10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2025; 21(9):4098-4116. doi:10.7150/ijbs.115932 This issue Cite
Review
Orchestration of Tumor-Associated Macrophages in the Tumor Cell-Macrophage-CD8+ T Cell Loop for Cancer Immunotherapy
1. Cancer Center, Faculty of Health Sciences, University of Macau, Macau SAR, China.
2. Centre for Precision Medicine Research and Training, Faculty of Health Sciences, University of Macau, Macau SAR, China.
3. MOE Frontier Science Centre for Precision Oncology, University of Macau, Macau SAR, China.
4. Medical Sciences Division, Macau University of Science and Technology, Macau SAR, China.
5. Precision Regenerative Medicine Research Centre, Macau University of Science and Technology, Macau SAR, China.
Received 2025-4-17; Accepted 2025-5-24; Published 2025-6-12
Abstract
The tumor microenvironment is densely populated with tumor-associated macrophages (TAMs), which exhibit various phenotypes at different stages of tumor progression. TAMs are highly plastic and intricately linked to the antitumor activity and functionality of CD8+ T cells. Tumor cells, TAMs and CD8+ T cells constitute a feedback loop that monitors the tumor immune surveillance. Modulation of several chief signaling pathways within TAMs can steer them towards either an immunoinflammatory or immunosuppressive state. This can be achieved indirectly through cancer therapies or by directly targeting TAMs. New detailed insights into the immunostimulatory reprogramming of TAMs inspire the design of novel combinatory strategies that can be extrapolated to bolster cancer immunotherapy.
Keywords: tumor-associated macrophage, CD8+ T cell, tumor microenvironment, cancer immunotherapy
1. Introduction
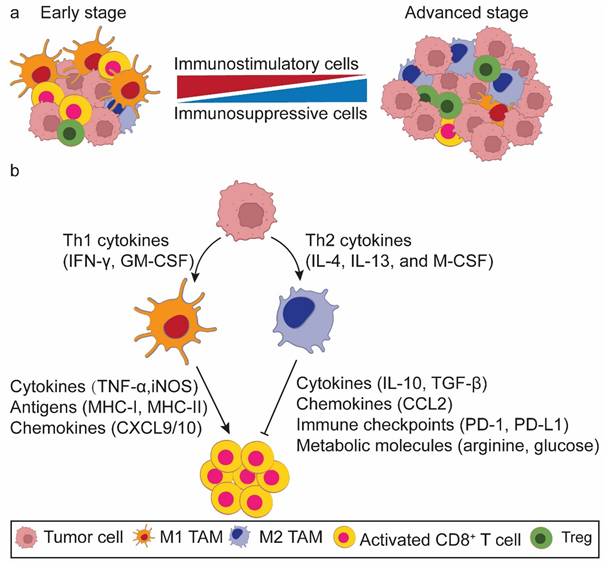
Immune cells are generally classified into innate and adaptive immune cells, with macrophages and T cells mapping to main representatives of each category, respectively. Macrophages serve as the body's first line of defense against pathogens and cancers[1]. They directly eliminate senescent cells and tumor cells through phagocytosis or indirectly kill them via revitalizing CD8+ T cells. The phagocytosis of macrophages is reliant on “eat me” and “don't eat me” signals[2]. Tumor cells frequently overexpress CD47, which, upon binding to SIRPα on the surface of macrophages, transmits a “don't eat me” signal. This interaction between CD47 and SIRPα attenuates the phagocytic activity of macrophages, enabling tumor cells to obviate destruction[3]. As such, macrophages further partake in both innate and adaptive immunity[4]. These immune cells play drastically distinct roles at different stages of tumor progression by altering their phenotypes. In the early stage a high proportion of proinflammatory tumor-associated macrophages (M1 TAMs) and activated CD8+ T cells accumulate to remove the initially formed tumor cells. Conversely, in the late stage the TME is characterized by an increasing body of immunosuppressive TAMs (M2 TAMs) and exhausted CD8+ T cells, which promote tumor progression and metastasis. M1 TAMs potentiate T cell responses, whereas M2 TAMs attenuate T cell function and cancer immune surveillance within the TME (Figure 1a). Therefore, TAMs play dual roles in immune activation and suppression[5, 6]. It is important to note that macrophage phenotypes exist along as a dynamic continuum rather than as rigid binary classification. This spectrum reflects their functional plasticity in response to microenvironmental cues.
Macrophages are highly plastic immune cells within the TME[7, 8]. Lipopolysaccharide (LPS), either alone or in combination with Th1 cytokines such as interferon-γ (IFN-γ) and granulocyte-macrophage colony-stimulating factor (GM-CSF), facilitates the polarization of TAMs towards the M1 phenotype. This polarization is characterized by the production of proinflammatory cytokines [interlukin-6 (IL-6), IFN-γ, tumor necrosis factor-α (TNFα), and inducible nitric oxide synthase (iNOS)], augmented antigen presentation, and heightened phagocytic activity[9]. Conversely, Th2 cytokines such as IL-4, IL-13, and macrophage colony-stimulating factor (M-CSF) reprogram TAMs towards the M2 phenotype, leading to the secretion of anti-inflammatory cytokines like IL-10 and transforming growth factor-β (TGF-β), and elevated expression of CD163, CD206, and Arg-1[9].
Currently, it is envisaged that TAMs modulate the CD8+ T cell function through five principal underpinnings (Figure 1b): (1) cytokine secretion[10, 11], (2) antigen presentation[12], (3) chemokine production to recruit CD8+ T cells[13], (4) immune checkpoint modulation [e.g., programmed death receptor 1 (PD-1), programmed death ligand 1 (PD-L1), and B7-H3 (CD276)][12, 14, 15], and (5) metabolism modulation (e.g., arginine, glucose, and lactate)[14-16]. Ideally, TAMs and activated CD8+ T cells work synergistically to identify and eliminate primary and metastatic tumor cells, potentially maximizing the tumoricidal potency.
Interactions between tumor cells and immune cells play a crucial role in controlling tumorigenesis. Reprogramming immune cells within the TME may enhance the efficacy of cancer immunotherapy. In this review, we propose a tumor cell-macrophage-CD8+ T cell loop by wiring a connection between TAMs and CD8+ T cells across multiple tumor landscapes. Several crucial signaling pathways within TAMs that regulate their immunostimulatory or immunosuppressive polarization are further discussed. Orchestration of these signaling pathways can yield a myriad of treatment scenarios to overcome TAM-mediated immunosuppressive TME. Finally, we focus on strategies to immunostimulate the accumulation and functionality of TAMs and CD8+ T cells within the TME, exploring potential therapeutic avenues for combining immune checkpoint blockade (ICB) therapy.
A conceptual model of the tumor cell-macrophage-CD8+ T cell loop in the tumor microenvironment (TME). a. Alterations of macrophage phenotype and CD8+ T cell functionality in the TME during tumorigenesis. In the early stage of tumorigenesis, the TME is predominantly populated by M1 tumor associated macrophages (TAMs) and activated CD8+ T cells. Conversely, in the advanced stage, M2 TAMs and exhausted CD8+ T cells pervade the TME. b. Macrophages act as the bridge to orchestrates the interaction of tumor cells and CD8+ T cells. Tumor cells secrete an array of cytokines to educate the polarization state of TAMs. These TAMs, in turn, change the antitumor efficacy of CD8+ T cells via multiple mechanisms.
2. Tumor Cell-Macrophage-CD8+ T Cell Loop
Tumors with diverse gene expression demonstrate significant correlations with the congregation of distinct phenotypes of TAMs and CD8+ T cells (Table 1). Elevated expression of MICAL2 in pancreatic cancer is associated with a heightened infiltration of M2 TAMs and a diminished presence of CD8+ T cells[17]. YBX1+ luminal breast cancer tissues demonstrate substantial infiltration of M2 TAMs and elevated expression of T cell exhaustion markers, such as indoleamine 2,3-dioxygenase 1 and cytotoxic T-lymphocyte-associated protein 4 (CTLA4)[18]. In patients with intrahepatic cholangiocarcinoma, GM-CSFRα is positively correlated with CD8+ T cell infiltration, while inversely associated with the presence of M2 TAMs and myeloid-derived suppressor cells (MDSCs)[19]. KCTD5 shows a positive correlation with the accumulation of CD8+ T cells and TAMs in patients with lung adenocarcinoma (LUAD)[20]. Breast cancers with overexpression of ARHGAP39 exhibit low infiltration levels of CD8+ T cells and macrophages, but high infiltration of CD4+ T cells[21]. OPN deficiency in glioma cells reduces M2 TAM populations and increases CD8+ T cell cytotoxicity[22]. MTA1-overexpressing colon cancer weakens CD8+ T cell responses by inducing TAMs[23]. In hepatocellular carcinoma (HCC) tissues with high MISP expression, M2 TAMs are scarce, while CD8+ T cells are diffusely infiltrated[24]. GPC3-overexpressing ovarian tumor enhances M1 TAM infiltration and triggers a specific CD8+ T cell response, thereby improving the long-term survival of mice[25].
The correlation of macrophages and CD8+ T cells in different cancer types.
| Cancer subtype | Macrophage status | T cell status | Refs |
|---|---|---|---|
| MICAL2 in pancreatic cancer | Increased presence of M2 TAMs | Decreased presence of CD8+ T cells | 17 |
| YBX1+ luminal breast cancer | Increased presence of M2 TAMs | Increased T cell exhaustion | 18 |
| GM-CSFRα+ Intrahepatic cholangiocarcinoma | Decreased presence of M2 TAMs | Increased presence of CD8+ T cells | 19 |
| KCTD5+ lung adenocarcinoma | Increased presence of TAMs | Increased presence of CD8+ T cells | 20 |
| ARHGAP39+ breast cancer | Decreased presence of TAMs | Decreased presence of CD8+ T cells | 21 |
| OPN- glioma | Decreased presence of M2 TAMs | Increased CD8+ T cells cytotoxicity | 22 |
| MTA1+ Colon Cancer | Increased presence of TAMs | Decreased CD8+ T cells cytotoxicity | 23 |
| MISP+ hepatocellular carcinoma | Decreased presence of M2 TAMs | Increased presence of CD8+ T cells | 24 |
| GPC3+ ovarian cancer | Increased presence of M1 TAMs | Increased CD8+ T cells cytotoxicity | 25 |
Given the proinflammatory and anti-inflammatory characteristics, specific molecular biomarkers for TAMs have been delineated through advanced molecular biology techniques. These newly characterized TAMs exhibit a robust correlation with the presence of CD8+ T cells in different tumor types. TIM4+ macrophages are predominantly located within the T-cell zones of tertiary lymphoid structures associated with various malignancies, showing a positive correlation with CD8+ T cell infiltration[26]. CD163+ M2 TAMs accumulate within the stromal compartments at the tumor-stroma interface of clear cell renal cell carcinoma and are positively correlated with an increased proportion of TIM3+CD8+ T cells, indicating terminal exhaustion[27]. JMJD8+ M2 TAMs exhibit a positive correlation with the presence of immunosuppressive cells and the suppression of CD8+ T cell function across various cancer types[28].
Cumulatively, modulating the expression levels of specific genes in tumor cells can profoundly influence the TAM phenotype and CD8+ T cell antitumor immunity. Likewise, genetic alterations in TAMs closely changes CD8+ T cell presence and activation within the TME. Tumor cells, macrophages, and CD8+ T cells constitute a feedback loop that orchestrates tumor immune surveillance; wherein, TAMs are pivotal determinants. Consequently, the phenotypic modulation of TAMs is critical for either reversing or propelling tumor immune evasion.
Recent studies have elucidated valuable insights into the temporal and spatial heterogeneity of TAMs and their interactions with CD8+ T cells, which are pivotal for comprehending the cancer-TAM-CD8+ T cell loop and its role in tumor immunity. In the early stages of tumor development, the TME is characterized by the presence of M1 TAMs, activated CD8+ T cells, and proinflammatory cytokines, which can aid in controlling tumor growth[29]. As tumors progress, the TME undergoes profound alterations, leading to an immunosuppressive state. This shift is marked by the increased presence of regulatory T cells (Tregs), MDSCs, M2 TAMs, and immunosuppressive cytokines. These changes inhibit the function of CD8+ T cells and promote tumor growth and metastasis[29, 30].
The spatial distribution of TAMs within the TME can significantly modulate their interactions with CD8+ T cells. For example, TAMs located near blood vessels may have different functional properties compared to those in hypoxic regions of the tumor. These regional disparities can influence the recruitment, activation, and suppression of T cells[31]. Advanced techniques, such as single-cell RNA sequencing, have revealed the diversity of TAM subtypes and their distinct roles in various tumor regions. These studies have shown that TAMs can either potentiate or inhibit T cell function depending on their location and the specific signals they receive from the TME[32]. Reciprocally, immunotherapy-activated CD8+ T cells recruit TAMs to their vicinity via CCR5 signaling and educates them into M1 phenotypes. Therefore, effective immunotherapy requires coordinated functional modulation of both CD8+ T cells and TAMs[33]. Activated CD8+ T cells also secret cytokines to promote the M1 TAM polarization. Understanding the temporal and spatial heterogeneity of TAMs and their interactions with CD8+ T cells is essential for the development of effective cancer therapies.
3. M1 TAM Polarization Signaling Pathways
3.1. NF-κB signaling activation
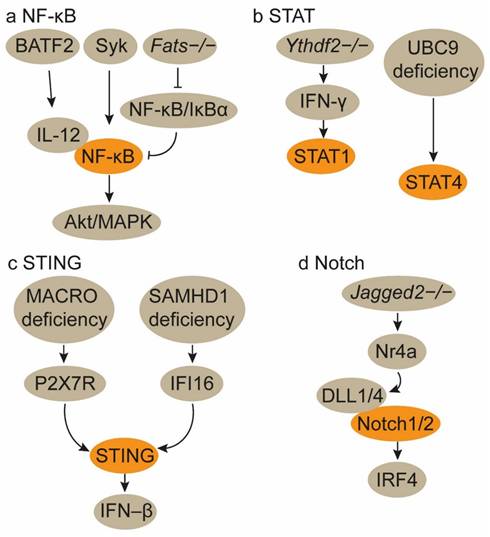
The basic leucine zipper transcription factor ATF-like 2 (Batf2) triggers CD8+ T cell antitumor immunity by upregulating IL-12 p40 in TAMs. Mechanistically, BATF2 interacts with p50 and p65 in macrophages, upregulating their activity in the nucleus. This interaction promotes IL-12 p40 expression in macrophages via nuclear factor kappa-B (NF-κB) binding site in the Il12b promoter[34]. ApoC3TG mice-derived macrophages, when co-cultured with CD8+ T cells, significantly enhance CD8+ T cell activation. ApoC3 binds to Toll-like receptor 2/4 (TLR2/TLR4), inducing the spleen tyrosine kinase (Syk) activation, which promotes inflammasome activation in macrophages through the NF-κB/Akt/MAPK and cPLA2/NOX2 pathways[35]. Fats-/- mice experience a shift of TAMs from an M2 to an M1 phenotype. This polarization is mediated by the stimulation and prolonged activation of NF-κB through the disruption of NF-κB/IκBα negative feedback loops, thereby enhancing the adaptive immune response of CD8+ T cells[36]. Morus alba L. fruit extract enhances the secretion of inflammatory cytokines and promotes M1 polarization in macrophages through the TLR4 downstream MAPKinase and NF-κB signaling pathways. This, in turn, amplifies CD8+ T cell activity and IFN-γ production[37]. An H2 receptor antagonist Ranitidine may activate the phosphoinositide 3-kinase (PI3K)-Akt2 signal, which subsequently regulates the NF-κB and GSK3β/Dynamin1 pathways to promote TAM polarization to the M1 phenotype and enhance macrophage endocytosis (Figure 2a)[38]. These findings collectively demonstrate that upregulation of NF-κB signaling pathway favors M1 TAM polarization and augments TAM-specific CD8+ T cell antitumor immunity (Table 2).
However, in certain instances, the inactivation of the NF-κB signaling pathway is conducive to M1 TAM polarization and CD8+ T cell-mediated antitumor responses. In glioblastoma multiforme (GBM) mouse models, NF-κB p65 knockout significantly increases M1 TAMs and CD8+ T cells, while reducing the populations of M2 TAMs and MDSCs[39]. In vitro co-culture models demonstrate that abrogating myeloid cell-associated NF-κB signaling enhances T cell proliferation and activation, as well as educates M2 to M1 polarization[39].
Potential signaling pathways for reprogramming tumor-associated macrophages.
| M1 TAM polarization | M2 TAM polarization |
|---|---|
| NF-κB signaling activation35-38 | NF-κB signaling inactivation51-53 |
| STAT1/4 signaling activation40-42 | STAT3/6 signaling activation57, 59, 60, 68-71 |
| STING signaling activation43, 44 | Mincle pathway activation73, 74 |
| Notch1/2 signaling activation47 | Syk-PI3K signaling activation75-80 |
| PRR signaling activation48, 49 |
3.2. STAT1/4 signaling activation
The signal transducer and activator of transcription (STAT) family of transcription factors plays distinct roles in modulating TAMs and CD8+ T cells within the TME. Activation of the STAT1 and STAT4 pathways orchestrates TAMs towards M1 phenotypes (Table 2). The loss of Ythdf2 in TAMs reprograms them towards an antitumor phenotype and enhances their antigen presentation crosstalk to CD8+ T cells by upregulating IFN-γ-STAT1 signaling, thereby increasing CD8+ T cell responses (Figure 2b)[40]. C1q+ macrophages express a repertoire of immunomodulatory ligands via METTL14-YTHDF2 axis-mediated N6-methyladenosine (m6A) methylation on Ebi3, thereby preserving the functionality of CD8+ T cells. Consequently, Mettl14 or Ythdf2 deficiency in C1q+ macrophages thwarts cytotoxic CD8+ T cell infiltration and facilitates the accumulation of dysfunctional CD8+ T cells[41].
UBC9 in TAMs impedes their polarization towards the M1 phenotype by facilitating STAT4 SUMOylation. The targeted ablation of UBC9 in TAMs promotes M1 polarization and augments TAM-CD8+ T cell interactions, thereby amplifying CD8+ T cell responses (Figure 2b)[42].
3.3. STING signaling activation
MACRO inhibits type I IFN secretion and antigen presentation in TAMs. Mechanistically, MACRO diminishes the accumulation of tumor-derived cGAMP and ATP in the TME, thereby impeding P2X7R-mediated activation of the stimulator of interferon genes (STING)-IFN-β pathway. Utilizing anti-MACRO neutralizing antibodies can restore the phagocytic activity and antigen presentation capabilities of TAMs, leading to increased CD8+ T cell infiltration[43]. However, SAMHD1 deficiency in tumor cells triggers type I IFN production via the activation of the cytosolic IFI16-STING pathway, concurrently fostering the polarization of TAMs towards the M1 phenotype and augmenting CD8+ T cell accumulation (Figure 2c)[44]. Therefore, STING signaling activation in macrophages and cancer cells both primes type I IFN secretion and M1 TAM polarization (Table 2). RON expression in breast cancer tissues, which inhibits IRAK4-mediated type I IFN production, gives rise to sparse infiltration of macrophages and CD8+ T cells[45].
3.4. Notch1/2 signaling activation
Jagged1-expressing tumor cells activate the Notch signaling pathway, culminating in the secretion of pluralistic cytokines that facilitate TAM recruitment. These TAMs subsequently attenuate the proliferation and cytotoxicity of CD8+ T cells. Consequently, in triple-negative breast cancer models with elevated Jagged1 expression, TAM infiltration is pronounced while CD8+ T cell presence is markedly assuaged[46]. In non-small cell lung carcinoma models, the absence of Jagged2, rather than Jagged1, shields CD8+ T cell functionality. Jagged2-deficient lung cancers exhibit increased infiltration of M1 TAMs and CD8+ T cells. Mechanistically, deletion of Jagged2 triggers Nr4a-mediated induction of the Notch ligands DLL1/4 in cancer cells. DLL1/4 activates Notch1/2 signaling in macrophages, inducing the expression of the transcription factor IRF4 to sustain their immunostimulatory phenotype (Figure 2d)[47]. These findings suggest that Jagged1 and Jagged2 respectively modulate the Notch pathway in tumor cells and macrophages to maintain an immunosuppressive TME mediated by macrophages and CD8+ T cells (Table 2).
Signaling pathways for the polarization of TAMs towards the M1 phenotype. a. Activation of the NF-κB signaling pathway. b. Activation of the STAT1/4 signaling pathways. The activation of the NF-κB and STAT1/4 signaling pathways in TAMs induces their polarization towards the M1 phenotype. c. Activation of the STING signaling pathway. MACRO deficiency in TAMs promotes M1 polarization by activating the P2X7R-mediated STING-IFN-β pathway. Concurrently, the genetic deletion of SAMHD1 in tumor cells enhances the activation of the FI16-STING pathway, which subsequently polarizes TAMs to the M1 phenotype. d. Activation of the Notch pathway. Jagged 2-/- lung tumor cells trigger Nr4a-mediated induction of the Notch ligands DLL1/4 in cancer cells. DLL1/4 activates Notch1/2 signaling in macrophages, leading to M1 TAM polarization.
3.5. Pattern recognition receptor signaling activation
Pattern recognition receptors (PRRs), including TLRs, Nod-like receptors (NLRs), C-type lectin receptors (CLRs), and RIG-I-like receptors (RLRs), recognize specific pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) and play dual roles in macrophage polarization and immunomodulation[48]. PRRs orchestrate the immune response by regulating the production of cytokines and chemokines. M1 TAMs are induced by PRR signaling in response to microbial products and proinflammatory cytokines, leading to the production of inflammatory mediators like TNF-α and IL-6. Conversely, M2 TAMs are promoted by PRR signaling in response to anti-inflammatory signals, resulting in the production of anti-inflammatory cytokines like IL-10 and TGF-β[48, 49]. These regulatory mechanisms facilitate the recruitment and activation of other immune cells, such as T cells and dendritic cells, thereby shaping the overall immune response[49]. In the context of cancer, PRRs on TAMs can either promote or inhibit tumor progression. For example, PRR activation can lead to the production of cytokines that enhance antitumor immunity or, alternatively, create an immunosuppressive environment that supports tumor growth[50]. The role of PRRs in macrophages is crucial for developing targeted therapies that can modulate macrophage function in various diseases, including cancer.
4. M2 TAM Polarization Signaling Pathways
TAMs primarily appear as an M2 phenotype within the TME at late stages. This phenomenon is largely by virtue of the myriad of materials produced during tumor progression, which, upon embedding into macrophages, alter the activation states of several critical signaling pathways (Table 2). In turn, these M2 TAMs secrete extracellular vesicles and cytokines that foster tumor immune evasion.
4.1. NF-κB signaling inactivation
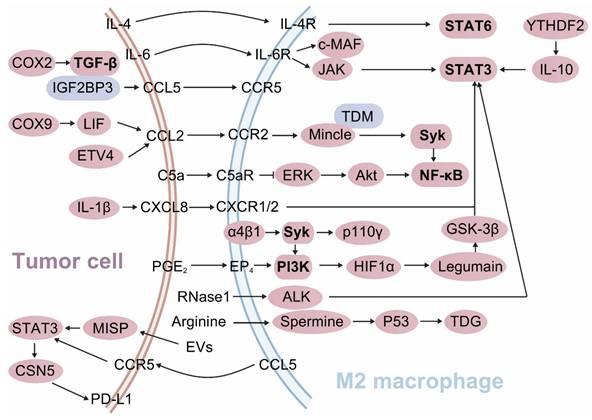
The complement component 5a-complement component 5a receptor (C5a-C5aR) axis modulates macrophage function and antitumor immunity. C5a facilitates M2 TAM infiltration and tumor cell metastasis. Conversely, C5aR deficiency reinstates TAM antitumor activity and augments TAM-mediated CD8+ T cell responses. Mechanistically, the C5a-C5aR axis suppresses macrophage C-X-C motif chemokine ligand 9 (CXCL9) secretion by activating C/EBPβ and inhibiting the ERK/Akt/NF-κB signaling pathways[51]. In macrophages, annexin A1 (ANXA1) promotes TAM polarization towards the M2 phenotype by downregulating the NF-κB and Notch1 pathways, while upregulating the JAK-STAT, Akt, and ERK pathways. ANXA1 deficiency increases the M1/M2 ratio and augments CD8+ T cell activation (Figure 3)[52]. Phospholipase A2 Group VII (PLA2G7) promotes the polarization of TAMs towards the M2 phenotype in HCC candidates by downregulating the NF-κB pathway, thereby suppressing CD8+ T cell responses[53].
The cyclooxygenase-2 (COX-2)-derived oncogenic promoter PGE2 augments the affinity of NF-κB to the PD-1 promoter in both macrophages and CD8+ T cells via the EP4-PI3K-Akt signaling cascade, thereby upregulating their PD-1 expression. Conversely, the EP4-PI3K-Akt signaling blockade enhances macrophage phagocytosis and CD8+ T cell proliferation and activation in CRC models[54]. However, in SIGLEC10+ macrophages, Akt/P38/Erk signaling activity is suppressed, which educates their polarization towards the M2 phenotype and mitigates the CD8+ T cell proliferation and activation (Figure 3)[55]. Akt phosphorylates MAPK-activating death domain protein, which subsequently activates Rab27a, leading to enhanced secretion of PD-L1-enriched exosomes from TAMs[56].
4.2. STAT3/6 signaling activation
Activation of the STAT3 and STAT6 signaling pathways induces M2 phenotype polarization and fosters an immunosuppressive TME. The STAT3 signaling pathway is crucial for maintaining the M2 phenotype of TAMs across various tumor models. Tumor cell-derived RNase1 induces the polarization of TAMs from the M1 to the M2 phenotype by activating ALK/STAT3 signaling and attenuating STAT1 phosphorylation[57]. In macrophages, the m6A reader YTHDF2 educates an anti-inflammatory phenotype by upregulating IL-10-STAT3 signaling[40]. Hypoxia inducible factor 1 α (HIF1α) transcriptionally upregulates Legumain in TAMs, which subsequently induces M2 polarization by activating the GSK-3β-STAT3 signaling pathway (Figure 3). Disrupting the HIF1α-Legumain axis can attenuate M2 TAM polarization and potentiate CD8+ T cell antitumor immunity[58]. YAP and STAT3 are overactivated and form a complex in breast cancers. Inhibition of the YAP/STAT3 complex induces M1 TAM polarization, curtails Tregs populations, and amplifies CD8+ T cell activity[59]. Progranulin markedly upregulates PD-L1 expression on macrophages and drives their polarization towards the M2 phenotype via the JAK/STAT3 signaling pathway. This process inhibits CD8+ T cell proliferation and activation through the PD-1/PD-L1 interaction. Conversely, this effect can be abrogated by the STAT3 inhibitor Stattic[60].
Within the senescent TME, the senescence-associated secretory phenotype-associated proinflammatory cytokine IL-6 modulates CD73 expression in TAMs via the JAK/STAT3 signaling cascade (Figure 3). This elevates adenosine levels and attenuates CD8+ T cell antitumor immunity[61]. The c-MAF and STAT3 signaling pathways are crucial for IL-6 to sustain the LYVE-1+ TAM phenotype. IL-6 induces LYVE-1+ TAMs to express the immunosuppressive enzyme heme oxygenase-1 and form LYVE-1+ TAM nests via a CCR5-dependent signaling axis, thereby suppressing CD8+ T cell recruitment[62]. However, early IL-6 signal blockade weakens M1 TAM functionality through reducing SOCS3 levels and increasing SIRP levels, and thus decreasing the CD8+ T cell responses[63-65].
STAT6 is another regulator of the M2 TAM transcriptional program within the STAT family. In the nucleus, phosphorylated STAT6 induces the transcription of M2 signature genes such as Arg1, Ccl17, and Mrc1, while concurrently inhibiting the activation of M1 signature genes like Nos2, Ccl5, and Nlrp3[66, 67]. Multiple cells within the TME produce IL-4, which polarizes TAMs towards the M2 phenotype via activating the STAT6 pathway (Figure 3)[68, 69]. However, Stat6-/- tumor-bearing mice-derived TAMs exhibit an M1 phenotype[70]. Plus, exoASO-STAT6 treatment increases the M1/M2 ratio and CD8+ T cell activation in mice, further substantiating the role of STAT6 in the M2 polarization[71].
4.3. Mincle pathway activation
Tumor cells secrete various cytokines and chemokines that recruit and activate macrophages[72]. Once recruited to the TME, macrophages can express Mincle (Clec4e), a CLR that recognizes certain glycolipids (particularly trehalose dimycolate) and damaged cell components[73]. Upon activation, Mincle triggers downstream signaling pathways that modulate macrophage behavior[73]. Specifically, Mincle engages Syk to subsequently activates the NF-κB signaling pathway. This pathway is crucial for the transcription of genes associated with the M2 TAMs (Figure 3)[73]. The activation of the Mincle/Syk/NF-κB signaling axis promotes the expression of genes that are characteristic of M2 TAMs[73, 74]. Conversely, silencing Mincle has been shown to enhance the proinflammatory and antitumor activities of M1 TAMs[73]. This suggests that Mincle signaling actively suppresses the functionality of M1 TAMs while contributes to the polarization of M2 TAMs.
Tumor cell-macrophage interactions reprogram M2 TAM polarization. Tumor cells release a panel of chemokines, cytokines, PEG2, RNase1, and arginine to educate macrophages to M2 phenotypes. These molecules engulfed by macrophages induce their immunosuppressive polarization by activating STAT3/6, Syk-PI3K signaling pathways and suppressing NF-κB signaling activity. Moreover, tumor cell-derived arginine orchestrates macrophages towards M2 TAMs via activating arginine-polyamine- thymine DNA glycosylase (TDG) axis. In turn, M2 TAMs secrete extracellular vesicles (EVs) and chemokines (blue color) into tumor cells, which upregulate their PD-L1 expression by activating the STAT3 signaling pathway, thereby facilitating tumor immune evasion.
4.4. Syk-PI3K signaling activation
The activation of Syk-PI3K axis in macrophages drives their polarization towards the M2 phenotype, thereby establishing an immunosuppressive microenvironment in vivo. Pharmacological dual-targeting Syk and PI3K in macrophages can reprogram them towards the M1 phenotype. This intervention disrupts the α4β1-Syk-p110γ axis in macrophages, creating the destabilization of HIF1α and ultimately restoring the functionality of CD8+ T cells[75]. PI3Kγ signaling in macrophages leads to NF-κB inactivation and C/EBPβ activation via Akt/mTOR axis, thus initiating an immunosuppressive transcriptional program that undermines CD8+ T cell antitumor immunity (Figure 3)[76]. Clever-1 deficiency in macrophages amplifies their immunostimulatory activity by enhancing mTOR signaling, thereby reactivating CD8+ T cell responses[77].
Genetic deletion of Syk in macrophages educates them towards the M1 phenotype, therefore augmenting CD8+ T cell infiltration and activation. Similarly, the FDA-approved Syk inhibitor R788 induces TAM polarization towards the M1 phenotype and promotes CD8+ T cell activation in pancreatic ductal adenocarcinoma (PDAC) mice[78]. PI3Kγ is a marker of TAMs in PDAC and drives their polarization towards an immunosuppressive phenotype. Inhibition of PI3Kγ reprograms the transcriptional profile of TAMs, thereby activating CD8+ T cell-mediated immune surveillance[79]. In PDAC mice, B cell-macrophage crosstalk promotes TAM polarization towards the M2 phenotype via the activation of PI3Kγ/BTK axis (Figure 3). Thus, PI3Kγ inhibitor or BTK inhibitor treatment induces macrophage polarization towards the M1 phenotype and restores CD8+ T cell cytotoxicity[80]. Therefore, the suppression of the Syk/PI3K axis activity in macrophages reverses their immunosuppressive phenotype and rescues the tumor immune evasion.
4.5. Enhanced crosstalk of chemokines and their receptors
TAMs facilitate PD-1 expression on CD8+ T cells through IRF8-dependent antigen presentation, thereby precipitating CD8+ T cell exhaustion[81]. IL-1β in the TME enhances CXCL8 secretion by tumor cells and the chemotaxis of M2 TAMs. Tumor-derived CXCL8 fosters M2 TAM polarization by activating the STAT3 signaling pathway and concurrently hurdles PD-1+ CD8+ T cell recruitment[82]. TAM-derived CCL5 facilitates immune evasion in colorectal cancer (CRC) through the p65/STAT3-CSN5-PD-L1 pathway[83]. Tumor cell-derived exosomes orchestrate the differentiation of monocytes into PD-1+ TAMs, which experience an M2-like phenotype with decreased phagocytic capacity and effectively suppress CD8+ T cell responses[84]. M2 TAM-derived extracellular vesicles enhance IQGAP1 nuclear translocation and activate STAT3 phosphorylation by downregulating MISP in HCC. This process attenuates CD8+ T cell responses and upregulates PD-L1 expression in tumor cells, thereby facilitating tumor immune evasion (Figure 3)[24]. Act1 downregulation in macrophages increases CXCL9/10 and PD-L1 expressions by activating the STAT3 signaling pathway. Additionally, anti-Act1 macrophages facilitate the benign-to-malignant transition in CRC cells via the CXCL9/10-CXC chemokine receptor 3 (CXCR3) axis and induce CD8+ T cell exhaustion through the PD-L1/PD-1 axis[85].
Tumor cell-derived SOX9 orchestrates the polarization of TAMs towards the M2 phenotype through the paracrine secretion of leukemia inhibitory factor (LIF), thereby attenuating CD8+ T cell function. LIF is abundantly present in malignant ascites. The ablation of SOX9 can curtail the levels of M2 TAM-induced immunosuppressive cytokines, such as C-C motif chemokine ligand 2 (CCL2) and IL10, and reinvigorate CD8+ T cell responses[86]. Inhibiting the CCL2/C-C motif chemokine receptor 2 (CCR2) axis can reprogram TAM polarization towards the M2 phenotype and revitalize CD8+ T cell responses, thereby mitigating the immunosuppressive state[87]. ETV4 upregulation in tumor cells augments the recruitment of TAMs and MDSCs via the CCL2/CCR2 axis, while concurrently inhibiting CD8+ T cell accumulation (Figure 3). Moreover, ETV4 propels HCC metastasis through an FGF19-ETV4-FGFR4 positive feedback loop[88]. In a triple-negative breast cancer model, CD8+ T cells induce PD-L1 expression in TAMs at the marginal TME through the CCL2/PD-L1 axis[89]. Innate αβ T cells (iαβTs) demonstrate tumor-protective properties by reprogramming immunogenic macrophages in a CCR5-dependent manner and inhibiting CD8+ T cell activation through PD-L1/PD-1 interactions[90]. Hedgehog (Hh) signaling in TAMs drives M2 polarization through the downstream transcription factor Gli1, which modulates Krüppel-like factor 4 (Klf4). Klf4 deficiency in macrophages manifests as an M1 phenotype. Tumor cell-derived Hh ligand sonic hedgehog further amplifies M2 TAM polarization. Hh-induced M2 TAMs attenuate the production of CXCL9 and CXCL10, thereby impeding CD8+ T cell recruitment[91]. Nasopharyngeal carcinoma (NPC) cells secrete IFN-stimulated gene 15 (ISG15), which remodels macrophages into the M2 subtype by activating the LFA-1/SFK/CCL18 axis. These ISG15+ M2 TAMs significantly impair CD8+ T cell responses. Clinically, the presence of ISG15+ M2 TAMs is frequently correlated with poor prognosis in NPC patients[92].
In advanced CRC patients, TAMs-derived IL8 alters CD8+ T cell function by downregulating TIM3 expression through the IL8-CXCR2 axis[93]. NLRP3 signaling in macrophages drives the differentiation of CD4+ T cells into Tregs and inhibits CD8+ T cell activation, processes dependent on IL-10[94]. Emp3-overexpressing macrophages produce elevated levels of TNF-α, which downregulates IL-2Rα expression on CD8+ T cells, thereby mitigating their proliferation and activation[95].
4.6. TGF-β signaling upregulation and metabolic dysregulation in tumor cells
IGF2BP3, by binding to CCL5 or TGF-β1, orchestrates the polarization of TAMs towards the M2 phenotype, therefore suppressing CD8+ T cell functionality[96]. Elevated COX-2 expression in HCC drives TAM polarization towards the M2 phenotype and precipitates CD8+ T cell exhaustion via the TGF-β signaling pathway (Figure 3)[97]. Conversely, COX-2 deficiency in TAMs promotes their polarization towards the M1 phenotype, thereby augmenting CD8+ T cell activity and immune surveillance[98].
Another critical mechanism involves the regulation of immune responses by metabolic enzymes and metabolites. In the tumor microenvironment (TME), breast cancer cells act as the primary source of arginine, which polarizes M2 TAMs and suppresses CD8+ T cell-mediated antitumor activity. Therapeutic targeting of the arginine-polyamine-thymine DNA glycosylase (TDG) axis between cancer cells and M2 TAMs significantly inhibits breast cancer growth (Figure 3)[99]. Pathways associated with immunometabolic circuits are increasingly recognized as critical regulators of TAM-T cell interaction. These pathways integrate metabolic reprogramming and immune signaling to shape the functional dynamics of the tumor microenvironment.
5. Post-Transcriptional Regulations
The 2024 Nobel Prize in Physiology or Medicine was awarded jointly to Dr. Victor Ambros and Dr. Gary Ruvkun for their seminal discovery of microRNAs (miRNAs) for their pivotal roles in post-transcriptional gene regulation. This monumental work has profoundly enhanced our understanding of gene expression and its implications for a plethora of diseases, including the polarization of TAMs and CD8+ T cell responses within the TME.
miR-155 is highly expressed in M1 TAMs[100, 101] and plays a pivotal role in the polarization of TAMs towards the M1 phenotype[102, 103]. Additionally, miR-155 expression in T cells enhances TAM activation by inducing IFN-inducible genes. A triple combination of anti-PD-1/PD-L1/CTLA-4 ICB therapy significantly restores antitumor immunity in miR-155 T cell conditional knockout mice through the activation of both T cells and TAMs[104]. miR-506 reprograms M2 TAMs into M1 phenotypes by targeting STAT3 signaling, thus promoting CD8+ T cell infiltration and enhancing the sensitivity of PDAC patients to PD-1 ICB therapy[105]. Extracellular vesicles, replete with bioactive substances, act as essential mediators of intercellular molecular transport and communication[106]. Extracellular vesicle-derived miR-155-5p from LUAD cells reprograms TAMs to an immunostimulatory phenotype and enhances CD8+ T cell activation, thereby inhibiting immune escape in immunocompetent mice[107].
However, some tumor cell-derived miRNAs engulfed by TAMs lead to an education of M2 phenotype. CRC cell-derived extracellular vesicles contain miR-21-5p and miR-200a, which, upon uptake by TAMs, induce M2 polarization and upregulate PD-L1 expression through the PTEN/Akt and SOCS1/STAT1 signaling pathways. This process leads to diminished CD8+ T cell responses and promotes tumor progression[108]. Endoplasmic reticulum (ER) stress in tumor cells facilitates immune escape in solid tumors by modulating the TME. ER-stressed HCC cell-derived exosomes harbor high levels of miR-23a-3p. Mechanistically, miR-23a-3p upregulates PD-L1 expression in macrophages via the PTEN/PI3K/Akt signaling pathway, thereby inhibiting T cell function and increasing T cell apoptosis[109].
The competing endogenous RNAs (ceRNAs), such as long non-coding RNAs (lncRNA) and circRNAs, influence the post-transcriptional regulation of miRNAs through miRNA response elements[110-112], ultimately modulating the functions of TAMs and CD8+ T cells[113, 114]. M2 TAMs-derived exosomes contain LINC01592, which can be transported into tumor cells and subsequently facilitate tumor growth by inhibiting the E2F6/NBR1/MHC-I signaling pathway. Consequently, inhibiting LINC01592 increases the MHC-I expression on the surface of tumor cells, thereby augmenting the efficacy of CD8+ T cell reinfusion therapy against tumors[113]. M2 TAMs exhibit high expression of circRNA MERTK, which mechanistically upregulates IL-10 expression in macrophages by sponging miR-125a-3p. This process leads to increased apoptosis of CD8+ T cells and fosters an immunosuppressive TME[114].
6. Strategies for Repolarizing M2 TAMs to M1 Phenotypes
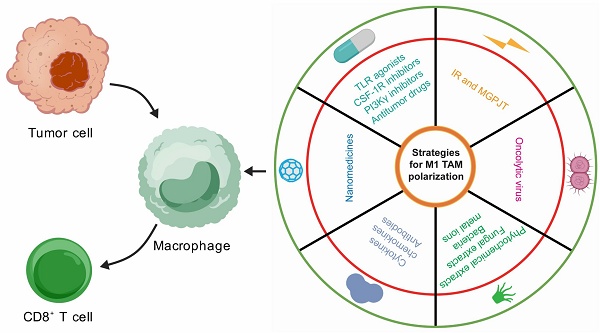
The M1/M2 ratio elucidates the immune architecture of the TME, with M1 TAMs being indicative of favorable patient prognoses, while M2 TAMs are emblematic of poor clinical outcomes[115]. Current strategies for TAM-related cancer therapy primarily encompass (1) the direct eradication of M2 TAMs, (2) the reduction of M2 TAM recruitment, and (3) the repolarization of M2 TAMs to the M1 phenotype. These modalities can effectively enhance CD8⁺ T antitumor immune responses and improve patient survival. By modulating the activity of the aforementioned signaling pathways, an increasing body of therapeutic paradigms are harnessed to repolarize TAM phenotypes and further potentiate the antitumor efficacy of CD8+ T cells (Table 3; Figure 4).
6.1. Small-molecular drugs
TLR agonists trigger innate immune responses in macrophages and can reprogram M2 TAMs into M1 phenotypes, thereby diminishing Treg populations and promoting antigen-specific CD8+ T cell activation[116-118]. Additionally, they can attenuate PD-L1 expression in TAMs[116] and facilitate the secretion of proinflammatory cytokines by TAMs[118]. A TLR3-specific adjuvant, in conjunction with the VISTA-specific monoclonal antibody 13F3, markedly enhances the CD8+ T cell/Treg and M1/M2 ratios. Furthermore, this combination therapy upregulates the expression of immunostimulatory molecules while concurrently downregulating immunosuppressive molecules[119]. A folate-targeted TLR7 agonist selectively delivers the drug to folate receptor-β positive macrophages in vivo, facilitating TAM polarization towards the M1 phenotype and enhancing CD8+ T cell infiltration. This synthetic compound significantly extends mouse survival without evident toxicity[120].
Strategies for M1 TAM polarization. Multifaceted treatment avenues have confirmed their potential to drive M1 tumor-associated macrophage (TAM) polarization, including small-molecule drugs, nanomedicines, molecular therapies, natural products, oncolytic viruses, and ionizing radiation. Specifically, Toll-like receptor (TLR) agonists, colony-stimulating factor-1 receptor (CSF-1R) inhibitors, and phosphoinositide 3-kinase γ (PI3Kγ) inhibitors are representative small-molecule drugs. Nanomedicines refer to the use of nanomaterials to deliver drugs, specific antibodies, and even gene-editing plasmids to reshape the TAM phenotype. Many proinflammatory cytokines and chemokines, as well as immune checkpoint blockade antibodies, constitute the mainstay of molecular therapy. Natural products comprise of phytochemical and fungal extracts, bacteria, and metal ions.
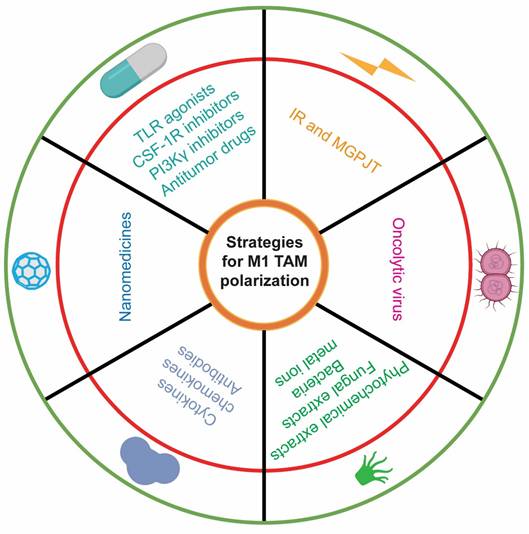
The detailed avenues for M1 TAM polarization.
| Classifications | Subclassifications | Treatment strategies | Effects | Study status | Refs |
|---|---|---|---|---|---|
| Small-molecular drugs | TLR agonists | TLR3 Agonist | Enhanced TAM antigen presentation, and increased CD8+ T cell activation | Preclinical | 116 |
| SMU-L11 (TLR7 agonist) | M1 TAM polarization, and increased CD8+ T cell proliferation and activation | Preclinical | 118 | ||
| Folate-targeted TLR7 agonist | Increased M1/M2 ratio, and increased CD8+ T cell infiltration | Preclinical | 120 | ||
| CSF1R kinase inhibitor | Q702 | Increased M1/M2 ratio, and increased CD8+ T cell populations | Preclinical | 125 | |
| Inhibitor of FGFR1/2/3 and CSF-1R | 3D185 | Increased M1/M2 ratio, and increased CD8+ T cell activation | Preclinical | 126 | |
| Targeting the Mincle pathway | USMB-shMincle | M1 TAM polarization | Preclinical | 132 | |
| PI3Kγ inhibitor | AZD3458 | Enhanced TAM activation, and increased CD8+ T cell antitumor activity | Preclinical | 135 | |
| Pan-PI3K inhibitor | Copanlisib | Enhanced CD8+ T cell/Treg and M1/M2 ratios, and increased infiltration of CD8+ T cells and TAMs | Preclinical | 136 | |
| Nanomedicines | TLR7/8 agonist | R848@LNPs | Increased M1 TAM and CD8+ T cell populations | Preclinical | 145 |
| TLR3 agonist, NF-κB activation | FP-NPs | M1 TAM polarization | Preclinical | 146 | |
| NF-κB activation | Ferumoxytol | Increased M1 TAM populations | Preclinical | 147 | |
| TLR7/8 agonist | Telratolimod | Increased M1 TAM populations, and increased CD8+ T cell antitumor activity | Preclinical | 148 | |
| TLR9 agonist | LCpG | M1 TAM polarization, and increased CD8+ T cell proliferation | Preclinical | 149 | |
| TLR7 agonist | T7-Exo/siGalectin-9 | M1 TAM polarization | Preclinical | 150 | |
| STAT3 signaling blockade | CpGgel-siSTAT3 | Enhanced M1 TAM activation, and increased infiltration of CD8+ T cells | Preclinical | 151 | |
| STAT3 signaling blockade | CS/LyP-1-PC | Decreased infiltration of Tregs, M2 TAMs, and MDSCs | Preclinical | 155 | |
| Targeting splenic CD169+ macrophages | GM3-αGC-OVA | Increased CD8+ T cell antitumor immunity | Preclinical | 158, 160 | |
| Delivering siPDL1 into M2 TAMs | 7D12-mExo-M2pep-siPDL1 | M1 TAM polarization, and increased CD8+ T cell antitumor immunity | Preclinical | 161 | |
| Nanodelivery of PD-L1 expression in M2 TAMs | nano-PD-L1 trap | Reduced M2 TAM proportion, and increased CD8+ T cell activation | Preclinical | 162 | |
| Targeting Wnt/β-catenin signaling | XAV-Np | Increased M1/M2 ratio, and increased CD8+ T cell proliferation | Preclinical | 163 | |
| Molecular therapy | TLR3 agonist | Poly-ICLC | M1 TAM polarization | Preclinical | 164 |
| TLR4 agonist | Monophosphoryl lipid A | M1 TAM polarization, and increased CD+ 8 T cell activation | Preclinical | 165 | |
| Type I IFN | Increased CD8+ T cell activation | Preclinical | 168 | ||
| IFN-γ | Increased infiltration of M1 TAMs and CD8+ T cells | Preclinical | 169 | ||
| Dual-targeting CD47/PD-L1 antibody | Increased CD8+ T cell activation, and increased infiltration of M1 TAMs | Preclinical | 171 | ||
| Combination of dual-targeting CD47/PD-L1 antibody and FOLFOX | Decreased infiltration of Tregs and MDSCs, increased M1/M2 ratio, and increased CD8+ T cell activation | Preclinical | 172 | ||
| Combination of dual-targeting PD-L1/CTLA4 antibody and TGF-β inhibitor | Increased infiltration of M1 TAMs | Preclinical | 173 | ||
| Dual-targeting HER2/CD47 CAR-Ms | Shift of TAM phenotype, and increased CD8+ T cell activation | Preclinical | 177 | ||
| Natural products | Chinese medicines | Compound Kushen Injection | Increased CD8+ T cell proliferation and activation | Preclinical | 178 |
| Resveratrol | Reduced proportion of M2 TAMs, and increased CD8+ T cell activation | Preclinical | 179 | ||
| Terminalia bellirica | M1 TAM polarization, and increased CD8+ T cell antitumor immunity | Preclinical | 180 | ||
| Naringenin | Lymph node CD169+ macrophage activation and increased infiltration of CD8+ T cells | Preclinical | 181 | ||
| phytohemagglutinin | Increased CD8+ T cell proliferation | Preclinical | 182 | ||
| Cannabigerol | Remodeling M1 TAMs, and increased CD8+ T cell activation | Preclinical | 183 | ||
| Fungal extracts | Lentinan | Enhanced TAM activation, and increased CD8+ T cell proliferation | Preclinical | 184 | |
| L-ergothioneine | Increased infiltration of M1 TAMs and increased CD8+ T cell activation | Preclinical | 186 | ||
| Bacteria | Streptococcus salivarius | M1 TAM polarization and enhanced antigen presentation, and increased CD8+ T cell proliferation and activation | Preclinical | 187 | |
| BCG hydrogel | M1 TAM polarization and enhanced antigen presentation | Preclinical + clinical | 188 | ||
| Metal ions | Manganese | Enhanced M1 TAM maturation and antigen presentation, increased CD8+ T cell differentiation and activation | Clinical | 189 | |
| Oncolytic virus | CARG-2020 | Increased M1 TAM differentiation, decreased MDSC expansion, and increased CD8+ T cell antitumor immunity | Preclinical | 191 | |
| IR and MGPJT | IR | Combination of VPA/HPTA and radiotherapy | M1 TAM polarization, and increased CD8+ T cell activation | Preclinical | 192 |
| MGPJT | MGPJT | M1 TAM polarization | Preclinical | 199 |
This table is related to Figure 4, which provides all strategies for M1 TAM polarization.
The CSF-1/CSF-1R axis is crucial for the differentiation, proliferation, and survival of M2 TAMs[121, 122]. The CSF1R fosters an immunosuppressive TME by increasing the intratumoral infiltration of M2 TAMs and MDSCs. Consequently, CSF-1R inhibition emerges as a promising strategy to specifically target M2 TAMs and counteract tumor immune evasion[123, 124]. The selective CSF1R inhibitors remodels the TME into an immunostimulatory state by increasing M1 TAMs and CD8+ T cells while reducing M2 TAMs and MDSCs[125, 126]. Fusing IL-10 with a CSF-1R blockade antibody generates a bifunctional protein that effectively depletes TAMs and augments CD8+ T cell antitumor immunity, demonstrating significant antitumor activity across various solid tumors, particularly in head and neck cancers[127].
As previously described, targeting the Mincle pathway is beneficial for reversing the polarization of M2 TAMs. Syk inhibitors can disrupt the Mincle signaling pathway[128, 129]. Aptamers, which are short, single-stranded DNA or RNA molecules, can bind to specific targets with high affinity. Mincle-specific aptamers have demonstrated therapeutic potential by selectively inhibiting Mincle activation[130]. Ultrasound-Mediated Bubble (USMB) technology enhances the delivery of therapeutic agents to specific tissues using ultrasound and microbubbles. It has shown promise in improving the efficiency and targeting of anti-Mincle agents[131, 132]. Potential therapeutic approaches targeting the Mincle pathway highlight their relevance in modulating TAM activity and enhancing antitumor immunity.
PI3Kγ inhibition can repolarize M2 TAMs to M1 phenotypes, thereby triggering an antitumor immune response[76, 133, 134]. The PI3Kγ inhibitors stimulates M1 TAM activation and antigen-presentation and augments the CD8+ T/Treg and M1/M2 ratios, while reducing IL-10 secretion by M2 TAMs. As a monotherapy, it further enhances the CD8+ T cell activation and boosts the ICB antitumor activity[135, 136].
A panel of canonical (e.g., Vinblastine and Doxorubicin[137, 138]) and non-canonical antitumor drugs (e.g., Hydroxychloroquine, Verteporfin, and Sulfasalazine[139-141]) can remodel the TME by immunostimulating the functions of TAMs and CD8+ T cells. Consequently, their combination with PD-1/PD-L1 ICB therapy reconfigures the TME by increasing the CD8+ T cell/Treg and M1/M2 ratios, contributing to the robust antitumor responses[142-144].
6.2. Nanomedicines
Given the substantial advantages of nanomaterials in cancer therapy, such as enhanced targeting precision, superior drug stability and bioavailability, and the augmented permeability and retention effect, researchers are endeavoring to design an array of nanomedicines to reprogram TAM phenotypes and augment CD8+ T cell functionality. Many nanomedicines repolarize M2 TAMs to M1 phenotype via activating NF-κB and TLR signaling pathways[145-150], or obstructing STAT3 signaling pathway[151], thereby reactivating CD8+ T cell-mediated immune responses.
Liposomes and lipid-coated calcium phosphate represent the promising antigen delivery systems and have been validated as an efficacious platform for vaccinations and drugs[152-156]. For example, Paclitaxel induces the polarization of M1 TAMs to M2 phenotypes and diminishes the expression of CXCL9/10 on macrophages[157]. Conversely, the concomitant delivery of Paclitaxel and Cryptotanshinone via liposomes inhibits STAT3 activation, thereby reversing the immunosuppressive TME[155]. CD169+ macrophages are situated in the marginal zone of the spleen and the subcapsular sinus of lymph nodes. GM3-αGC-OVA liposomes increase CD8+ T cell responses, which is closely related to CD169+ macrophages and the CD169 receptor[158]. A liposomal platform targeting CD169 for selective delivery to macrophages can enhance CD8+ T cell proliferation and regulate the activation ratio of CD4+/CD8+ T cells in vivo[159, 160].
PD-L1 is an emerging target for TAM-directed therapies. Delivering siPD-L1 to M2 TAMs via nanocarriers facilitates their reprogramming to M1 TAMs[161]. The antitumor efficacy of the nano-PD-L1 trap surpasses that of PD-L1 monoclonal antibodies. Unlike PD-L1 monoclonal antibodies, the nano-PD-L1 trap can sustainably reduce the intratumoral accumulation of M2 TAMs and MDSCs[162]. Activation of Wnt/β-catenin signaling in antigen-presenting cells such as DCs and macrophages preferentially primes Tregs over CD8+ T cells. Utilizing β-catenin-targeting nanocarriers significantly upregulates CD80 and CD86 expression on macrophages while inhibiting CD206 and PD-L1 expression. Consequently, in an in vitro co-culture system, these macrophages can enhance CD8+ T cell proliferation[163].
6.3. Molecular Therapies
Proinflammatory cytokines and chemokines are extensively utilized to modulate TAMs and CD8+ T cells within the TME. Some vaccine adjuvants enhance the antitumor immune response of TAMs and CD8+ T cells by activating TLR3[164] and TLR4[165]. Type I and Type II IFNs can induce macrophage polarization towards the M1 phenotype, augments their phagocytic activity and the production of proinflammatory cytokines[166-169]. The combination of anti-CD20 and a mutated IL-2 (no-alpha mutein) promotes the release of proinflammatory cytokines by CD8+ T cells and the expression of immunostimulatory molecules on the surface of TAMs[170].
Dual-targeting ICB therapy effectively reverses the immunosuppressive ecosystem via stimulating TAM functionality. Dual-targeting CD47/PD-L1 ICB therapy or combined with FOLFOX strategy markedly increases the M1/M2 ratio and activated CD8+ T cell populations in the TME[171, 172]. Dual-targeting PD-L1/CTLA4 antibody plus TGF-β inhibitor significantly increases M1 TAM populations[173]. However, dual-targeting PD-1/CTLA-4 ICB therapy-amplified CD8+ T cell infiltration and antitumor response are weakened by depleting CXCL9 expression on TAMs[174].
Targeting specific biomarkers on M2 TAMs has shown potential to reprogram them to the M1 subtype, thereby enhancing CD8+ T cell responses. MARCO is predominantly expressed on macrophages, particularly M2 TAMs, where it enhances IL-10 production and Treg proliferation[175]. Targeting MARCO on TAMs with antibody can re-educate them towards the M1 subtype[176]. Chimeric antigen receptor macrophages (CAR-Ms) dual-targeting HER2/CD47 phagocytose antigen-specific tumor cells. These engineered CAR-Ms also reprogram M1 TAM polarization and stimulate CD8+ T cells to secrete a plethora of proinflammatory molecules[177].
6.4. Natural Products
Many phytochemical extracts in nature, renowned for their anti-inflammatory and antioxidant properties, modulate TAMs and CD8+ T cells within the TME. Several traditional Chinese medicines (e.g., Compound Kushen Injection, Resveratrol, and Terminalia bellirica) drives the polarization of M2 TAMs to M1 phenotypes and augments the activation of intratumoral CD8+ T cells, ultimately leading to the shrinkage of solid tumors[178-180]. Moreover, Naringenin and Phytohemagglutinin potentiate CD8+ T cell activation by stimulating macrophages in the TME, likely through improved antigen processing and presentation[181, 182]. Cannabigerol can reduce the secretion of CSF-1 in solid tumors, such as melanoma, thereby remodeling M1 TAMs and reinstating CD8+ T cell activation[183].
Fungal extracts exhibit potent efficacy in educating M1 TAM polarization and invigorating CD8+ T cell antitumor immunity[184, 185]. L-ergothioneine lacks intrinsic immunostimulatory properties but can potentiate TLR responses in macrophages, thereby eliciting robust innate immune activity[185]. When conjugated with a TLR2-containing cancer vaccine, it markedly enhances M1 TAM infiltration and CD8+ T cell activation[186].
A variety of bacteria (e.g., Streptococcus salivarius and Bacillus Calmette-Guérin lysate) and metal ions (e.g., Mn²⁺) both polarize TAMs to M1 phenotype and augments their maturation and antigen presentation, thus inducing memory CD8+ T cell amplification, and CD8+ T cell activation[187-189]. Conversely, the microbiome in PDAC patients creates an immunosuppressive TME by differentially activating specific TLRs in macrophages. However, bacterial ablation can immunostimulate the tumor immune microenvironment and enhance the antitumor efficacy of PD-1 ICB therapy[190].
6.5. Oncolytic Virus
Oncolytic viruses are a class of viruses that selectively target and eradicate tumor cells while sparing jeopardy to normal tissues. Additionally, they can activate TAMs and CD8+ T cells to further eradicate residual tumor cells. For example, CARG-2020 is a self-amplifying virus-like vesicle that encodes immune regulatory genes to modulate various immune signaling pathways. It educates M2 TAMs polarizing towards M1 phenotypes and augments CD8+ T cell responses[191].
6.6. Ionizing radiation (IR) and medical gas plasma jet technology (MGPJT)
IR not only directly disrupts the DNA replication of tumor cells but also reconfigures the TME through influencing TAM phenotypes and CD8+ T cell activity. Valproic acid (VPA) and its derivative HPTA are potent immune activators for radiotherapy. The combination of VPA/HPTA and radiotherapy can induce the polarization of TAMs towards the M1 phenotype, increase the number of activated CD8+ T cells and elicit a substantial production of inflammatory cytokines during the early stage of treatment[192]. Conversely, two weeks of radiotherapy increases STAT3-activated TAMs and reduces CD8+ T cells. Targeting local TLR9/STAT3 signaling promotes the local accumulation of M1 TAMs and CD8+ T cells[193]. Notably, targeting local STAT3 is challenging because, although STAT3 diminishes CD8+ T cell cytotoxicity and enhances Treg tolerance, it is indispensable for the expansion of memory T cells and long-term tumor immunity[194-197]. ATM inhibition intensifies IR-induced DNA damage, which upregulates type I IFN expression in macrophages through the STING/IRF3 signaling pathway, thereby augmenting IR-induced CD8+ T cell antitumor immunity[198].
MGPJT is an innovative medical treatment method that harnesses plasma, often referred to as the fourth state of matter. This technology involves ionizing a gas using radio frequency or microwave energy to generate a plasma jet, which produces reactive oxygen and nitrogen species (ROS and RNS)[199]. These ROS and RNS can induce immunogenic cell death in tumor cells, thereby activating the immune system and polarize M2 TAM to M1 phenotype by releasing tumor antigens[200]. By modulating oxidative stress levels and cytokine profiles within the TME, gas plasma jets can potentiate the antitumor immune response of TAMs[199, 200]. The plasma jet-generated ROS and RNS can directly eradicate tumor cells, leading to the release of DAMPs[201]. These DAMPs can further stimulate the immune system and reprogram TAMs to bolster their antitumor activities[201, 202]. Consequently, MGPJT represents a promising approach to harnessing the body's intrinsic defenses against cancer.
7. Conclusions and Perspectives
We have posed and contextualized the tumor cell-macrophage-CD8+ T cell loop where macrophage is prone to be reprogrammed. Recent works that delve into the intricate mechanisms underlying TAM immunostimulatory and immunosuppressive polarization have been further explored. These mechanisms inform and enhance innovative or combinatory therapeutic strategies aimed at modulating TAMs towards the M1 phenotype. Such novel interventions can substantially ameliorate the immunosuppressive milieu of the TME, either as standalone treatments or in combination with ICB therapy. Emerging technologies, like single-cell RNA sequencing and spatial transcriptomics, are unraveling the functional diversity of TAMs and their crosstalk with CD8+ T cells. These insights provide a rational basis for optimizing combinatorial strategies that integrate TAM reprogramming with ICB therapy.
Future endeavors should expeditiously translate strategies for immunostimulatory reprogramming TAM phenotypes into tangible clinical benefits for cancer patients. Our advanced understanding of TAM polarization paves the way for the development of cutting-edge therapeutic approaches that functionally immunostimulate TAMs and subsequently augment CD8+ T cell antitumor immunity, potentially providing many promising avenues to better optimize clinical benefits for cancer patients undergoing ICB treatment.
Acknowledgements
Funding
This work was financially supported by by the Natural Science Foundation of China (82030094), and the Science and Technology Development Fund, Macau SAR (0009/2022/AKP and 0129/2024/RIA2).
Author contributions
All authors revised and approved the manuscript. Lin He prepared the figures and manuscript. Paul Kwong-Hang Tam participated in the manuscript writing. Chu-Xia Deng designed the review, supervised all figures, and cowrote the manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Demaria O, Cornen S, Daëron M, Morel Y, Medzhitov R, Vivier E. Harnessing innate immunity in cancer therapy. Nature. 2019;574:45-56
2. Pang G, Wei S, Zhao J. Improving nanochemoimmunotherapy efficacy by boosting "eat-me" signaling and downregulating "don't-eat-me" signaling with Ganoderma lucidum polysaccharide-based drug delivery. J Mater Chem B. 2023;11:11562-11577
3. Li Z, Li Y, Gao J, Fu Y, Hua P, Jing Y, Cai M, Wang H, Tong T. The role of CD47-SIRPα immune checkpoint in tumor immune evasion and innate immunotherapy. Life Sci. 2021;273:119150
4. Song Q, Javid A, Zhang G, Li Y. Applications of Magnetite Nanoparticles in Cancer Immunotherapies: Present Hallmarks and Future Perspectives. Front Immunol. 2021;12:701485
5. Ji ZZ, Chan MK, Chan AS, Leung KT, Jiang X, To KF, Wu Y, Tang PM. Tumour-associated macrophages: versatile players in the tumour microenvironment. Front Cell Dev Biol. 2023;11:1261749
6. Zhang W, Wang M, Ji C, Liu X, Gu B, Dong T. Macrophage polarization in the tumor microenvironment: Emerging roles and therapeutic potentials. Biomed Pharmacother. 2024;177:116930
7. Amit I, Winter DR, Jung S. The role of the local environment and epigenetics in shaping macrophage identity and their effect on tissue homeostasis. Nat Immunol. 2016;17:18-25
8. Tzetzo SL, Abrams SI. Redirecting macrophage function to sustain their "defender" antitumor activity. Cancer Cell. 2021;39:734-737
9. Locati M, Curtale G, Mantovani A. Diversity, Mechanisms, and Significance of Macrophage Plasticity. Annu Rev Pathol. 2020;15:123-147
10. Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593-604
11. Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14:1014-1022
12. Shen X, Zhou S, Yang Y, Hong T, Xiang Z, Zhao J, Zhu C, Zeng L, Zhang L. TAM-targeted reeducation for enhanced cancer immunotherapy: Mechanism and recent progress. Front Oncol. 2022;12:1034842
13. Pan Y, Yu Y, Wang X, Zhang T. Tumor-Associated Macrophages in Tumor Immunity. Front Immunol. 2020;11:583084
14. Du Y, Lin Y, Gan L, Wang S, Chen S, Li C, Hou S, Hu B, Wang B, Ye Y, Shen Z. Potential crosstalk between SPP1 + TAMs and CD8 + exhausted T cells promotes an immunosuppressive environment in gastric metastatic cancer. J Transl Med. 2024;22:158
15. Mortezaee K. B7-H3 immunoregulatory roles in cancer. Biomed Pharmacother. 2023;163:114890
16. Lecoultre M, Dutoit V, Walker PR. Phagocytic function of tumor-associated macrophages as a key determinant of tumor progression control: a review. J Immunother Cancer. 2020;8:e001408
17. Liu Z, Sun B, Xu A, Tang J, Zhang H, Gao J, Wang L. MICAL2 implies immunosuppressive features and acts as an independent and adverse prognostic biomarker in pancreatic cancer. Sci Rep. 2024;14:3177
18. Lv Z, Xue C, Zhang L, Sun J, Bo C. Elevated mRNA Level of Y-Box Binding Protein 1 Indicates Unfavorable Prognosis Correlated with Macrophage Infiltration and T Cell Exhaustion in Luminal Breast Cancer. Cancer Manag Res. 2021;13:6411-6428
19. Saranaruk P, Waraasawapati S, Chamgramol Y, Sawanyawisuth K, Paungpan N, Somphud N, Wongkham C, Okada S, Wongkham S, Vaeteewoottacharn K. Dense GM-CSFRα-expressing immune infiltration is allied with longer survival of intrahepatic cholangiocarcinoma patients. PeerJ. 2023;11:e14883
20. Shi YX, Zhang WD, Dai PH, Deng J, Tan LH. Comprehensive analysis of KCTD family genes associated with hypoxic microenvironment and immune infiltration in lung adenocarcinoma. Sci Rep. 2022;12:9938
21. Yao L, Li Y, Li S, Wang M, Cao H, Xu L, Xu Y. ARHGAP39 is a prognostic biomarker involved in immune infiltration in breast cancer. BMC Cancer. 2023;23:440
22. Wei J, Marisetty A, Schrand B, Gabrusiewicz K, Hashimoto Y, Ott M, Grami Z, Kong LY, Ling X, Caruso H. et al. Osteopontin mediates glioblastoma-associated macrophage infiltration and is a potential therapeutic target. J Clin Invest. 2019;129:137-149
23. Zhou Y, Nan P, Li C, Mo H, Zhang Y, Wang H, Xu D, Ma F, Qian H. Upregulation of MTA1 in Colon Cancer Drives A CD8(+) T Cell-Rich But Classical Macrophage-Lacking Immunosuppressive Tumor Microenvironment. Front Oncol. 2022;12:825783
24. Wang X, Ye X, Chen Y, Lin J. Mechanism of M2 type macrophage-derived extracellular vesicles regulating PD-L1 expression via the MISP/IQGAP1 axis in hepatocellular carcinoma immunotherapy resistance. Int Immunopharmacol. 2023;124:110848
25. Luo C, Shibata K, Suzuki S, Kajiyama H, Senga T, Koya Y, Daimon M, Yamashita M, Kikkawa F. GPC3 expression in mouse ovarian cancer induces GPC3-specific T cell-mediated immune response through M1 macrophages and suppresses tumor growth. Oncol Rep. 2014;32:913-921
26. Bugatti M, Bergamini M. A Population of TIM4+FOLR2+ Macrophages Localized in Tertiary Lymphoid Structures Correlates to an Active Immune Infiltrate Across Several Cancer Types. Cancer Immunol Res. 2022;10:1340-1353
27. Chakiryan NH, Kim Y, Berglund A. Geospatial characterization of immune cell distributions and dynamics across the microenvironment in clear cell renal cell carcinoma. J Immunother Cancer. 2023;11:e006195
28. Liang X, Zhang H, Wang Z, Zhang X, Dai Z, Zhang J, Luo P, Zhang L, Hu J, Liu Z. et al. JMJD8 Is an M2 Macrophage Biomarker, and It Associates With DNA Damage Repair to Facilitate Stemness Maintenance, Chemoresistance, and Immunosuppression in Pan-Cancer. Front Immunol. 2022;13:875786
29. Liu Y, Xu L, Dou Y, He Y. AXL: shapers of tumor progression and immunosuppressive microenvironments. Mol Cancer. 2025;24:11
30. Cesano A, Augustin R, Barrea L, Bedognetti D, Bruno TC, Carturan A. Advances in the understanding and therapeutic manipulation of cancer immune responsiveness: a Society for Immunotherapy of Cancer (SITC) review. J Immunother Cancer. 2025;13:e008876
31. Batchu S, Hanafy KA, Redjal N, Godil SS, Thomas AJ. Single-cell analysis reveals diversity of tumor-associated macrophages and their interactions with T lymphocytes in glioblastoma. Sci Rep. 2023;13:20874
32. Chu X, Tian Y, Lv C. Decoding the spatiotemporal heterogeneity of tumor-associated macrophages. Mol Cancer. 2024;23:150
33. van Elsas MJ, Middelburg J, Labrie C, Roelands J, Schaap G, Sluijter M, Tonea R, Ovcinnikovs V, Lloyd K, Schuurman J. et al. Immunotherapy-activated T cells recruit and skew late-stage activated M1-like macrophages that are critical for therapeutic efficacy. Cancer Cell. 2024;42:1032-1050.e1010
34. Kanemaru H, Yamane F, Fukushima K, Matsuki T, Kawasaki T, Ebina I, Kuniyoshi K, Tanaka H, Maruyama K, Maeda K. et al. Antitumor effect of Batf2 through IL-12 p40 up-regulation in tumor-associated macrophages. Proc Natl Acad Sci U S A. 2017;114:E7331-e7340
35. Hu X, Ding S, Lu G, Lin Z, Liao L, Xiao W, Ding Y, Zhang Y, Wang Z, Gong W, Jia X. Apolipoprotein C-III itself stimulates the Syk/cPLA2-induced inflammasome activation of macrophage to boost anti-tumor activity of CD8(+) T cell. Cancer Immunol Immunother. 2023;72:4123-4144
36. Zhang L, Zhang K, Zhang J, Zhu J, Xi Q, Wang H, Zhang Z, Cheng Y, Yang G, Liu H. et al. Loss of fragile site-associated tumor suppressor promotes antitumor immunity via macrophage polarization. Nat Commun. 2021;12:4300
37. Chang BY, Kim SB, Lee MK, Park H, Kim SY. Improved Chemotherapeutic Activity by Morus alba Fruits through Immune Response of Toll-Like Receptor 4. Int J Mol Sci. 2015;16:24139-24158
38. Li C, Wang S, Ma X, Wang T, Lu R, Jia X, Leng Z, Kong X, Zhang J, Li L. Ranitidine as an adjuvant regulates macrophage polarization and activates CTLs through the PI3K-Akt2 signaling pathway. Int Immunopharmacol. 2023;116:109729
39. Achyut BR, Angara K, Jain M, Borin TF, Rashid MH, Iskander ASM, Ara R, Kolhe R, Howard S, Venugopal N. et al. Canonical NFκB signaling in myeloid cells is required for the glioblastoma growth. Sci Rep. 2017;7:13754
40. Ma S, Sun B, Duan S. YTHDF2 orchestrates tumor-associated macrophage reprogramming and controls antitumor immunity through CD8(+) T cells. Nat Immunol. 2023;24:255-266
41. Dong L, Chen C, Zhang Y, Guo P, Wang Z, Li J, Liu Y, Liu J, Chang R, Li Y. et al. The loss of RNA N(6)-adenosine methyltransferase Mettl14 in tumor-associated macrophages promotes CD8(+) T cell dysfunction and tumor growth. Cancer Cell. 2021;39:945-957.e910
42. Xiao J, Sun F, Wang YN, Liu B, Zhou P, Wang FX, Zhou HF, Ge Y, Yue TT, Luo JH. et al. UBC9 deficiency enhances immunostimulatory macrophage activation and subsequent antitumor T cell response in prostate cancer. J Clin Invest. 2023;133:e158352
43. Ding L, Qian J, Yu X, Wu Q, Mao J, Liu X, Wang Y, Guo D, Su R, Xie H. et al. Blocking MARCO(+) tumor-associated macrophages improves anti-PD-L1 therapy of hepatocellular carcinoma by promoting the activation of STING-IFN type I pathway. Cancer Lett. 2024;582:216568
44. Li Y, Gao Y, Jiang X, Cheng Y, Zhang J, Xu L, Liu X, Huang Z, Xie C. SAMHD1 silencing cooperates with radiotherapy to enhance anti-tumor immunity through IFI16-STING pathway in lung adenocarcinoma. J Transl Med. 2022;20:628
45. Bourn JR, Ruiz-Torres SJ, Hunt BG, Benight NM, Waltz SE. Tumor cell intrinsic RON signaling suppresses innate immune responses in breast cancer through inhibition of IRAK4 signaling. Cancer Lett. 2021;503:75-90
46. Meng J, Jiang YZ, Zhao S, Tao Y, Zhang T, Wang X, Zhang Y, Sun K, Yuan M, Chen J. et al. Tumor-derived Jagged1 promotes cancer progression through immune evasion. Cell Rep. 2022;38:110492
47. Mandula JK, Sierra-Mondragon RA, Jimenez RV, Chang D, Mohamed E, Chang S, Vazquez-Martinez JA, Cao Y, Anadon CM, Lee SB. et al. Jagged2 targeting in lung cancer activates anti-tumor immunity via Notch-induced functional reprogramming of tumor-associated macrophages. Immunity. 2024;57:1124-1140.e1129
48. Chen S, Saeed A, Liu Q, Jiang Q, Xu H, Xiao GG, Rao L. Macrophages in immunoregulation and therapeutics. Signal Transduct Target Ther. 2023;8:207
49. Zhao Q, Wang Q, Wang T, Xu J, Li T, Liu Q, Yao Q, Wang P. Pattern Recognition Receptors (PRRs) in Macrophages Possess Prognosis and Immunotherapy Potential for Melanoma. Front Immunol. 2021;12:765615
50. Allavena P, Chieppa M, Monti P, Piemonti L. From pattern recognition receptor to regulator of homeostasis: the double-faced macrophage mannose receptor. Crit Rev Immunol. 2004;24:179-192
51. Luan X, Lei T, Fang J, Liu X, Fu H, Li Y, Chu W, Jiang P, Tong C, Qi H, Fu Y. Blockade of C5a receptor unleashes tumor-associated macrophage antitumor response and enhances CXCL9-dependent CD8(+) T cell activity. Mol Ther. 2024;32:469-489
52. Song Z, Wang X, Liu X, Luo Y, Qiu J, Yin A, Liu Y, Yi H, Xiao Z, Li A. Targeting of Annexin A1 in Tumor-associated Macrophages as a therapeutic strategy for hepatocellular carcinoma. Biochem Pharmacol. 2023;213:115612
53. Zhang F, Liu W, Meng F, Jiang Q, Tang W, Liu Z, Lin X, Xue R, Zhang S, Dong L. Inhibiting PLA2G7 reverses the immunosuppressive function of intratumoral macrophages and augments immunotherapy response in hepatocellular carcinoma. J Immunother Cancer. 2024;12:e008094
54. Wei J, Zhang J, Wang D, Cen B, Lang JD. The COX-2-PGE2 Pathway Promotes Tumor Evasion in Colorectal Adenomas. Cancer Prev Res (Phila). 2022;15:285-296
55. Guo Y, Ke S, Xie F, Chen J, Liu X, Wang Z, Xu D, Shen Y, Zhao G, Zhao W, Lu H. SIGLEC10(+) macrophages drive gastric cancer progression by suppressing CD8(+) T cell function. Cancer Immunol Immunother. 2023;72:3229-3242
56. Zhong W, Lu Y, Han X, Yang J, Qin Z, Zhang W, Yu Z, Wu B, Liu S, Xu W. et al. Upregulation of exosome secretion from tumor-associated macrophages plays a key role in the suppression of anti-tumor immunity. Cell Rep. 2023;42:113224
57. Liu C, Zhou C, Xia W, Zhou Y, Qiu Y, Weng J, Zhou Q, Chen W, Wang YN. Targeting ALK averts ribonuclease 1-induced immunosuppression and enhances antitumor immunity in hepatocellular carcinoma. Nat Commun. 2024;15:1009
58. Pang L, Guo S, Khan F, Dunterman M, Ali H, Liu Y, Huang Y, Chen P. Hypoxia-driven protease legumain promotes immunosuppression in glioblastoma. Cell Rep Med. 2023;4:101238
59. Wang C, Shen N, Guo Q, Tan X, He S. YAP/STAT3 inhibited CD8(+) T cells activity in the breast cancer immune microenvironment by inducing M2 polarization of tumor-associated macrophages. Cancer Med. 2023;12:16295-16309
60. Fang W, Zhou T, Shi H, Yao M, Zhang D, Qian H, Zeng Q, Wang Y, Jin F, Chai C, Chen T. Correction to: Progranulin induces immune escape in breast cancer via up-regulating PD-L1 expression on tumor-associated macrophages (TAMs) and promoting CD8(+) T cell exclusion. J Exp Clin Cancer Res. 2022;41:93
61. Deng Y, Chen Q, Yang X, Sun Y, Zhang B, Wei W, Deng S, Meng J, Hu Y, Wang Y. et al. Tumor cell senescence-induced macrophage CD73 expression is a critical metabolic immune checkpoint in the aging tumor microenvironment. Theranostics. 2024;14:1224-1240
62. Anstee JE, Feehan KT, Opzoomer JW, Dean I, Muller HP, Bahri M, Cheung TS, Liakath-Ali K, Liu Z, Choy D. et al. LYVE-1(+) macrophages form a collaborative CCR5-dependent perivascular niche that influences chemotherapy responses in murine breast cancer. Dev Cell. 2023;58:1548-1561.e1510
63. Beyranvand Nejad E, Labrie C, van Elsas MJ, Kleinovink JW. IL-6 signaling in macrophages is required for immunotherapy-driven regression of tumors. J Immunother Cancer. 2021;9:e002460
64. Croker BA, Krebs DL, Zhang JG, Wormald S, Willson TA, Stanley EG, Robb L, Greenhalgh CJ, Förster I, Clausen BE. et al. SOCS3 negatively regulates IL-6 signaling in vivo. Nat Immunol. 2003;4:540-545
65. Kong XN, Yan HX, Chen L, Dong LW, Yang W, Liu Q, Yu LX, Huang DD, Liu SQ, Liu H. et al. LPS-induced down-regulation of signal regulatory protein {alpha} contributes to innate immune activation in macrophages. J Exp Med. 2007;204:2719-2731
66. Czimmerer Z, Daniel B, Horvath A, Rückerl D, Nagy G, Kiss M, Peloquin M, Budai MM, Cuaranta-Monroy I, Simandi Z. et al. The Transcription Factor STAT6 Mediates Direct Repression of Inflammatory Enhancers and Limits Activation of Alternatively Polarized Macrophages. Immunity. 2018;48:75-90.e76
67. Piccolo V, Curina A, Genua M, Ghisletti S, Simonatto M, Sabò A, Amati B, Ostuni R, Natoli G. Opposing macrophage polarization programs show extensive epigenomic and transcriptional cross-talk. Nat Immunol. 2017;18:530-540
68. Ito SE, Shirota H, Kasahara Y, Saijo K, Ishioka C. IL-4 blockade alters the tumor microenvironment and augments the response to cancer immunotherapy in a mouse model. Cancer Immunol Immunother. 2017;66:1485-1496
69. Binnemars-Postma K, Bansal R, Storm G, Prakash J. Targeting the Stat6 pathway in tumor-associated macrophages reduces tumor growth and metastatic niche formation in breast cancer. Faseb J. 2018;32:969-978
70. Sinha P, Clements VK, Ostrand-Rosenberg S. Reduction of myeloid-derived suppressor cells and induction of M1 macrophages facilitate the rejection of established metastatic disease. J Immunol. 2005;174:636-645
71. Kamerkar S, Leng C, Burenkova O, Jang SC. Exosome-mediated genetic reprogramming of tumor-associated macrophages by exoASO-STAT6 leads to potent monotherapy antitumor activity. Sci Adv. 2022;8:eabj7002
72. Kerneur C, Cano CE, Olive D. Major pathways involved in macrophage polarization in cancer. Front Immunol. 2022;13:1026954
73. Li C, Xue VW, Wang QM, Lian GY, Huang XR, Lee TL, To KF, Tang PM, Lan HY. The Mincle/Syk/NF-κB Signaling Circuit Is Essential for Maintaining the Protumoral Activities of Tumor-Associated Macrophages. Cancer Immunol Res. 2020;8:1004-1017
74. Li W, Yuan Q, Li M, He X, Shen C, Luo Y, Tai Y, Li Y, Deng Z, Luo Y. Research advances on signaling pathways regulating the polarization of tumor-associated macrophages in lung cancer microenvironment. Front Immunol. 2024;15:1452078
75. Joshi S, Liu KX. Macrophage Syk-PI3Kγ Inhibits Antitumor Immunity: SRX3207, a Novel Dual Syk-PI3K Inhibitory Chemotype Relieves Tumor Immunosuppression. Mol Cancer Ther. 2020;19:755-764
76. Kaneda MM, Messer KS, Ralainirina N, Li H, Leem CJ, Gorjestani S, Woo G, Nguyen AV, Figueiredo CC, Foubert P. et al. PI3Kγ is a molecular switch that controls immune suppression. Nature. 2016;539:437-442
77. Viitala M, Virtakoivu R, Tadayon S, Rannikko J, Jalkanen S, Hollmén M. Immunotherapeutic Blockade of Macrophage Clever-1 Reactivates the CD8(+) T-cell Response against Immunosuppressive Tumors. Clin Cancer Res. 2019;25:3289-3303
78. Rohila D, Park IH. Syk Inhibition Reprograms Tumor-Associated Macrophages and Overcomes Gemcitabine-Induced Immunosuppression in Pancreatic Ductal Adenocarcinoma. Cancer Res. 2023;83:2675-2689
79. Kaneda MM, Cappello P, Nguyen AV, Ralainirina N, Hardamon CR, Foubert P, Schmid MC, Sun P, Mose E, Bouvet M. et al. Macrophage PI3Kγ Drives Pancreatic Ductal Adenocarcinoma Progression. Cancer Discov. 2016;6:870-885
80. Gunderson AJ, Kaneda MM, Tsujikawa T, Nguyen AV, Affara NI, Ruffell B, Gorjestani S, Liudahl SM, Truitt M, Olson P. et al. Bruton Tyrosine Kinase-Dependent Immune Cell Cross-talk Drives Pancreas Cancer. Cancer Discov. 2016;6:270-285
81. Nixon BG, Kuo F, Ji L, Liu M, Capistrano K, Do M, Franklin RA, Wu X, Kansler ER, Srivastava RM. et al. Tumor-associated macrophages expressing the transcription factor IRF8 promote T cell exhaustion in cancer. Immunity. 2022;55:2044-2058.e2045
82. Shao Y, Lan Y, Chai X, Gao S, Zheng J, Huang R, Shi Y, Xiang Y, Guo H, Xi Y. et al. CXCL8 induces M2 macrophage polarization and inhibits CD8(+) T cell infiltration to generate an immunosuppressive microenvironment in colorectal cancer. Faseb J. 2023;37:e23173
83. Liu C, Yao Z, Wang J, Zhang W, Yang Y, Zhang Y, Qu X, Zhu Y, Zou J, Peng S. et al. Macrophage-derived CCL5 facilitates immune escape of colorectal cancer cells via the p65/STAT3-CSN5-PD-L1 pathway. Cell Death Differ. 2020;27:1765-1781
84. Wang F, Li B, Wei Y, Zhao Y, Wang L, Zhang P, Yang J, He W, Chen H, Jiao Z, Li Y. Tumor-derived exosomes induce PD1(+) macrophage population in human gastric cancer that promotes disease progression. Oncogenesis. 2018;7:41
85. Wang S, Kuai Y, Lin S, Li L, Gu Q, Zhang X, Li X, He Y, Chen S, Xia X. et al. NF-κB Activator 1 downregulation in macrophages activates STAT3 to promote adenoma-adenocarcinoma transition and immunosuppression in colorectal cancer. BMC Med. 2023;21:115
86. Fan Y, Li Y, Yao X, Jin J, Scott A, Liu B, Wang S, Huo L, Wang Y, Wang R. et al. Epithelial SOX9 drives progression and metastases of gastric adenocarcinoma by promoting immunosuppressive tumour microenvironment. Gut. 2023;72:624-637
87. Li X, Yao W, Yuan Y, Chen P, Li B, Li J, Chu R, Song H, Xie D, Jiang X, Wang H. Targeting of tumour-infiltrating macrophages via CCL2/CCR2 signalling as a therapeutic strategy against hepatocellular carcinoma. Gut. 2017;66:157-167
88. Xie M, Lin Z, Ji X, Luo X, Zhang Z, Sun M, Chen X, Zhang B, Liang H, Liu D. et al. FGF19/FGFR4-mediated elevation of ETV4 facilitates hepatocellular carcinoma metastasis by upregulating PD-L1 and CCL2. J Hepatol. 2023;79:109-125
89. Suzuki K, Ohe R, Kabasawa T, Kitaoka T, Kawai M, Motoi F, Futakuchi M. Histological spatial analysis on the induction of PD-L1(+) macrophages by CD8(+) T cells at the marginal microenvironment of triple-negative breast cancer. Breast Cancer. 2023;30:1094-1104
90. Hundeyin M, Kurz E, Mishra A, Rossi JAK, Liudahl SM, Leis KR, Mehrotra H, Kim M, Torres LE, Ogunsakin A. et al. Innate αβ T Cells Mediate Antitumor Immunity by Orchestrating Immunogenic Macrophage Programming. Cancer Discov. 2019;9:1288-1305
91. Petty AJ, Li A, Wang X, Dai R, Heyman B, Hsu D, Huang X, Yang Y. Hedgehog signaling promotes tumor-associated macrophage polarization to suppress intratumoral CD8+ T cell recruitment. J Clin Invest. 2019;129:5151-5162
92. Chen RH, Xiao ZW, Yan XQ, Han P, Liang FY, Wang JY, Yu ST, Zhang TZ, Chen SQ, Zhong Q, Huang XM. Tumor Cell-Secreted ISG15 Promotes Tumor Cell Migration and Immune Suppression by Inducing the Macrophage M2-Like Phenotype. Front Immunol. 2020;11:594775
93. Zhao C, Wang D, Li Z, Zhang Z, Xu Y, Liu J, Lei Q, Han D, Huo Y, Liu S. et al. IL8 derived from macrophages inhibits CD8(+) T-cell function by downregulating TIM3 expression through IL8-CXCR2 axis in patients with advanced colorectal cancer. Int Immunopharmacol. 2023;121:110457
94. Daley D, Mani VR, Mohan N, Akkad N, Pandian G. NLRP3 signaling drives macrophage-induced adaptive immune suppression in pancreatic carcinoma. J Exp Med. 2017;214:1711-1724
95. Kusumoto Y, Okuyama H, Shibata T, Konno K, Takemoto Y, Maekawa D, Kononaga T, Ishii T, Akashi-Takamura S, Saitoh SI. et al. Epithelial membrane protein 3 (Emp3) downregulates induction and function of cytotoxic T lymphocytes by macrophages via TNF-α production. Cell Immunol. 2018;324:33-41
96. Ma L, Jiang J, Si Q, Chen C, Duan Z. IGF2BP3 Enhances the Growth of Hepatocellular Carcinoma Tumors by Regulating the Properties of Macrophages and CD8(+) T Cells in the Tumor Microenvironment. J Clin Transl Hepatol. 2023;11:1308-1320
97. Xun X, Zhang C, Wang S, Hu S, Xiang X, Cheng Q, Li Z, Wang Y, Zhu J. Cyclooxygenase-2 expressed hepatocellular carcinoma induces cytotoxic T lymphocytes exhaustion through M2 macrophage polarization. Am J Transl Res. 2021;13:4360-4375
98. Chen EP, Markosyan N, Connolly E, Lawson JA, Li X, Grant GR, Grosser T, FitzGerald GA, Smyth EM. Myeloid Cell COX-2 deletion reduces mammary tumor growth through enhanced cytotoxic T-lymphocyte function. Carcinogenesis. 2014;35:1788-1797
99. Zhu Y, Zhou Z, Du X, Lin X, Liang ZM, Chen S, Sun Y, Wang Y, Na Z, Wu Z. et al. Cancer cell-derived arginine fuels polyamine biosynthesis in tumor-associated macrophages to promote immune evasion. Cancer Cell. 2025;43(6):1045-1060.e7
100. Zonari E, Pucci F, Saini M, Mazzieri R, Politi LS, Gentner B, Naldini L. A role for miR-155 in enabling tumor-infiltrating innate immune cells to mount effective antitumor responses in mice. Blood. 2013;122:243-252
101. O'Connell RM, Taganov KD, Boldin MP, Cheng G, Baltimore D. MicroRNA-155 is induced during the macrophage inflammatory response. Proc Natl Acad Sci U S A. 2007;104:1604-1609
102. He M, Xu Z, Ding T, Kuang DM, Zheng L. MicroRNA-155 regulates inflammatory cytokine production in tumor-associated macrophages via targeting C/EBPbeta. Cell Mol Immunol. 2009;6:343-352
103. Cai X, Yin Y, Li N, Zhu D, Zhang J, Zhang CY, Zen K. Re-polarization of tumor-associated macrophages to pro-inflammatory M1 macrophages by microRNA-155. J Mol Cell Biol. 2012;4:341-343
104. Huffaker TB, Lee SH, Tang WW, Wallace JA, Alexander M, Runtsch MC, Larsen DK, Thompson J, Ramstead AG, Voth WP. et al. Antitumor immunity is defective in T cell-specific microRNA-155-deficient mice and is rescued by immune checkpoint blockade. J Biol Chem. 2017;292:18530-18541
105. Yang T, Han Y, Chen J, Liang X, Sun L. MiR-506 Promotes Antitumor Immune Response in Pancreatic Cancer by Reprogramming Tumor-Associated Macrophages toward an M1 Phenotype. Biomedicines. 2023;11:2874
106. Xie F, Zhou X, Fang M, Li H, Su P, Tu Y, Zhang L. Extracellular Vesicles in Cancer Immune Microenvironment and Cancer Immunotherapy. Adv Sci (Weinh). 2019;6:1901779
107. Li X, Wang S, Mu W, Barry J, Han A, Carpenter RL, Jiang BH, Peiper SC, Mahoney MG, Aplin AE. et al. Reactive oxygen species reprogram macrophages to suppress antitumor immune response through the exosomal miR-155-5p/PD-L1 pathway. J Exp Clin Cancer Res. 2022;41:41
108. Yin Y, Liu B, Cao Y, Yao S, Liu Y, Jin G, Qin Y, Chen Y, Cui K, Zhou L. et al. Colorectal Cancer-Derived Small Extracellular Vesicles Promote Tumor Immune Evasion by Upregulating PD-L1 Expression in Tumor-Associated Macrophages. Adv Sci (Weinh). 2022;9:2102620
109. Liu J, Fan L, Yu H, Zhang J, He Y, Feng D, Wang F, Li X, Liu Q, Li Y. et al. Endoplasmic Reticulum Stress Causes Liver Cancer Cells to Release Exosomal miR-23a-3p and Up-regulate Programmed Death Ligand 1 Expression in Macrophages. Hepatology. 2019;70:241-258
110. Salmena L, Poliseno L, Tay Y, Kats L, Pandolfi PP. A ceRNA hypothesis: the Rosetta Stone of a hidden RNA language? Cell. 2011;146:353-358
111. Han TS, Hur K. Epigenetic Associations between lncRNA/circRNA and miRNA in Hepatocellular Carcinoma. Cancers (Basel). 2020;12:2622
112. He L, Chang H, Qi Y, Zhang B, Shao Q. ceRNA Networks: The Backbone Role in Neoadjuvant Chemoradiotherapy Resistance/Sensitivity of Locally Advanced Rectal Cancer. Technol Cancer Res Treat. 2021;20:15330338211062313
113. Qiao X, Cheng Z, Xue K, Xiong C, Zheng Z, Jin X, Li J. Tumor-associated macrophage-derived exosomes LINC01592 induce the immune escape of esophageal cancer by decreasing MHC-I surface expression. J Exp Clin Cancer Res. 2023;42:289
114. Zhu M, Zhu Z, Jiang P, Zheng J, Yan F, Feng J. CircMERTK modulates the suppressive capacity of tumor-associated macrophage via targeting IL-10 in colorectal cancer. Hum Cell. 2023;36:276-285
115. Oshi M, Tokumaru Y. M1 Macrophage and M1/M2 ratio defined by transcriptomic signatures resemble only part of their conventional clinical characteristics in breast cancer. Sci Rep. 2020;10:16554
116. Thomas G, Micci L, Yang W, Katakowski J, Oderup C, Sundar P, Wang X, Geles KG, Potluri S, Salek-Ardakani S. Intra-Tumoral Activation of Endosomal TLR Pathways Reveals a Distinct Role for TLR3 Agonist Dependent Type-1 Interferons in Shaping the Tumor Immune Microenvironment. Front Oncol. 2021;11:711673
117. He Y, Zhan L, Shi J, Xiao M, Zuo R, Wang C, Liu Z, Gong W, Chen L, Luo Y. et al. The Combination of R848 with Sorafenib Enhances Antitumor Effects by Reprogramming the Tumor Immune Microenvironment and Facilitating Vascular Normalization in Hepatocellular Carcinoma. Adv Sci (Weinh). 2023;10:e2207650
118. Ou J, Zheng L, Chen Y, Fu Q, Tan L, Liang E, Huang L, Pan Y, Ke J, Chen Z, Cheng K. Heterocyclic-Modified Imidazoquinoline Derivatives: Selective TLR7 Agonist Regulates Tumor Microenvironment against Melanoma. J Med Chem. 2024;67:3321-3338
119. Wang B, Ou Z. Effective Antitumor Immunity Can Be Triggered by Targeting VISTA in Combination with a TLR3-Specific Adjuvant. Cancer Immunol Res. 2023;11:1656-1670
120. Cresswell GM, Wang B, Kischuk EM. Folate Receptor Beta Designates Immunosuppressive Tumor-Associated Myeloid Cells That Can Be Reprogrammed with Folate-Targeted Drugs. Cancer Res. 2021;81:671-684
121. Chitu V, Stanley ER. Colony-stimulating factor-1 in immunity and inflammation. Curr Opin Immunol. 2006;18:39-48
122. Ries CH, Cannarile MA, Hoves S, Benz J, Wartha K, Runza V, Rey-Giraud F, Pradel LP, Feuerhake F, Klaman I. et al. Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell. 2014;25:846-859
123. Maldonado MDM, Schlom J. Blockade of tumor-derived colony-stimulating factor 1 (CSF1) promotes an immune-permissive tumor microenvironment. Cancer Immunol Immunother. 2023;72:3349-3362
124. Ao JY, Zhu XD, Chai ZT, Cai H, Zhang YY, Zhang KZ, Kong LQ, Zhang N, Ye BG, Ma DN, Sun HC. Colony-Stimulating Factor 1 Receptor Blockade Inhibits Tumor Growth by Altering the Polarization of Tumor-Associated Macrophages in Hepatocellular Carcinoma. Mol Cancer Ther. 2017;16:1544-1554
125. Jeon Y, Kang H, Yang Y, Park D, Choi B, Kim J, Kim J, Nam K. A Novel Selective Axl/Mer/CSF1R Kinase Inhibitor as a Cancer Immunotherapeutic Agent Targeting Both Immune and Tumor Cells in the Tumor Microenvironment. Cancers (Basel). 2022;14:4821
126. Peng X, Hou P, Chen Y, Dai Y, Ji Y, Shen Y, Su Y, Liu B, Wang Y, Sun D. et al. Preclinical evaluation of 3D185; a novel potent inhibitor of FGFR1/2/3 and CSF-1R, in FGFR-dependent and macrophage-dominant cancer models. J Exp Clin Cancer Res. 2019;38:372
127. Chang YW, Hsiao HW, Chen JP, Tzeng SF, Tsai CH, Wu CY, Hsieh HH, Carmona SJ, Andreatta M, Di Conza G. et al. A CSF-1R-blocking antibody/IL-10 fusion protein increases anti-tumor immunity by effectuating tumor-resident CD8(+) T cells. Cell Rep Med. 2023;4:101154
128. Liu D, Mamorska-Dyga A. Syk inhibitors in clinical development for hematological malignancies. J Hematol Oncol. 2017;10:145
129. Issara-Amphorn J, Chancharoenthana W, Visitchanakun P, Leelahavanichkul A. Syk Inhibitor Attenuates Polymicrobial Sepsis in FcgRIIb-Deficient Lupus Mouse Model, the Impact of Lupus Characteristics in Sepsis. J Innate Immun. 2020;12:461-479
130. Stephens M, Keane K, Roizes S, Liao S, Weid PV. Mincle-binding DNA aptamer demonstrates therapeutic potential in a model of inflammatory bowel disease. Mol Ther Nucleic Acids. 2022;28:935-947
131. Wu Y, Liu Y, Wu H, Tong M, Du L, Ren S, Che Y. Advances in Ultrasound-Targeted Microbubble Destruction (UTMD) for Breast Cancer Therapy. Int J Nanomedicine. 2025;20:1425-1442
132. Xue VW, Chung JY, Tang PC, Chan AS, To TH, Chung JS, Mussal F, Lam EW, Li C, To KF. et al. USMB-shMincle: a virus-free gene therapy for blocking M1/M2 polarization of tumor-associated macrophages. Mol Ther Oncolytics. 2021;23:26-37
133. De Henau O, Rausch M, Winkler D, Campesato LF, Liu C, Cymerman DH, Budhu S, Ghosh A, Pink M, Tchaicha J. et al. Overcoming resistance to checkpoint blockade therapy by targeting PI3Kγ in myeloid cells. Nature. 2016;539:443-447
134. Zheng W, Pollard JW. Inhibiting macrophage PI3Kγ to enhance immunotherapy. Cell Res. 2016;26:1267-1268
135. Carnevalli LS, Taylor MA. Macrophage Activation Status Rather than Repolarization Is Associated with Enhanced Checkpoint Activity in Combination with PI3Kγ Inhibition. Mol Cancer Ther. 2021;20:1080-1091
136. Heller S, Glaeske S, Gluske K, Paul J, Böhme A, Janzer A, Roider HG, Montebaur A, Nicke B, Lesche R. et al. Pan-PI3K inhibition with copanlisib overcomes Treg- and M2-TAM-mediated immune suppression and promotes anti-tumor immune responses. Clin Exp Med. 2023;23:5445-5461
137. Wang YN, Wang YY, Wang J, Bai WJ, Miao NJ, Wang J. Vinblastine resets tumor-associated macrophages toward M1 phenotype and promotes antitumor immune response. J Immunother Cancer. 2023;11:e007253
138. Sun JH, Liang X, Cai M, Yan L, Chen Z, Guo L, Jing L, Wang Y, Zhou D. Protein-Crowned Micelles for Targeted and Synergistic Tumor-Associated Macrophage Reprogramming to Enhance Cancer Treatment. Nano Lett. 2022;22:4410-4420
139. Li Y, Cao F, Li M, Li P, Yu Y, Xiang L, Xu T, Lei J, Tai YY, Zhu J. et al. Hydroxychloroquine induced lung cancer suppression by enhancing chemo-sensitization and promoting the transition of M2-TAMs to M1-like macrophages. J Exp Clin Cancer Res. 2018;37:259
140. Golino JL, Wang X. Anti-Cancer Activity of Verteporfin in Cholangiocarcinoma. Cancers (Basel). 2023;15:2454
141. Ishii T, Mimura I, Nagaoka K, Naito A, Sugasawa T, Kuroda R, Yamada D, Kanki Y, Kume H, Ushiku T, Kakimi K. Effect of M2-like macrophages of the injured-kidney cortex on kidney cancer progression. Cell Death Discov. 2022;8:480
142. Vuong L, Kouverianou E, Rooney CM, McHugh BJ, Howie SEM, Gregory CD, Forbes SJ, Henderson NC. An Orally Active Galectin-3 Antagonist Inhibits Lung Adenocarcinoma Growth and Augments Response to PD-L1 Blockade. Cancer Res. 2019;79:1480-1492
143. Wang Y, Wei B, Gao J. Combination of Fruquintinib and Anti-PD-1 for the Treatment of Colorectal Cancer. J Immunol. 2020;205:2905-2915
144. Nunes SP, Morales L, Rubio C, Munera-Maravilla E, Lodewijk I. Modulation of tumor microenvironment by targeting histone acetylation in bladder cancer. Cell Death Discov. 2024;10:1
145. Figueiredo P, Lepland A, Scodeller P, Fontana F, Torrieri G, Tiboni M, Shahbazi MA, Casettari L, Kostiainen MA, Hirvonen J. et al. Peptide-guided resiquimod-loaded lignin nanoparticles convert tumor-associated macrophages from M2 to M1 phenotype for enhanced chemotherapy. Acta Biomater. 2021;133:231-243
146. Zhao J, Zhang Z, Xue Y, Wang G, Cheng Y, Pan Y, Zhao S, Hou Y. Anti-tumor macrophages activated by ferumoxytol combined or surface-functionalized with the TLR3 agonist poly (I: C) promote melanoma regression. Theranostics. 2018;8:6307-6321
147. Zanganeh S, Hutter G, Spitler R, Lenkov O, Mahmoudi M, Shaw A, Pajarinen JS, Nejadnik H, Goodman S, Moseley M. et al. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat Nanotechnol. 2016;11:986-994
148. Wen D, Liang T, Chen G, Li H, Wang Z, Wang J, Fu R, Han X, Ci T, Zhang Y. et al. Adipocytes Encapsulating Telratolimod Recruit and Polarize Tumor-Associated Macrophages for Cancer Immunotherapy. Adv Sci (Weinh). 2023;10:e2206001
149. Xu X, Yi C, Feng T, Ge Y, Liu M, Wu C, Yu H, Chen X, Gopinath SCB, Zhang W. et al. Regulating tumor microenvironments by a lymph node-targeting adjuvant via tumor-specific CTL-derived IFNγ. Clin Immunol. 2023;253:109685
150. Li C, Guan N, Liu F. T7 peptide-decorated exosome-based nanocarrier system for delivery of Galectin-9 siRNA to stimulate macrophage repolarization in glioblastoma. J Neurooncol. 2023;162:93-108
151. Zhang Q, Guo Y, Zhu L, Liu X, Yang J, Li Y, Zhu X. A nucleic acid nanogel dually bears siRNA and CpG motifs for synergistic tumor immunotherapy. Biomater Sci. 2021;9:4755-4764
152. Joshi MD, Unger WJ, Storm G, van Kooyk Y, Mastrobattista E. Targeting tumor antigens to dendritic cells using particulate carriers. J Control Release. 2012;161:25-37
153. De Serrano LO, Burkhart DJ. Liposomal vaccine formulations as prophylactic agents: design considerations for modern vaccines. J Nanobiotechnology. 2017;15:83
154. Gu Z, Da Silva CG. Liposome-Based Drug Delivery Systems in Cancer Immunotherapy. Pharmaceutics. 2020;12:1054
155. Luo K, Yang L, Yan C, Zhao Y, Li Q, Liu X, Xie L, Sun Q, Li X. A Dual-Targeting Liposome Enhances Triple-Negative Breast Cancer Chemoimmunotherapy through Inducing Immunogenic Cell Death and Inhibiting STAT3 Activation. Small. 2023;19:e2302834
156. Zhang Y, Bush X, Yan B, Chen JA. Gemcitabine nanoparticles promote antitumor immunity against melanoma. Biomaterials. 2019;189:48-59
157. Kim SH, Kim SJ. Reprograming of Tumor-Associated Macrophages in Breast Tumor-Bearing Mice under Chemotherapy by Targeting Heme Oxygenase-1. Antioxidants (Basel). 2021;10:470
158. Grabowska J, Affandi AJ, van Dinther D, Nijen Twilhaar MK, Olesek K, Hoogterp L, Ambrosini M, Heijnen DAM, Klaase L, Hidalgo A. et al. Liposome induction of CD8(+) T cell responses depends on CD169(+) macrophages and Batf3-dependent dendritic cells and is enhanced by GM3 inclusion. J Control Release. 2021;331:309-320
159. Edgar LJ, Kawasaki N, Nycholat CM, Paulson JC. Targeted Delivery of Antigen to Activated CD169(+) Macrophages Induces Bias for Expansion of CD8(+) T Cells. Cell Chem Biol. 2019;26:131-136.e134
160. Grabowska J, Stolk DA. Liposomal Nanovaccine Containing α-Galactosylceramide and Ganglioside GM3 Stimulates Robust CD8(+) T Cell Responses via CD169(+) Macrophages and cDC1. Vaccines (Basel). 2021;9:56
161. Chen Y, Gong L, Cao Y, Liu Z, Wang Y, Cheng H, Feng Y, Yao S, Yin Y, Wu Z, Huang Z. Reprogramming tumor-associated macrophages by a dually targeted milk exosome system as a potent monotherapy for cancer. J Control Release. 2024;366:395-409
162. Liu X, Zhou J, Wu H, Chen S, Zhang L, Tang W, Duan L, Wang Y, McCabe E, Hu M. et al. Fibrotic immune microenvironment remodeling mediates superior anti-tumor efficacy of a nano-PD-L1 trap in hepatocellular carcinoma. Mol Ther. 2023;31:119-133
163. Pundkar C, Antony F, Kang X, Mishra A, Babu RJ, Chen P, Li F, Suryawanshi A. Targeting Wnt/β-catenin signaling using XAV939 nanoparticles in tumor microenvironment-conditioned macrophages promote immunogenicity. Heliyon. 2023;9:e16688
164. Liu B, Wang X, Chen TZ, Li GL, Tan CC, Chen Y, Duan SQ. Polarization of M1 tumor associated macrophage promoted by the activation of TLR3 signal pathway. Asian Pac J Trop Med. 2016;9:484-488
165. Jagodinsky JC, Bates AM, Clark PA. Local TLR4 stimulation augments in situ vaccination induced via local radiation and anti-CTLA-4 checkpoint blockade through induction of CD8 T-cell independent Th1 polarization. J Immunother Cancer. 2022;10:e005103
166. Hervas-Stubbs S, Perez-Gracia JL, Rouzaut A, Sanmamed MF, Le Bon A, Melero I. Direct effects of type I interferons on cells of the immune system. Clin Cancer Res. 2011;17:2619-2627
167. Pylaeva E, Lang S, Jablonska J. The Essential Role of Type I Interferons in Differentiation and Activation of Tumor-Associated Neutrophils. Front Immunol. 2016;7:629
168. Liao J, Zeng DN, Li JZ, Hua QM, Huang CX, Xu J, Wu C, Zheng L, Wen WP, Wu Y. Type I IFNs repolarized a CD169(+) macrophage population with anti-tumor potentials in hepatocellular carcinoma. Mol Ther. 2022;30:632-643
169. Xu H, Piao L, Wu Y, Liu X. IFN-γ enhances the antitumor activity of attenuated salmonella-mediated cancer immunotherapy by increasing M1 macrophage and CD4 and CD8 T cell counts and decreasing neutrophil counts. Front Bioeng Biotechnol. 2022;10:996055
170. Casadesús AV, Deligne C, Diallo BK, Sosa K, Josseaume N, Mesa C, León K, Hernández T, Teillaud JL. A rationally-engineered IL-2 improves the antitumor effect of anti-CD20 therapy. Oncoimmunology. 2020;9:1770565
171. Nan Y, Zhang X, Wang S, Xu C, Wang Y, Han L, Luan J, Hu X, Chen W, Cao Z. et al. Targeting CD47 enhanced the antitumor immunity of PD-L1 blockade in B-cell lymphoma. Immunotherapy. 2023;15:175-187
172. Alimohammadi R, Mahmoodi Chalbatani G, Alimohammadi M, Ghaffari-Nazari H, Rahimi A, Mortaz E, Mossafa N, Boon L, Jalali SA. Dual blockage of both PD-L1 and CD47 enhances the therapeutic effect of oxaliplatin and FOLFOX in CT-26 mice tumor model. Sci Rep. 2023;13:2472
173. Rana M, Kansal R, Chaib M, Teng B, Morrrison M, Hayes DN, Stanfill AG, Shibata D, Carson JA, Makowski L, Glazer ES. The pancreatic cancer immune tumor microenvironment is negatively remodeled by gemcitabine while TGF-β receptor plus dual checkpoint inhibition maintains antitumor immune cells. Mol Carcinog. 2022;61:549-557
174. House IG, Savas P, Lai J, Chen AXY, Oliver AJ, Teo ZL, Todd KL, Henderson MA, Giuffrida L, Petley EV. et al. Macrophage-Derived CXCL9 and CXCL10 Are Required for Antitumor Immune Responses Following Immune Checkpoint Blockade. Clin Cancer Res. 2020;26:487-504
175. La Fleur L, Botling J, He F, Pelicano C. Targeting MARCO and IL37R on Immunosuppressive Macrophages in Lung Cancer Blocks Regulatory T Cells and Supports Cytotoxic Lymphocyte Function. Cancer Res. 2021;81:956-967
176. Cassetta L, Kitamura T. Targeting Tumor-Associated Macrophages as a Potential Strategy to Enhance the Response to Immune Checkpoint Inhibitors. Front Cell Dev Biol. 2018;6:38
177. Chen Y, Zhu X, Liu H, Wang C, Chen Y, Wang H, Fang Y, Wu X, Xu Y, Li C. et al. The application of HER2 and CD47 CAR-macrophage in ovarian cancer. J Transl Med. 2023;21:654
178. Yang Y, Sun M, Yao W, Wang F, Li X, Wang W, Li J, Gao Z, Qiu L, You R. et al. Compound kushen injection relieves tumor-associated macrophage-mediated immunosuppression through TNFR1 and sensitizes hepatocellular carcinoma to sorafenib. J Immunother Cancer. 2020;8:e000317
179. Zhang W, Zhang R, Chang Z, Wang X. Resveratrol activates CD8(+) T cells through IL-18 bystander activation in lung adenocarcinoma. Front Pharmacol. 2022;13:1031438
180. Chang Z, Zhang Q, Hu Q, Liu Y, Zhang L, Liu R. Tannins in Terminalia bellirica inhibits hepatocellular carcinoma growth via re-educating tumor-associated macrophages and restoring CD8(+)T cell function. Biomed Pharmacother. 2022;154:113543
181. Kawaguchi S, Kawahara K. Naringenin potentiates anti-tumor immunity against oral cancer by inducing lymph node CD169-positive macrophage activation and cytotoxic T cell infiltration. Cancer Immunol Immunother. 2022;71:2127-2139
182. Lomakova YD, Londregan J, Maslanka J, Goldman N, Somerville J, Riggs JE. PHA eludes macrophage suppression to activate CD8(+) T cells. Immunobiology. 2019;224:94-101
183. Wyrobnik I, Steinberg M, Gelfand A, Rosenblum R, Eid Mutlak Y, Sulimani L, Procaccia S, Ofran Y, Novak-Kotzer H, Meiri D. Decreased melanoma CSF-1 secretion by Cannabigerol treatment reprograms regulatory myeloid cells and reduces tumor progression. Oncoimmunology. 2023;12:2219164
184. Sun M, Bu R, Zhang B, Cao Y, Liu C, Zhao W. Lentinan Inhibits Tumor Progression by Immunomodulation in a Mouse Model of Bladder Cancer. Integr Cancer Ther. 2020;19:1534735420946823
185. Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41:49-61
186. Yoshida S, Shime H, Matsumoto M, Kasahara M, Seya T. Anti-oxidative Amino Acid L-ergothioneine Modulates the Tumor Microenvironment to Facilitate Adjuvant Vaccine Immunotherapy. Front Immunol. 2019;10:671
187. Wang J, Yang L, Mao X, Li Z, Lin X, Jiang C. Streptococcus salivarius-mediated CD8(+) T cell stimulation required antigen presentation by macrophages in oral squamous cell carcinoma. Exp Cell Res. 2018;366:121-126
188. Kremenovic M, Chan AA, Feng B, Bäriswyl L, Robatel S, Gruber T, Tang L, Lee DJ, Schenk M. BCG hydrogel promotes CTSS-mediated antigen processing and presentation, thereby suppressing metastasis and prolonging survival in melanoma. J Immunother Cancer. 2022;10:e004133
189. Lv M, Chen M, Zhang R, Zhang W, Wang C, Zhang Y, Wei X, Guan Y. Manganese is critical for antitumor immune responses via cGAS-STING and improves the efficacy of clinical immunotherapy. Cell Res. 2020;30:966-979
190. Pushalkar S, Hundeyin M, Daley D, Zambirinis CP, Kurz E, Mishra A, Mohan N, Aykut B, Usyk M, Torres LE. et al. The Pancreatic Cancer Microbiome Promotes Oncogenesis by Induction of Innate and Adaptive Immune Suppression. Cancer Discov. 2018;8:403-416
191. Alvero AB, Fox A. Immune Modulation of Innate and Adaptive Responses Restores Immune Surveillance and Establishes Antitumor Immunologic Memory. Cancer Immunol Res. 2024;12:261-274
192. Cai Z, Lim D, Liu G, Chen C, Jin L, Duan W, Ding C, Sun Q, Peng J, Dong C. et al. Valproic Acid-Like Compounds Enhance and Prolong the Radiotherapy Effect on Breast Cancer by Activating and Maintaining Anti-Tumor Immune Function. Front Immunol. 2021;12:646384
193. Moreira D, Sampath S, Won H, White SV, Su YL, Alcantara M, Wang C, Lee P, Maghami E, Massarelli E, Kortylewski M. Myeloid cell-targeted STAT3 inhibition sensitizes head and neck cancers to radiotherapy and T cell-mediated immunity. J Clin Invest. 2021;131:e137001
194. Kortylewski M, Xin H, Kujawski M, Lee H, Liu Y, Harris T, Drake C, Pardoll D, Yu H. Regulation of the IL-23 and IL-12 balance by Stat3 signaling in the tumor microenvironment. Cancer Cell. 2009;15:114-123
195. Kujawski M, Zhang C, Herrmann A, Reckamp K, Scuto A, Jensen M, Deng J, Forman S, Figlin R, Yu H. Targeting STAT3 in adoptively transferred T cells promotes their in vivo expansion and antitumor effects. Cancer Res. 2010;70:9599-9610
196. Kaech SM, Cui W. Transcriptional control of effector and memory CD8+ T cell differentiation. Nat Rev Immunol. 2012;12:749-761
197. Huynh J, Chand A. Therapeutically exploiting STAT3 activity in cancer - using tissue repair as a road map. Nat Rev Cancer. 2019;19:82-96
198. Gao Y, Li Y, Lin Z, Zeng Y, Huang Z, Han L, Zhong Y, Gong Y, Wu Q, Xie C. Ataxia telangiectasia mutated kinase inhibition promotes irradiation-induced PD-L1 expression in tumour-associated macrophages through IFN-I/JAK signalling pathway. Immunology. 2023;168:346-361
199. Bekeschus S, Clemen R, Nießner F, Sagwal SK, Freund E, Schmidt A. Medical Gas Plasma Jet Technology Targets Murine Melanoma in an Immunogenic Fashion. Adv Sci (Weinh). 2020;7:1903438
200. Bekeschus S, Roessler K, Kepp O, Freund E. Gas Plasma Technology and Immunogenic Cell Death: Implications for Chordoma Treatment. Cancers. 2025;17:681
201. Wang T, Xu H. Multi-faced roles of reactive oxygen species in anti-tumor T cell immune responses and combination immunotherapy. Exploration of Medicine. 2022;3:77-98
202. O'Reilly A, Zhao W, Wickström S. Reactive oxygen species: Janus-faced molecules in the era of modern cancer therapy. J Immunother Cancer. 2024;12:e009409
Author contact
![]() Corresponding author: cxdengedu.mo.
Corresponding author: cxdengedu.mo.

 Global reach, higher impact
Global reach, higher impact