10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2023; 19(3):897-915. doi:10.7150/ijbs.81609 This issue Cite
Review
An Overview: The Diversified Role of Mitochondria in Cancer Metabolism
1. Tongji University Cancer Center, Shanghai Tenth People's Hospital of Tongji University, School of Medicine, Tongji University, Shanghai 200092, China.
2. Department of Urology, Shanghai Tenth People's Hospital, School of Medicine, Tongji University, Shanghai, China.
3. Institute of Artificial Intelligence, Hefei Comprehensive National Science Center, Hefei, China.
4. Xi Chen, Children's Nutrition Research Center, Department of Pediatrics, Baylor College of Medicine, Houston, TX, 77030, USA.
5. Department of Thoracic Surgery, Shanghai Pulmonary Hospital, School of Medicine, Tongji University, 200433, Shanghai, China.
6. Department of Pharmacology and Toxicology, University of Mississippi Medical Center, 39216, Jackson, Mississippi, USA.
7. Department of Pathology and Medical Biology, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands.
8. Department of Critical Care Medicine, Sichuan Academy of Medical Science and Sichuan Provincial People's Hospital, University of Electronic Science and Technology of China, Chengdu, China.
9. Clinical Center for Brain and Spinal Cord Research, Tongji University, Shanghai 200092, China.
#These authors contributed equally to this work.
Abstract
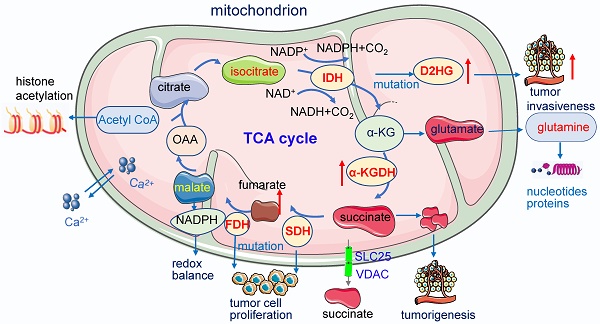
Mitochondria are intracellular organelles involved in energy production, cell metabolism and cell signaling. They are essential not only in the process of ATP synthesis, lipid metabolism and nucleic acid metabolism, but also in tumor development and metastasis. Mutations in mtDNA are commonly found in cancer cells to promote the rewiring of bioenergetics and biosynthesis, various metabolites especially oncometabolites in mitochondria regulate tumor metabolism and progression. And mutation of enzymes in the TCA cycle leads to the unusual accumulation of certain metabolites and oncometabolites. Mitochondria have been demonstrated as the target for cancer treatment. Cancer cells rely on two main energy resources: oxidative phosphorylation (OXPHOS) and glycolysis. By manipulating OXPHOS genes or adjusting the metabolites production in mitochondria, tumor growth can be restrained. For example, enhanced complex I activity increases NAD+/NADH to prevent metastasis and progression of cancers. In this review, we discussed mitochondrial function in cancer cell metabolism and specially explored the unique role of mitochondria in cancer stem cells and the tumor microenvironment. Targeting the OXPHOS pathway and mitochondria-related metabolism emerging as a potential therapeutic strategy for various cancers.
Keywords: mitochondria, cancer, tumor metastasis, tumor metabolism

 Global reach, higher impact
Global reach, higher impact