10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2019; 15(6):1215-1224. doi:10.7150/ijbs.29741 This issue Cite
Research Paper
The Nuclear Export and Ubiquitin-Proteasome-Dependent Degradation of PPARγ Induced By Angiotensin II
1. Tianjin Medical University General Hospital; Tianjin Neurological Institute; Key Laboratory of Post-trauma Neuro-repair and Regeneration in Central Nervous System, Ministry of Education and Tianjin City, Tianjin, 300052, PR China
2. Murad Research Institute for Modernized Chinese Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai, 201203, PR China
3. Department of Biochemistry and Molecular Medicine, The George Washington University, Ross Hall 2300 Eye Street, NW, Washington, DC 20037, USA
Abstract
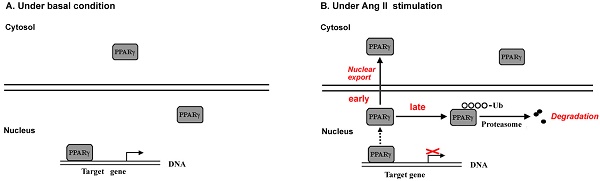
Evidence has documented local angiotensin II (Ang II) as a pro-oxidant and pro-inflammatory molecule contributes to progressive deterioration of organ function in diseases. Peroxisome proliferator-activated receptor γ (PPARγ), a ligand-activated transcription factor, plays crucial roles in protection against oxidative stress and inflammation. Ang II stimulation decreases PPARγ protein in multiple types of cells, while the regulatory role of Ang II on PPARγ is not clear. Here we show that Ang II down-regulated PPARγ in ECV304 cells through 2 actions, inducing nuclear export and loss of protein. The nuclear export of PPARγ occurred transiently in the early phase, while the reduction in PPARγ protein happened in the later phase and was more persistent. Both alterations in PPARγ were accompanied by the decrease in PPARγ-DNA binding activity. Reduction of PPARγ protein levels was also coupled with the inhibition of PPARγ target genes. In addition, activation of PPARγ by its ligand troglitazone could completely counteract both 2 actions of Ang II on PPARγ. Further studies demonstrated that the decline of PPARγ protein was in association with ubiquitin-proteasome-dependent degradation, which was supported by the increase in polyubiquitin-PPARγ conjugates and the inhibitory effect of lactacystin, a specific proteasome inhibitor, on the loss of PPARγ. Taken together, this study uncovers a novel means by which Ang II down-regulates PPARγ. This down-regulation disrupts nuclear PPARγ function, which may lead to the loss of beneficial effects of PPARγ in response to Ang II stress.
Keywords: angiotensin II, peroxisome proliferator-activated receptor γ, nuclear export, proteasome, ubiquitin

 Global reach, higher impact
Global reach, higher impact