Bi T, Cui Y, Liu S. et al. Ligand-Enabled Pd-Catalyzed sp3 C-H Macrocyclization: Synthesis and Evaluation of Macrocyclic Sulfonamide for the Treatment of Parkinson's Disease. Angew Chem Int Ed Engl. 2024;63(45):e202412296 doi:10.1002/anie.202412296
388. Pan JS-C, Huang L, Belousova T. et al. Stanniocalcin-1 inhibits renal ischemia/reperfusion injury via an AMP-activated protein kinase-dependent pathway. J Am Soc Nephrol. 2015;26(2):364-378 doi:10.1681/ASN.2013070703
389. Liu Z, Liu H, Xiao L, Liu G, Sun L, He L. STC-1 ameliorates renal injury in diabetic nephropathy by inhibiting the expression of BNIP3 through the AMPK/SIRT3 pathway. Lab Invest. 2019;99(5):684-697 doi:10.1038/s41374-018-0176-7
390. Suliman H, Ma Q, Zhang Z. et al. Annexin A1 Tripeptide Mimetic Increases Sirtuin-3 and Augments Mitochondrial Function to Limit Ischemic Kidney Injury. Front Physiol. 2021;12:683098 doi:10.3389/fphys.2021.683098
Author contact
![]() Corresponding author: Zhong Wang, Department of General Practice, Beijing Tsinghua Changgung Hospital, School of Clinical Medicine, Tsinghua University, Beijing 100084, China. Email: wangzhong523163.com.
Corresponding author: Zhong Wang, Department of General Practice, Beijing Tsinghua Changgung Hospital, School of Clinical Medicine, Tsinghua University, Beijing 100084, China. Email: wangzhong523163.com.

 Global reach, higher impact
Global reach, higher impact